Abstract
Background
Bacteria are associated with the gut, fat bodies and reproductive organs of stored product mites (Acari: Astigmata). The mites are pests due to the production of allergens. Addition of antibiotics to diets can help to characterize the association between mites and bacteria.
Methodology and Principal Findings
Ampicillin, neomycin and streptomycin were added to the diets of mites and the effects on mite population growth (Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae) and associated bacterial community structure were assessed. Mites were treated by antibiotic supplementation (1 mgg−1 of diet) for 21 days and numbers of mites and bacterial communities were analyzed and compared to the untreated control. Bacterial quantities, determined by real-time PCR, significantly decreased in antibiotic treated specimens from 5 to 30 times in A. siro and T. putrescentiae, while no decline was observed in L. destructor. Streptomycin treatment eliminated Bartonella-like bacteria in the both A. siro and T. putrescentiae and Cardinium in T. putrescentiae. Solitalea-like bacteria proportion increased in the communities of neomycin and streptomycin treated A. siro specimens. Kocuria proportion increased in the bacterial communities of ampicillin and streptomycin treated A. siro and neomycin and streptomycin treated L. destructor.
Conclusions/Significance
The work demonstrated the changes of mite associated bacterial community under antibiotic pressure in pests of medical importance. Pre-treatment of mites by 1 mgg−1 antibiotic diets improved mite fitness as indicated accelerated population growth of A. siro pretreated streptomycin and neomycin and L. destructor pretreated by neomycin. All tested antibiotics supplemented to diets caused the decrease of mite growth rate in comparison to the control diet.
Introduction
Interactions between arthropods and microbes result in symbiotic associations, which involve both vertical and horizontal transfer mechanisms [1]. Specialized feeding on highly restricted diets and utilization of low digestible substrates (e.g. cellulose, lignin) require association with microorganisms [2], [3]. The resulting associations aid microorganisms in obtaining nitrogen, sterols, vitamins and essential amino acids [4], [5], [6], while microbial digestive enzymes support digestive systems of arthropods by interacting with their endogenous enzymes [7].
Stored product mites successfully colonize human made habitats. Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae feed and develop on stored plant and animal material including grain, oil seeds, dog feed, cheese, dried milk and dry ham, as well as on microscopic fungi [8]. The food sources are nutritionally unbalanced, providing high amount of some nutrients, but lacking others and for such situation the symbiosis with microorganisms is beneficial [9].
The mite associated bacteria were hypothesized to interact with their hosts in the utilization of chitin and cellulose, the most abundant structural polysaccharides in plant and fungal cell walls present in soil and man-made habitats [10], [11], [12]. The stored product mites can feed on Gram-possitive bacteria [13], however, supplementation of the rearing diet with Micrococcus luteus accelerated the population growth of L. destructor but not of A. siro and T. putrescentiae [14]. The associated bacteria enhance mite colonization of human habitats and utilization of new food sources. Except of those bacteria, the mites are attacked by pathogenic or parasitic bacteria [15]. However, associations between mites and bacteria have not yet been assessed in detail.
Antibiotics represent a tool for exploring the ecology of animal gut-associated communities [16] because they may help to understand the role of microbial communities in arthropods [9]. The host-microbe interactions were studied using antibiotics in termites [17], carabid beetles [9], hemiptera [18] and other insects. It is suggested that antibiotics cause (i) direct antibiotic toxicity (ii) decreased food intake, (iii) indirect effect on the symbiotic bacteria involved in basic physiological functions, such as food digestion, (iv) and infection resulted from an antibiotic-resistant pathogenic microorganism [19].
The work focused on demonstrating the effects of antibiotics on mites of medical importance. The selected antibiotics (ampicillin, neomycin and streptomycin) have different spectrum of target taxa but all are broad spectrum antibiotics effective on Gram-positive and negative bacteria [20]. Ampicillin is a broad-spectrum antibiotic inhibiting peptidoglycan synthesis. Streptomycin targets mainly Gram positive. Neomycin is a broad-spectrum antibiotic inhibiting translation and again [20]. The goal of the study was to evaluate changes of mite fitness after antibiotic treatments and determine effects on associated bacterial communities caused by three antibiotics with different spectra and mode of action.
