Abstract
Simple catalysts that use atom-economical oxygen as the terminal oxidant to accomplish selective ortho–ortho, ortho–para, or para–para homo-couplings of phenols are described. In addition, chromium salen catalysts have been discovered as uniquely effective in the cross-coupling of different phenols with high chemo- and regioselectivity.
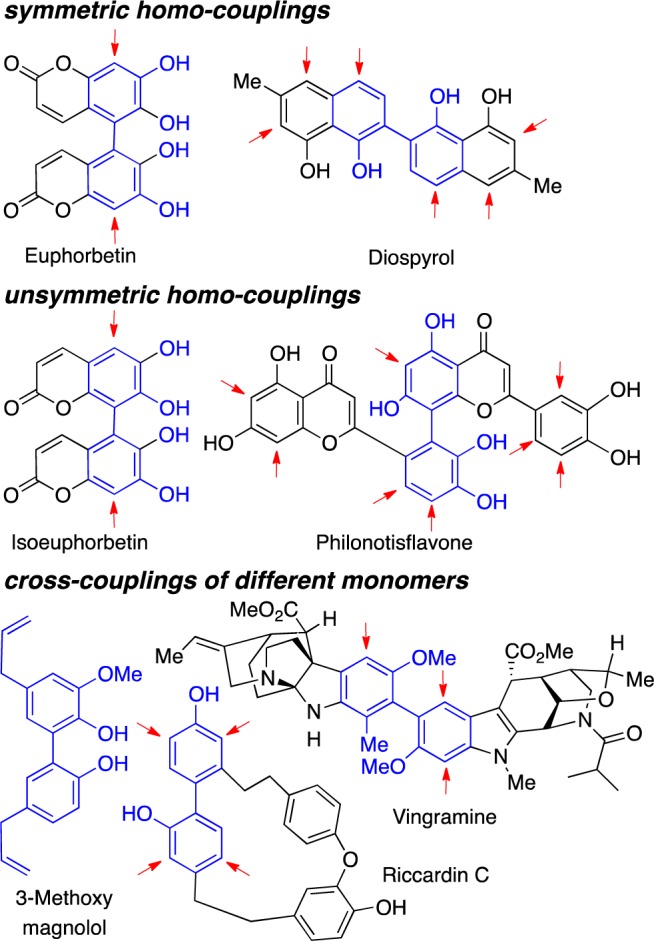
Since the groundbreaking work by Barton and Erdtmann that phenol oxidation is a key step in the biosynthesis of several natural product classes,1 chemists have been inspired to develop laboratory analogues of these important processes.2 Numerous natural products can be constructed via different oxidative phenol couplings including homo-coupling at the same site, homo-coupling at different sites, and cross-coupling of different phenols (Chart 1).2c−2f Due to the vast array of useful biological activities associated with these compounds, especially their antibacterial and antifungal properties, these compounds remain the subject of intense interest.2c−2f While many stoichiometric phenolic oxidations have been studied,2a,3 the coupling selectivities are typically low when multiple coupling sites are available (see red arrows in Chart 1). Furthermore, the use of superstoichiometric reagents is undesirable.4 Herein, we disclose simple catalysts that use atom-economical oxygen as the terminal oxidant to accomplish selective ortho–ortho, ortho–para, or para–para homo-couplings of phenols. In addition, chromium salen catalysts have been found to be exceptional in cross-coupling two different phenols with high selectivity.
Chart 1. Phenolic Coupling Natural Products.
