Abstract
Nutritional requirements for growth at 30 C of Leptospira pomona and L. canicola have been determined. Both pathogenic serotypes initially required bovine serum albumin (BSA) for growth in a medium (SM-4) which permitted growth of the water isolate B-16. Requirement for BSA was eliminated by (i) removing much of the apparent toxicity of free fatty acids in Tween 80 on an anion exchange column, (ii) decreasing extended lag periods observed from small inocula by incorporation of pyruvate into the medium, (iii) the addition of acetate to permit full utilization of substrate fatty acids in Tween 80, and (iv) the addition of glycerol to decrease generation times. Physiologic significance of these findings is discussed, and the possibility is suggested that apparent toxicity of fatty acids for leptospires may result from their auto-oxidation products. The resulting protein-free medium (SM-5) permitted the growth of pathogens at 30 C to high cell yields in low inocula. Highly virulent and avirulent strains from the same clone of L. canicola Moulton were used to determine additional growth requirements associated with virulence. As incubation temperatures were increased from 30 C to those of mammalian hosts, virulent cells required biotin at 35 C and higher levels of K+ and Mg2+ at 37 C. Additional Fe2+ eliminated the necessity for removing the toxicity of Tween 80 by anion exchange. Significance of these physiologic studies are discussed in relationship to virulence. The final protein-free medium (SM-6) grew highly virulent L. canicola from tissue to high yields from low inocula at 37 C with no loss in virulence over several transfers.
Full text
PDF

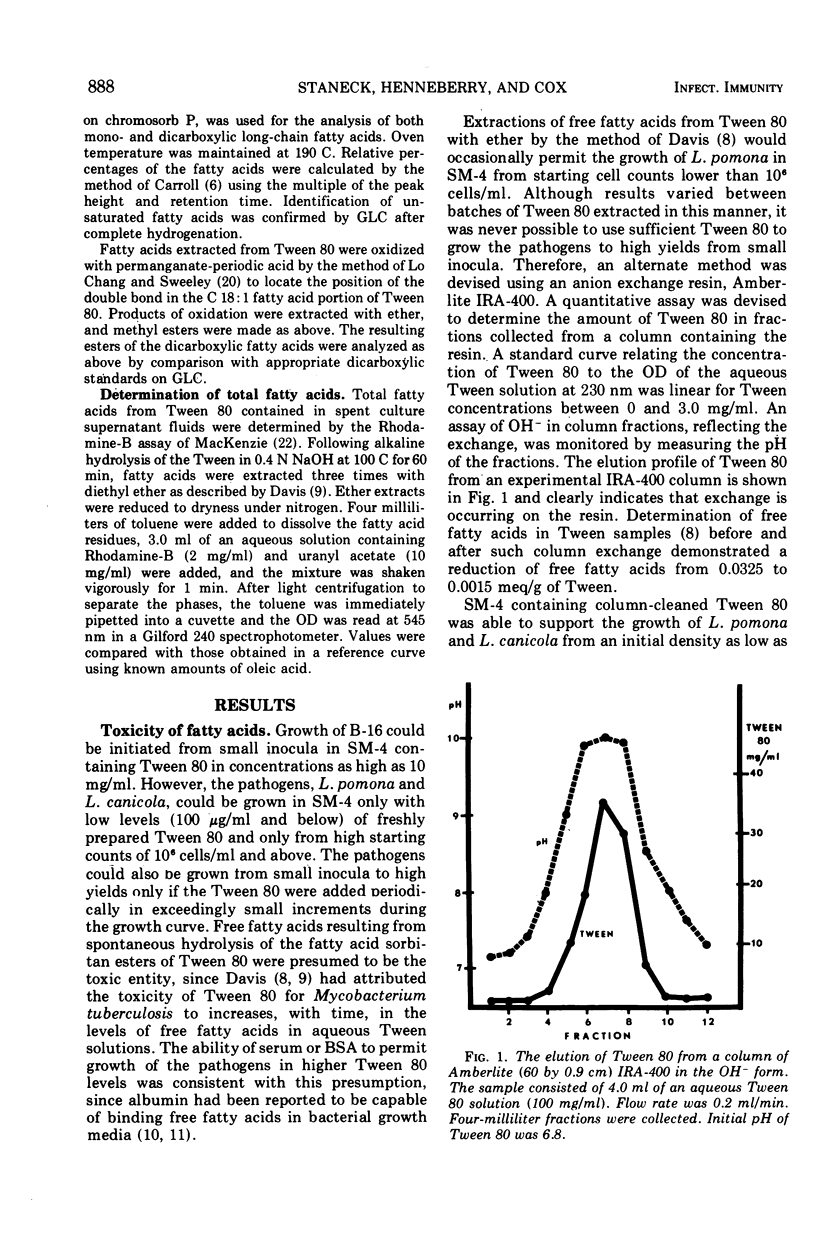


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auran N. E., Johnson R. C., Ritzi D. M. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect Immun. 1972 Jun;5(6):968–975. doi: 10.1128/iai.5.6.968-975.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBER A. A. Inhibition of lipid peroxide formation by vertebrate blood serum. Arch Biochem Biophys. 1961 Jan;92:38–43. doi: 10.1016/0003-9861(61)90215-6. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cox C. D. Intermediate energy metabolism of Leptospira. J Bacteriol. 1969 Mar;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cox C. D. Terminal electron transport in Leptospira. J Bacteriol. 1969 Mar;97(3):1001–1004. doi: 10.1128/jb.97.3.1001-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Henneberry R. C., Cox C. D. Isolation and growth of Leptospira on artificial media. J Bacteriol. 1966 Mar;91(3):1374–1375. doi: 10.1128/jb.91.3.1374-1375.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL K. K. Quantitative estimation of peak areas in gas-liquid chromatography. Nature. 1961 Jul 22;191:377–378. doi: 10.1038/191377a0. [DOI] [PubMed] [Google Scholar]