Materials and Methods
Mites
Stored product mites Acarus siro L., Tyrophagus putrescentiae (Schrank) (Acari: Acaridae), and Lepidoglyphus destructor (Schrank) (Acari: Glycyphagidae) came from laboratory cultures maintained at the Crop Research Institute, Prague, The Czech Republic (CZ). Both A. siro and T. putrescentiae were collected from the grain storage inBustehrad (50°9′19″N 14°11′19″E) CZ, in 1996; L. destructor was collected from Food processing factory, Prague (50°9′4″N 14°31′29″E), CZ, in 1965. All species were collected by Eva Zdarkova. The stored product mites are not protected and were not collected in the protected area. The collector Eva Zdarkova had a permission to collect samples on the farm issued by the owners. The mites were mass-reared in plastic chambers derived from IWAKI tissue cell cultures with filter cap plugs (P-Lab, Praha, CZ). The chambers were kept in desiccators (P-Lab) with a saturated KCl solution providing 85% relative humidity and incubated at 25±1°C in the dark [21] Before the experiments, the mites were collected from the rearing chambers using a paint brush and a dissection Stemi 2000 C stereomicroscope (C. Zeiss, Jena, Germany).
Rearing and experimental diets
The rearing diet contained a mixture of powdered oat flakes, wheat germ and Pangamin, a dried yeast biomass (Rapeto, Bezdružice, CZ) (10/10/1 w/w/w). The rearing diet (control) was powdered by blender, sieved at 0.5 mm sieves and sterilized by heating to 70°C for 0.5 h. The diet of 50±5 mg was placed to every IWAKI chamber. The antibiotics, ampicillin sodium salt (cat No. A0166, Sigma-Aldrich, Saint Louis, USA), neomycin trisulfate salt hydrate (cat No. N6386, Sigma-Aldrich) and streptomycin sulfate salt (cat No. S9137, Sigma-Aldrich) were added to the rearing diets, separately. The treatments included antibiotic concentrations of 0.1, 1, 10, and 30 mg of antibiotics per g of diet (mgg−1). The control was the rearing diet without supplementation.
Effect of antibiotic supplement on mite growth
Fifty unsexed adults from a 21 days old culture were added to each chamber. Ten replicates of each species, type of antibiotic and concentration were set. The chambers were incubated in desiccators at the same conditions as describe above for 21 days The experiment was terminated by addition of 10 mL of 80% ethanol per chamber. Mites were counted under the dissection stereomicroscope.
Effects of antibiotic pre-treatment on mites
Fifty unsexed adults from a 21 days old culture were added to each chamber on 100±10 mg of 1 mg g−1 antibiotic containing diets. The concentration was selected based on the previous experiments to ensure that there is a suppressive effect but the mites’ populations are still able to grow. The antibiotics ampicillin, neomycin and streptomycin diets were added separately. The control had no antibiotics. Ten replicates per antibiotic type and species of mites were set. The rearing conditions were the same as described above. After 21 days 10 adults were removed by brush from every chamber and placed to a new chamber with 50±5 mg of un-supplemented (control) rearing diet. The chambers were maintained in the same rearing conditions as in experiment assessing the population growth for 21 days. The experiment was terminated by addition of 10 mL of 80% ethanol to every chamber. Mites were counted under the dissection stereomicroscope.
Data analyses for biotests
For antibiotic treatment, the final population numbers (N) showed normal distribution and were analyzed by analysis of covariance (ANCOVA). Final population numbers were dependent, and type of antibiotics, concentration, mite species and their interactions were independent variables. The ANCOVA models showed higher R2 values when the antibiotic concentration was transformed according to the formula LN(concentration +1.10−7) than the models without transformation. Finally, the interaction of final population number and antibiotic concentration were analyzed separately per antibiotic type and mite species using regression models. Effective doses of antibiotic concentration reducing final population to 50% (EC50) in the comparison to control were estimated from the model with 95% confidence intervals.