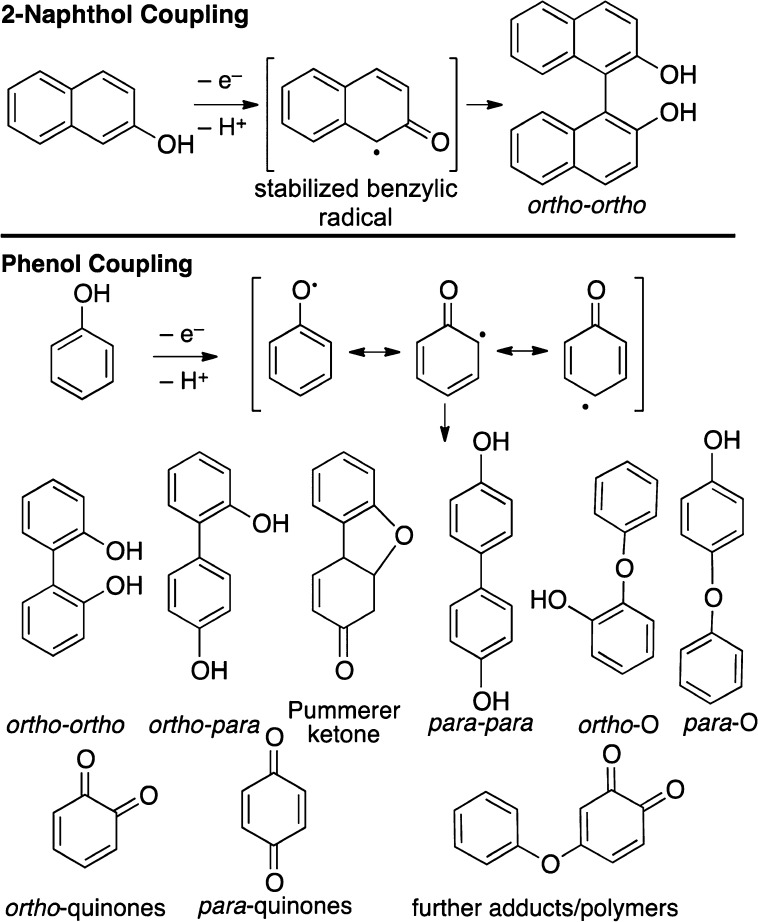
Few nonenzymatic catalytic systems have been reported for the oxidative coupling of the parent phenols, even though there are many for 2-naphthols.5 Due to the difference in oxidation potentials (naphthol = 1.87 eV, phenol = 2.10 eV),6 the oxidation of phenols is more difficult. In addition diverse product mixtures are observed due to similar stabilities of the different radical resonance forms relative to naphthol (Scheme 1).5a In addition, direct oxygenation of the aromatic ring to quinones and further adducts becomes competitive.
Scheme 1. Possible Outcomes in 2-Naphthol vs Phenol Oxidation.
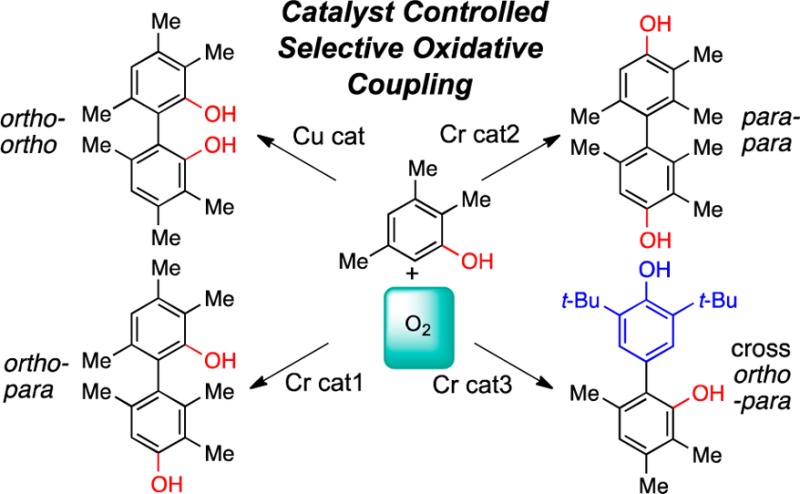
Our strategy to explore this challenging transformation centered on metal catalysts that are reoxidized readily by O2. Based on prior experience with 2-naphthol coupling, we elected to examine Cr, Cu, Fe, Mn, Ru, and V.5 An appropriate ligand framework that stabilizes the metal, is tuned easily and is oxidatively stable was crucial. For phenol coupling, the salen/salan scaffold7−9 proved superior. Due to the large number of variables (36 catalysts, Chart 2, R = H; solvent; additives; substrates), parallel microscale screening10 was used to rapidly identify trends (Figure 1). To test the premise that these catalysts are appropriate for phenol oxidation and that O2 was being effectively introduced into the reaction microvials, a substrate (Table 1, entry 1) that readily undergoes phenolic coupling to a single ortho–ortho product was tested first. Gratifyingly, almost all the catalysts were effective to some degree with this substrate (Figure 1, entry 1). Further bench scale optimization revealed a Ru catalyst as highly effective with oxygen for this substrate (Table 1, entry 1).