- COX C. D., LARSON A. D. Colonial growth of leptospirae. J Bacteriol. 1957 Apr;73(4):587–589. doi: 10.1128/jb.73.4.587-589.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAINE S. Virulence in leptospirae. III. Comparison of sensitivities of virulent and of avirulent Leptospira icterohaemorrhagiae to cultural conditons. J Bacteriol. 1959 May;77(5):599–603. doi: 10.1128/jb.77.5.599-603.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., CARLIN C. E. Studies on the nutrition and physiology of Pasteurella pestis. II. A defined medium for the growth of Pasteurella pestis. J Bacteriol. 1958 Apr;75(4):409–413. doi: 10.1128/jb.75.4.409-413.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLS G. M., SPURR E. D. The effect of temperature on the nutritional requirements of Pasteurella pestis. J Gen Microbiol. 1952 Feb;6(1-2):64–73. doi: 10.1099/00221287-6-1-2-64. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Baseman J. B., Cox C. D. Growth of a water strain of Leptospira in synthetic media. Antonie Van Leeuwenhoek. 1970;36(4):489–501. doi: 10.1007/BF02069051. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Cox C. D. Beta-oxidation of fatty acids by Leptospira. Can J Microbiol. 1970 Jan;16(1):41–45. doi: 10.1139/m70-007. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M., Anderson L. E., Holman R. T., Ismail I. A., Gunstone F. D. Effect of isomeric cis-octadecenoic acids on the growth of Leptospira interrogans serotype patoc. J Bacteriol. 1969 Jun;98(3):1026–1029. doi: 10.1128/jb.98.3.1026-1029.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOAT A. G., EMMONS E. K. The amino acid nutrition of yeast in relationship to biotin deficiency. J Bacteriol. 1954 Dec;68(6):687–690. doi: 10.1128/jb.68.6.687-690.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie R. D., Blohm T. R., Auxier E. M., Luther A. C. Rapid colorimetric micromethod for free fatty acids. J Lipid Res. 1967 Nov;8(6):589–597. [PubMed] [Google Scholar]
- SINCLAIR N. A., STOKES J. L. Factors which control maximal growth of bacteria. J Bacteriol. 1962 May;83:1147–1154. doi: 10.1128/jb.83.5.1147-1154.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STALHEIM O. H., WILSON J. B. CULTIVATION OF LEPTOSPIRAE. I. NUTRITION OF LEPTOSPIRA CANICOLA. J Bacteriol. 1964 Jul;88:48–54. doi: 10.1128/jb.88.1.48-54.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenberg E. Growth of pathogenic Leptospira in chemically defined media. J Bacteriol. 1967 May;93(5):1598–1606. doi: 10.1128/jb.93.5.1598-1606.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalheim O. H. Leptospiral selection, growth, and virulence in synthetic medium. J Bacteriol. 1966 Oct;92(4):946–951. doi: 10.1128/jb.92.4.946-951.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Roles of metallic ions in host-parasite interactions. Bacteriol Rev. 1966 Mar;30(1):136–151. doi: 10.1128/br.30.1.136-151.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