To evaluate the effect of antibiotic pre-treatment, final population numbers (N) were analyzed using analysis of variance (ANOVA) separately for each mite species. In the model, the dependent variable was the final population number and the factors were the pre-treatments (control, ampicillin, neomycin and streptomycin). Dunnet two-side test was applied to indicate the difference of the pre-treatment from the control.
The analyses were done in XLSTAT 2007 (Addinsoft USA, New York, NY, USA) and QC-Expert (TriloByte Statistical Software, s.r.o., Pardubice, CZ).
The samples of mites for PCR and plating
1,000±100 mites were reared in IWAKI chambers on 250±50 mg diets containing 1 mg g-1 of antibiotic as was describe above. Three replicates per antibiotic and mite species were set. The control diet contained no antibiotic. The mites in IWAKI chambers were cultivated under conditions as in experiment describing the effect of antibiotics on population growth. The experiment was terminated by collection of mites. The mites (10±1 mg) were removed by paint brush from plugs or inner surfaces of the chambers using the dissection stereomicroscope.
The mites were weighed using Mettler AE 240 microbalance (Mettler-Toledo, Columbus, OH, USA), with accuracy of 10 µg in Eppendorf tubes. Two tubes per one chamber were analyzed. Each tube was filled by 1 mL of bleach for 1 minute, the sample was centrifuged at 3,000 g (1 minute) and the bleach was replaced by 1 mL of absolute ethanol for 1 minute and centrifuges again 3,000 g (1 minute). The ethanol was replaced by PBST (phosphate buffered saline with the detergent Tween 20, 3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, 0.05% Tween 20).
The sample was cleaned 3 times with PBST using centrifugation. Then, the mites were homogenized in 100 µl of PBST by 2 mL glass homogenizer with Teflon-pestle (Kavalier, Sázava, CZ). The first set of tubes was used for total DNA extraction. DNA was extracted using Dneasy Tissue kit (Qiagen, Valencia, CA, USA), and cleaned with GeneClean Turbo kit (MP Biomedicals, Solon, USA).
The second sets of tubes contain the mite homogenates was used for plating. The homogenates were diluted in PBST and plated on Tryptic Soy Broth agar plates (HiMedia, Mumbai, India). The plates were incubated for 7 days at 25±0.5°C and colony forming units were checked. The colonies of different morphologies were identified using 16 S rRNA gene amplifications and sequencing [22].
PCR
PCR amplification of 16S rRNA gene was performed with universal bacterial primers – UF: 5′-AGA GTT TGA TYM TGGC- 3′ (position 8–23 according to E. coli) and UR: 5′-GYT ACC TTG TTA CGA CTT-3′ (position 1496–1514) [23]. Amplification was performed using C1000 Thermal Cycler (Bio-Rad, Hercules, USA). PCR reaction mixture contained in 25 µl total volume: 200 µM dNTPs, 3 mM MgCl2, forward and reverse primers (100 nM each), 0.5 unit Taq polymerase (all Promega, Madison, WI, USA) and 300 ng template DNA (i.e. mite genomic DNA with bacterial DNA). The amplification conditions were as follows: 2 min at 94°C, and 30 cycles of 90 s at 94°C, 90 s at 50°C, and 60 s at 72°C, followed by final extension for 10 min at 72°C and 4°C hold [23].
Resulting PCR products were purified with Wizard SV Gel and PCR Cleanup Kit (Promega). The PCR products from bacterial primers were cloned using pGEM-T Easy Vector (Promega) and sequenced by the Sanger dideoxy method (Macrogen, Seoul, Korea). Three independent samples per treatment (control, ampicillin, neomycin and streptomycin) and mite species were prepared and pooled for the subsequent analysis.