Chart 2. Catalyst Library.
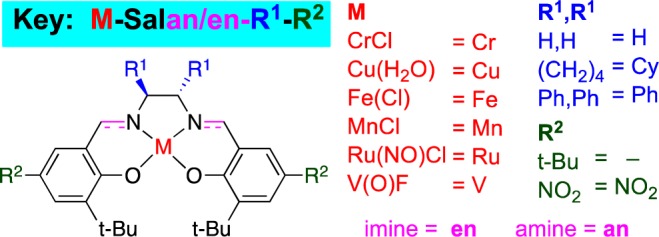
Figure 1.
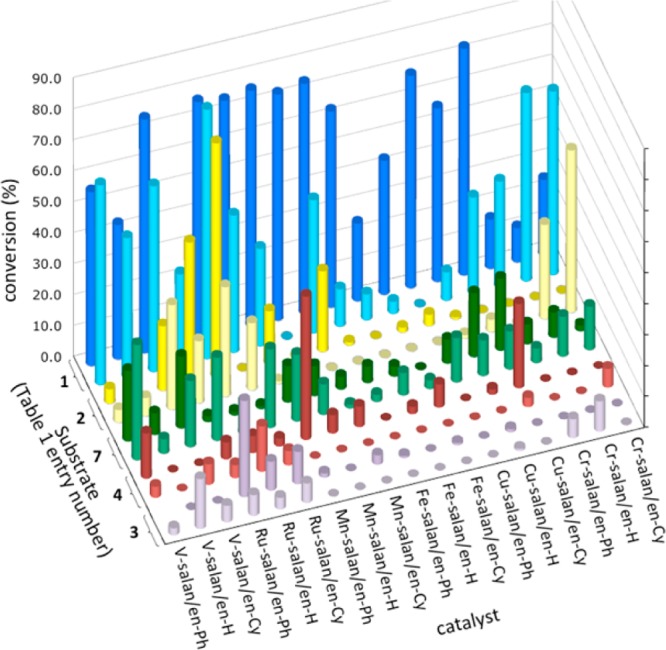
36 catalysts (20–30 mol %, 40–80 °C, DCE, 1 d) with five substrates in oxidative phenolic coupling using O2. For each substrate: top row = salan, bottom row = salen. Conversion is for the ortho–ortho products.
Table 1. Selective Phenol Homo-Couplings.