16S RNA gene library
Nearly full-length sequences of 16S rRNA gene were assembled with CodonCode Aligner, version 1.5.2 (CodonCode Corporation, Dedham, MA, USA) and assigned to bacterial taxa using Ribosomal database project naïve Bayesian rRNA classifier [24]. The sequences representing bacterial communities associated with A. siro (233 clones), L. destructor (189) and T. putrescentiae (280) were obtained. As a cultivable part of the respective bacterial communities, 177 isolates from A. siro (54 strains), L. destructor (68) and T. putrescentiae (55) were identified. The cloned sequences were deposited in GenBank under Accession Nos. JX064540–JX064766 and KM464000–KM464486, and the sequences from isolates under Accession Nos. JX064767–JX064911. Separately for clones and isolates, the 16S rRNA gene libraries were classified to operational taxonomic units (OTU97) using Mothur v. 1.32 program [25].
Quantitative PCR
The previously prepared DNA samples were used in three technical replicates per each sample. 5′-ACTCCTACGGGAGGCAGCAG-3′ and Eub518a: 5′-ATTACCGCGGCTGCTGG-3′. According to the previously published protocol [26], nearly full-length 16S RNA gene amplicon of Streptomyces sp. was used as a standard. The amplification was done on a StepOnePlus Real-Time PCR System (Life Technologies, Carlsbad, CA, USA) using 96-well plates with GoTaq qPCR Master Mix (Promega) containing SYBR Green as a double-stranded DNA binding dye.) The amplification consisted of 40 cycles including denaturation (30 s at 95°C), annealing (35 s at 54°C), and elongation (45 s at 72°C). The inhibition was tested by serial DNA dilution from each site. Melting curves were recorded to ensure qPCR specificity [26]. Baseline and threshold calculations were performed with the StepOnePLus software. To evaluate the effect of antibiotic treatment, the obtained numbers of copies were analyzed using the analysis of variance (ANOVA) separately for each mite species. Dunnet two-side test was applied to indicate the difference of the treatment from the control.
Results
The suppressive effect of antibiotics on growth rate of mites
All diets supplemented with antibiotics decreased the growth rate of A. siro, L. destructor and T. putrescentiae (Table 1). The ANCOVA model (F(22,427) = 104; P<0.0001) showed that the final population was significantly influenced by antibiotic concentration (F = 104; P<0.0001)) tested species (F = 33; P<0.0001), and type of antibiotic (F = 27; P<0.0001). The significant effect was observed for the interaction of species×type of antibiotic (F = 29; P<0.0001). Other interactions were not significant.
Table 1. The final population density (N) of species of mites on antibiotic additive diets and fitted concentration EC50 is an effective concentration of antibiotic s in diets for 50% population growth reduction in the comparison to growth on control diet.
| Species | Antibiotics (mgg−1) | Observed data (N) | Regression model | ||||||||
| 0 | 0.1 | 1 | 10 | 30 | R2 | a | b | c | Fit EC50 mgg−1 | ||
| Acarus siro | Ampicillin | 1183 | 728 | 484 | 275 | 200 | 0.95 | −2.95 | −89 | 513 | 0.37 |
| (71) | (111) | (25) | (47) | (56) | (−3.7/−2.2) | (−100/−78) | (483 /543) | (0.20/0.55) | |||
| Neomycin | 1179 | 1059 | 501 | 391 | 304 | 0.87 | −5 | −115 | 689 | 2.23 | |
| (74) | (89) | (73) | (24) | (57) | (−7/−4) | (−133/−96) | (640/739) | (1.11/3.67) | |||
| Streptomycin | 1280 | 1118 | 883 | 452 | 292 | 0.94 | −7 | −142 | 847 | 4.95 | |
| (102) | (97) | (49) | (71) | (41) | (−8 /−6) | (−155/129) | (811/882) | (3.52/7.03 | |||
| Lepidoglyphus destructor | Ampicillin | 1718 | 1398 | 1144 | 603 | 185 | 0.87 | −10 | −202 | 1060 | 2.66 |
| (108) | (208) | (129) | (216) | (57) | (−12/−8) | (−231/−173) | (982/1139) | (1.51/7.03) | |||
| Neomycin | 1685 | 414 | 338 | 197 | 116 | 0.98 | 2 | −56 | 304 | 0.0004 | |
| (105) | (60) | (60) | (40) | (26) | (1/3) | (−66/−45) | (275/332) | (0.0001/0.001) | |||
| Streptomycin | 1685 | 1465 | 1106 | 428 | 231 | 0.93 | −11 | −214 | 1050 | 2.38 | |
| (96) | (218) | (161) | (68) | (51) | (−12/−9) | (−236/−191) | (990/1110) | (1.7/3.07) | |||
| Tyrophagus putrescentiae | Ampicillin | 1164 | 415 | 297 | 156 | 131 | 0.97 | 0.1 | −51 | 293 | 0.003 |
| (95) | (36) | (18) | (20) | (18) | (−0.4/0.6) | (−60/−44) | (272/314) | (0.001/0.01) | |||
| Neomycin | 1271 | 751 | 639 | 290 | 139 | 0.95 | −4 | −108 | 569 | 0.76 | |
| (75) | (103) | (63) | (36) | (37) | (−5/−3) | (−120/−96) | (537/602) | (0.41/1.21) | |||
| Streptomycin | 1193 | 1111 | 411 | 355 | 148 | 0.85 | −7 | −140 | 661 | 1.58 | |
| (98) | (144) | (48) | (41) | (16) | (−8/−5) | (−164/−118) | (599/724) | (0.74/2.66) | |||
Legend: The final population density is expressed as mean ± standard deviation; the polynomial regression (r = ax2+bx+c; x = antibiotic concentration mgg−1 of diet) describing the suppressive effect of antibiotics to growth rate of tested mites. The antibiotic concentration was transformed logarithmically (LN concentration +1×10−7). The fitted parameters are showed with 95% confidence limits in parenthesis.
Polynomial regressions showed the effect of antibiotics on suppression of the growth rate separately for each type of antibiotic and species (Table 1). L. destructor was the most sensitive to antibiotic treatment, followed by T. putrescentiae and A. siro according to EC50 values. Neomycin was the most suppressive to population growth rate, followed by streptomycin and ampicillin.
The effect of antibiotic pre-treatment on mite growth
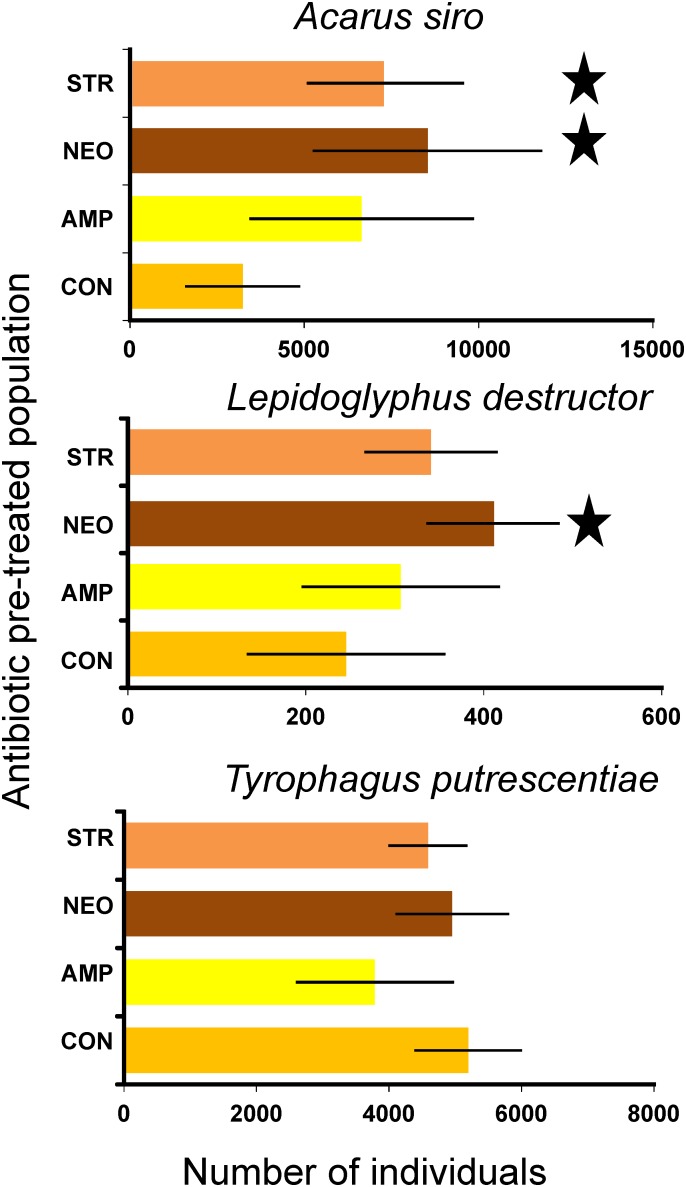
Pre-treatment of mites with diets containing antibiotics showed no effect on T. putrescentiae, (ANOVA: F(3,20) = 3; P = 0.061), but significant effects were observed for A. siro and L. destructor (ANOVA: F(3,20) = 4; P = 0.017 and F(3,20) = 3; P = 0.042, respectively) (Figure 1). The response of population growth was similar for both species; the highest population number was observed after neomycin pre-treatment, followed by streptomycin and ampicillin, the lowest population density was in the control (see Figure 1 for significant differences).