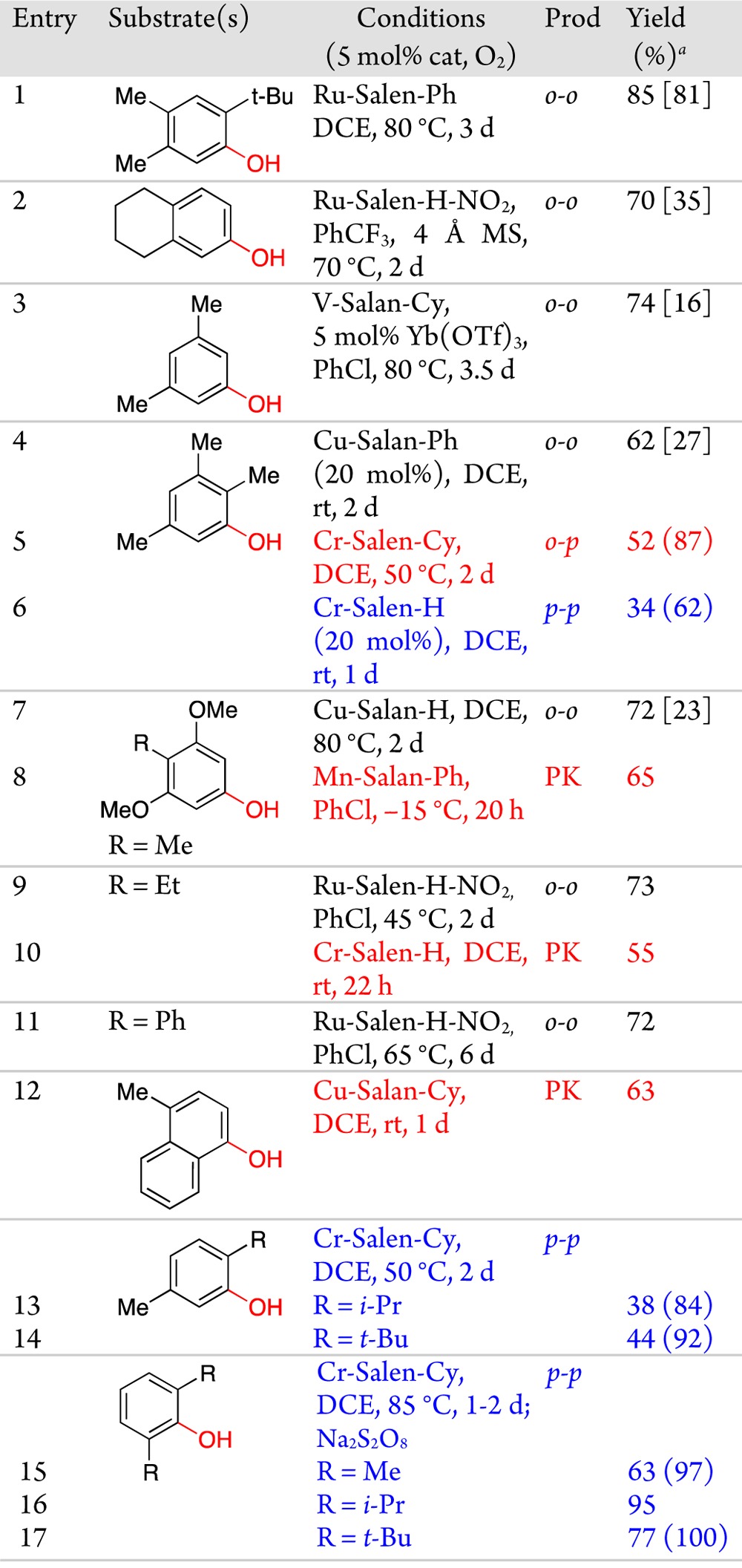
Parenthetical yields are based on recovered substrate. Bracketed yields are unoptimized parallel screening results.
With substrates that are not effectively coupled even with stoichiometric oxidants, the initial screen (Figure 1, bottom four entries) showed lower yields. However, the trends narrowed the focus for further optimization. By examining temperature, solvents, and additives,5eortho–ortho coupling of a range of substrates was achieved (Table 1, entries 2–4, 7). To improve reactivity for reluctant substrates, we theorized that an electron-withdrawing substituent NO2 (R2, Chart 2) would improve the oxidizing power of the Ru-Salen-H. With this second generation catalyst, higher yields were seen for entries 9 and 11. Overall, Ru salens are the most general for ortho–ortho coupling, but some substrates respond better to V or Cu catalysts.
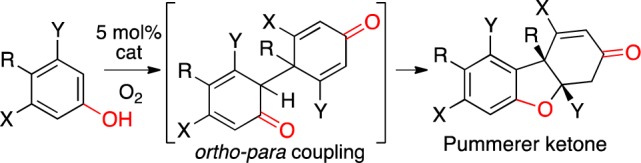
With entries 7 and 9 from Table 1, an additional major peak was seen in the HPLC spectra from the initial screening. Re-examination of the data rapidly identified catalysts selective for this compound (beige highlights in Figure 2). This material was ultimately determined to be the tricyclic Pummerer ketone1,2a (PK), which forms via ortho–para coupling followed by a 1,4-addition (Scheme 2). Optimized conditions provided this PK with high efficiency (Table 1, entries 8, 10, 12). Notably, this motif is found in several natural products such as the galanthamines and usnic acids.11 On the other hand, when the para-position is unsubstituted, ortho–para bisphenols are generated (entry 5). Notably, different catalysts permit control of ortho–ortho vs ortho–para coupling (Table 1, entries 4/5, 7/8, 9/10).
Figure 2.
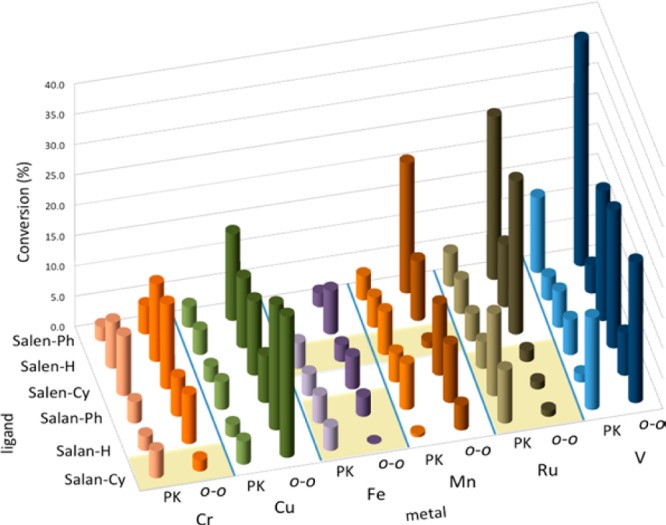
Amounts of ortho–ortho (o–o) and PK products from Table 1, entry 7 with 36 catalysts using O2. Beige shading indicates PK is the major product.
Scheme 2. Formation of PK.
The next challenge was identifying catalysts for para–para coupling. When there is competition between ortho- and para-sites, selective catalysts were found (Table 1, entries 6, 13, 14), but yields were modest due to low reactivity, a challenge that remains to be addressed. When the ortho-positions are blocked, the expected para-product is obtained (Table 1, entries 15–17). Most interestingly, selective catalysts for ortho–ortho, ortho–para, and para–para coupling of 2,3,5-trimethylphenol have been identified (Table 1, entries 4–6) showing the versatility of this catalytic aerobic coupling.
At this juncture, the question of cross-coupling different phenols arose, a very difficult venture since any catalyst must promote the cross-coupling much faster than either of the corresponding homo-couplings.2,12,13 Initially, phenols with only one open coupling site were used limiting the outcome to three coupling products (Table 2, entries 1–2). Remarkably, a Cr catalyst affected cross-coupling with high efficiency (75–85%) with only a 1.2:1 reactant stoichiometry.