Figure 1. The effect of antibiotics pre-treatment on the final population size after 21 days of growth on untreated diet, averages with respective standard deviations.
The Dunnet significant differences from the control are marked by asterisk; CON - untreated control, AMP –ampicillin, NEO – neomycin, STR – streptomycin.
Bacterial communities in stored product mites
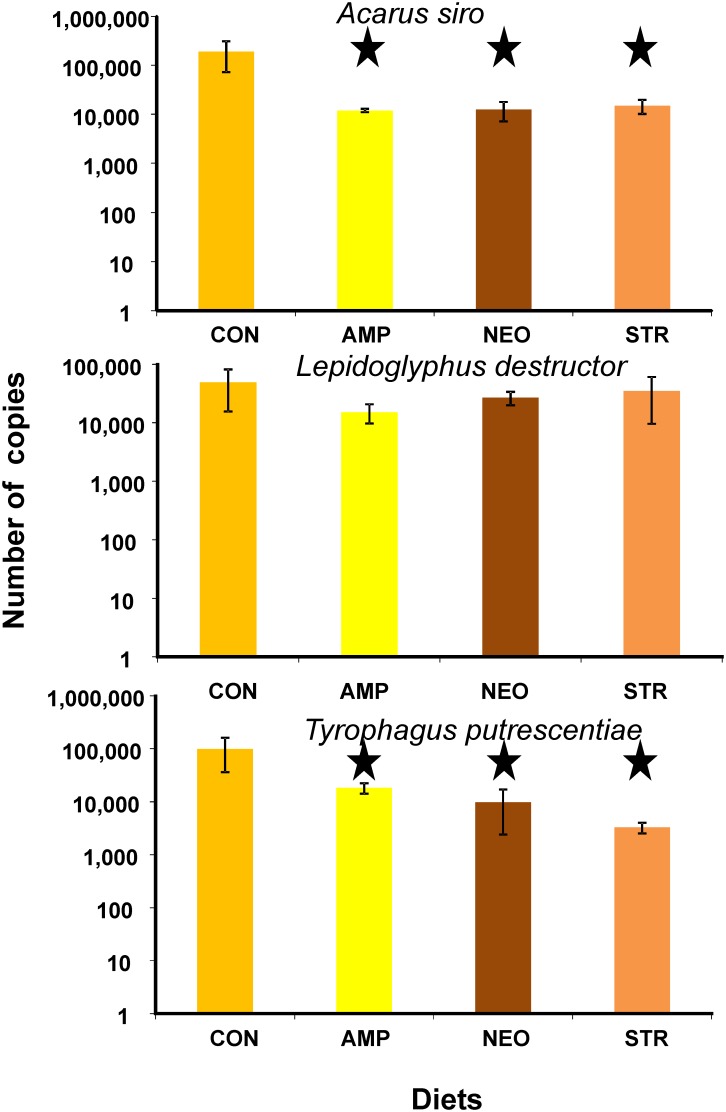
Numbers of 16S rRNA gene copies quantified by qPCR showed differences between mite species decreasing from A. siro (2×105) to T. putrescentiae (1×105) and L. destructor (5×104) (Figure 2). The 16S rRNA libraries from all species formed 11 OTUs97 of cloned sequences (Table 2) and 10 OTUs97 from isolated bacteria (Table 3). In A. siro, Bartonella-like bacteria and Solitalea-like bacteria OTUs97 were prevailing OTUs. Bartonelia-like bacteria together with Cardinium sp. were also prevailing OTU97 in T. putrescentiae. While Bacillus OTU97 was prevailing in L.destructor (Table 2). The 16S rRNA library of sequences from isolates were characterized by high a proportion of Staphylococcus nepalenesis OTU97 and Bacillus sp OTU97 in all species of mites (Table 3). In L destructor next 4 OTUs97 appeared with lower dominance then previously mentioned OTUs97.