Table 2. Cross-Coupling of Different Monomers.
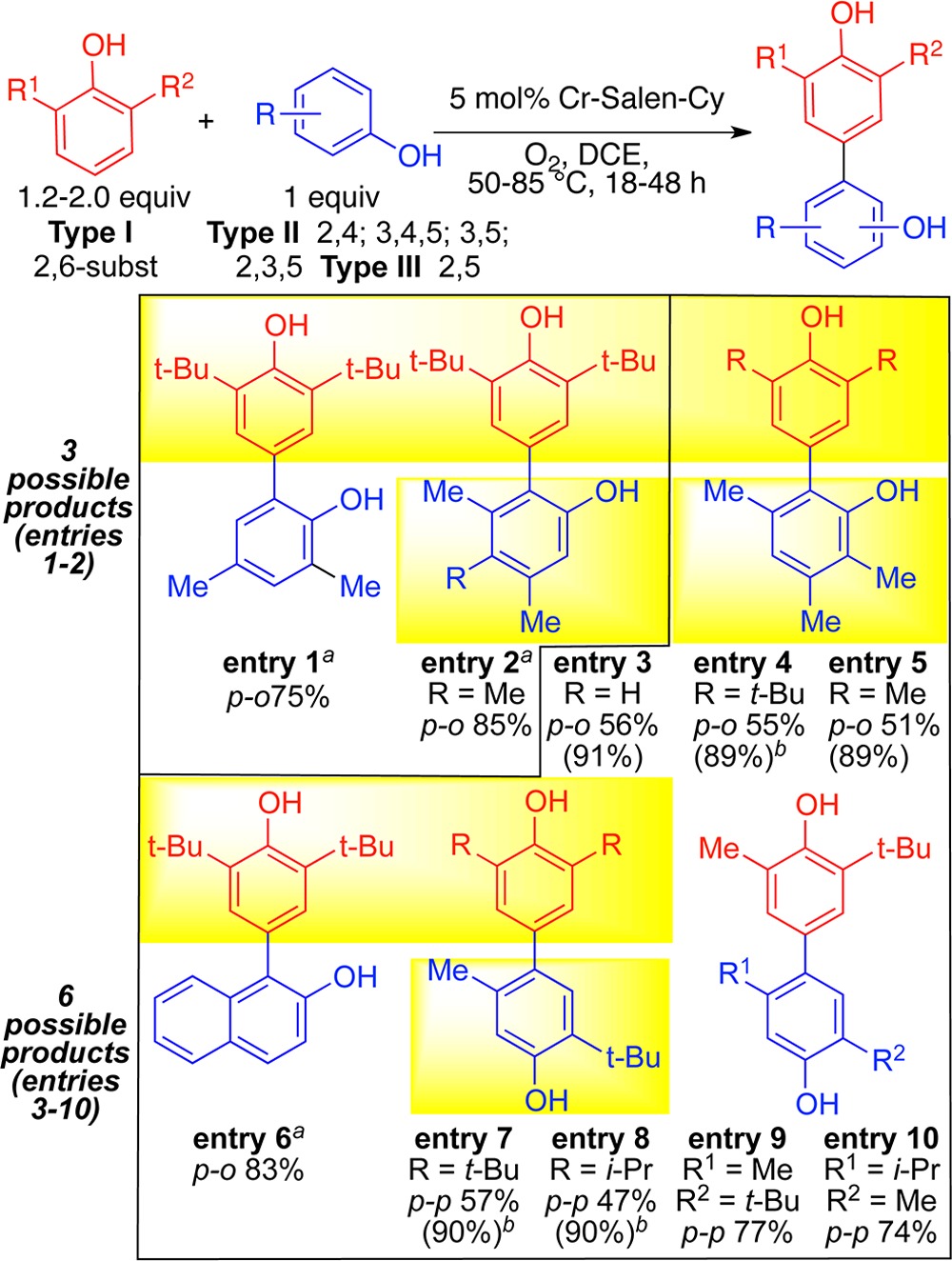
1.2 equiv of red coupling partner used. All others used 2.0 equiv.
Parenthetical yields based on recovered substrate.
Venturing to substrates where six products are possible led to the discovery that Cr-salen-Cy is broadly effective for cross-coupling (entries 3–10). A 2:1 stoichiometry of the coupling partners was well tolerated. Notably, selective cross-coupling was seen for many substrates (yellow highlights, Table 2) where selective homo-coupling had been achieved in Table 1. Selective cross-coupling requires a 2,6-disubstituted partner (Type I), which is postulated to add at the para-site to a metal bound radical or radical cation of the complementary partner (Type II or III), which has a less hindered phenol for metal binding (Scheme 3). Site selectivity occurs at the sterically least hindered site of this metal bound phenol (Type IIortho, Type IIIpara). To date, no other substitution patterns have been found effective for the Type I partner.
Scheme 3. Proposed Mechanism of the Cross-Coupling.
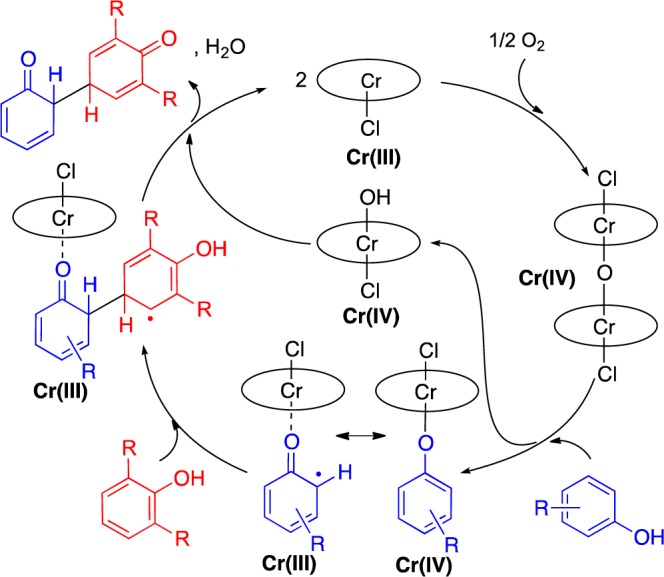
The degree of selectivity control in the catalysts described herein suggests significantly different mechanisms are operating. Further, preliminary studies with radical inhibitors reveal complex effects (see Supporting Information). For example, TEMPO inhibited reaction of the Cr catalyst with O2. Combined with the lack of reactivity of the Cr catalyst without O2 and the formation of product under N2 with a pregenerated Cr(IV) species,14 the data support the mechanism shown in Scheme 3 for the cross-coupling.
In summary, catalytic amounts of simple salen/salan complexes using O2 as the terminal oxidant provide access to phenolic dimers unattainable via conventional oxidants. The PK exemplifies oxidative coupling as a powerful strategy to rapidly build complexity without using leaving groups. The Cr salens, which have not been reported previously in oxidative phenolic coupling, exhibit unique cross-coupling activity enabling access to many unknown adducts. Further studies on the mechanisms to tailor catalysts for reactivity and selectivity are under way.
Acknowledgments
We are grateful to the NSF (CHE1213230, CHE0848460) for financial support. Partial instrumentation support was provided by the NIH for MS (1S10RR023444) and NMR (1S10RR022442) and the NSF for X-ray (CHE 0840438). T.C. thanks the Vietnam Education Foundation for a fellowship.
Supporting Information Available
Experimental procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Barton D. H. R.; Cohen T.. In Festschrift Prof. Dr. Arthur Stoll zum Siebzigsten Geburstag; Birkhäuser: Basel, 1957; p 117. [Google Scholar]; b Erdtman H.; Wachtmeister C. A.. In Festschrift Prof. Dr. Arthur Stoll zum Siebzigsten Geburstag; Birkhäuser: Basel, 1957; p 144. [Google Scholar]
- a Whiting D. A.Comprehensive Organic Synthesis; Trost B. M.; Fleming I., Pattenden G., Eds.; Pergamon: Oxford, 1991; Vol. 3, p 659. [Google Scholar]; b Allen S. E.; Walvoord R. R.; Padilla-Salinas R.; Kozlowski M. C. Chem. Rev. 2013, 113, 6234. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pal T.; Pal A. Curr. Sci. 1996, 71, 106. [Google Scholar]; d Keseru G. M.; Nogradi M. In Studies in Natural Products Chemistry; Atta-ur-Rahman F. R. S., Ed.; Elsevier: New York, 1998; Vol. 20, p 263. [Google Scholar]; e Bringmann G.; Günther C.; Ochse M.; Schupp O.; Tasler S. Prog. Chem. Org. Nat. Prod. 2001, 82, 1. [DOI] [PubMed] [Google Scholar]; f Bringmann G.; Gulder T.; Gulder T. M.; Breuning M. Chem. Rev. 2011, 111, 563. [DOI] [PubMed] [Google Scholar]
- a Armstrong D. R.; Cameron C.; Nonhebel D. C.; Perkins P. G. J. Chem. Soc. Perk. Trans. 2 1983, 563. [Google Scholar]; b Noshino H.; Itoh N.; Nagashima M.; Kurosawa K. Bull. Chem. Soc. Jpn. 1992, 65, 620. [Google Scholar]; c Morimoto K.; Sakamoto K.; Ohnishi Y.; Miyamoto T.; Ito M.; Dohi T.; Kita Y. Chem.—Eur. J. 2013, 19, 8726. [DOI] [PubMed] [Google Scholar]
- Catalytic oxidative phenol coupling where only a single coupling site is open:; a Liao B.-S.; Liu Y.-H.; Peng S.-M.; Liu S.-T. Dalton Trans. 2012, 41, 1158. [DOI] [PubMed] [Google Scholar]; b Jiang Q.; Sheng W.; Tian M.; Tang J.; Guo C. Eur. J. Org. Chem. 2013, 1861. [Google Scholar]; c Feng J.; Yang X.-B.; Liang S.; Zhang J.; Yu X.-Q. Tetrahedron Lett. 2013, 54, 355. [Google Scholar]
- a Kozlowski M. C.; Morgan B. J.; Linton E. C. Chem. Soc. Rev. 2009, 38, 3193. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Irie R.; Masutani K.; Katsuki T. Synlett 2000, 1433. [Google Scholar]; c Guo Q.-X.; Wu Z.-J.; Luo Z.-B.; Liu Q.-Z.; Ye J.-L.; Luo S.-W.; Cun L.-F.; Gong L.-Z. J. Am. Chem. Soc. 2007, 129, 13927. [DOI] [PubMed] [Google Scholar]; d Takizawa S.; Katayama T.; Sasai H. Chem. Commun. 2008, 4113. [DOI] [PubMed] [Google Scholar]; e Yan P.; Sugiyama Y.; Takahashi Y.; Kinemuchi H.; Temma T.; Habaue S. Tetrahedron 2008, 64, 4325. [Google Scholar]; f Egami H.; Katsuki T. J. Am. Chem. Soc. 2009, 131, 6082. [DOI] [PubMed] [Google Scholar]; g Egami H.; Matsumoto K.; Oguma T.; Kunisu T.; Katsuki T. J. Am. Chem. Soc. 2010, 132, 13633. [DOI] [PubMed] [Google Scholar]
- Bordwell F. G.; Cheng J. P. J. Am. Chem. Soc. 1991, 113, 1736. [Google Scholar]
- a Katsuki T. Chem. Soc. Rev. 2004, 33, 437. [DOI] [PubMed] [Google Scholar]; b Cozzi P. G. Chem. Soc. Rev. 2004, 33, 410. [DOI] [PubMed] [Google Scholar]
- Nonselective salen oxidative phenolic coupling:; a Tonami H.; Uyama H.; Kobayahsi S. Macromolecules 2004, 37, 7901. [Google Scholar]; b Bassoli A.; Di Gregorio G.; Rindone B.; Tollari S. J. Mol. Catal. 1989, 53, 173. [Google Scholar]; c Haikarainen A.; Sipilä J.; Pietikäinen P.; Pajunen A.; Mutikainen I. Biorg. Med. Chem. Lett. 2001, 9, 1633. [DOI] [PubMed] [Google Scholar]
- Although effective catalytic oxidants, Co salens were not studied due a strong tendency toward oxygenation. See ref (2a).
- Schmink J. R.; Bellomo A.; Berritt S. Aldrichimica Acta 2013, 46, 71. [Google Scholar]
- a Pelish H. E.; Westwood N. J.; Feng Y.; Kirchhausen T.; Shair M. D. J. Am. Chem. Soc. 2001, 123, 6740. [DOI] [PubMed] [Google Scholar]; b Cocchietto M.; Skert N.; Nimis P. L.; Sava G. Naturwissenschaften 2002, 89, 137. [DOI] [PubMed] [Google Scholar]; c Elo H.; Matikainen J.; Pelttari E. Naturwissenschaften 2007, 94, 465. [DOI] [PubMed] [Google Scholar]
- Bird C. W.; Chauhan Y.-P. Tetrahedron Lett. 1978, 19, 2133. [Google Scholar]
- For an alternate cross-coupling that appeared after this submission:Elsler B.; Schollmeyer D.; Dyballa K. M.; Franke R.; Waldvogel S. R.. Angew. Chem. Int. Ed. 10.1002/anie.201400627. [DOI] [Google Scholar]
- McGarrigle E. M.; Gilheany D. G. Chem. Rev. 2005, 105, 1563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.