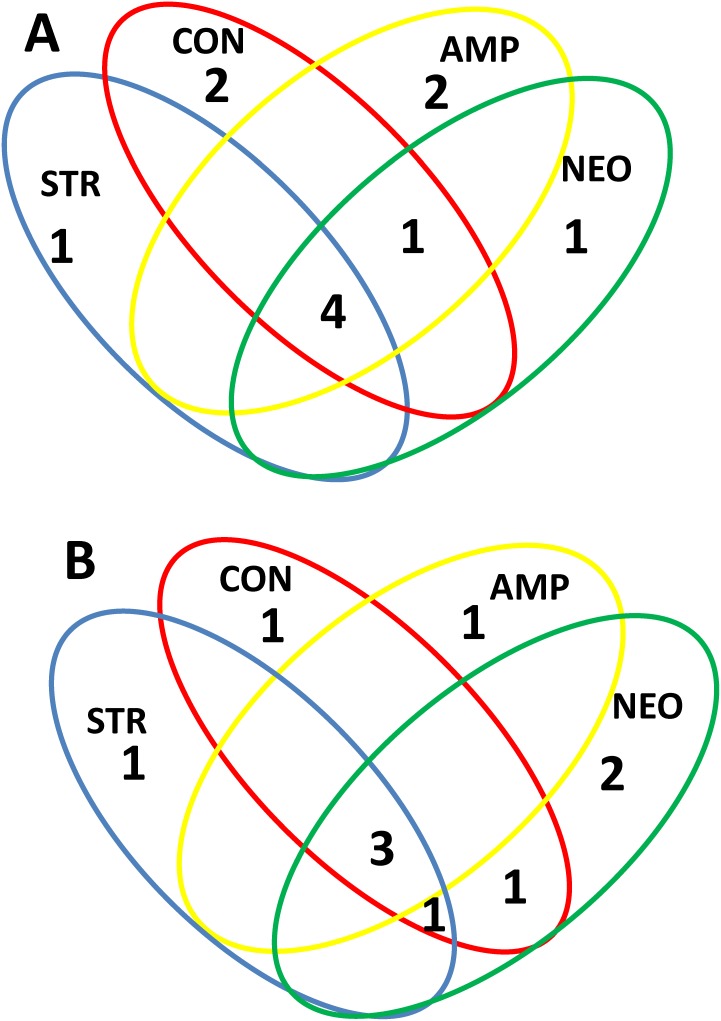
Figure 2. Venn’s diagram comparing the sequences in 16S rRNA library of the clones (A) and isolates (B) from mite on control and antibiotic treated diets.
Table 2. The analyses of 16S rRNA libraries from the clones from stored product mites reared on control diets.
| OTU97 | A. siro | L. destructor | T. putrescentiae | Total | GenBank match | ||||||||||||||
| C | A | N | S | C | A | N | S | C | A | N | S | C | A | N | S | I(%) | taxon | Ac. No. | |
| 1 | 2 | 7 | 1 | 50 | 60 | 14 | 31 | 15 | 44 | 47 | 47 | 67 | 111 | 62 | 78 | 99 | Bacillus cereus | KF150374 | |
| 2 | 38 | 26 | 15 | 38 | 17 | 15 | 1 | 76 | 43 | 30 | 1 | 99 | Bartonella-like | JN236499 | |||||
| 3 | 6 | 6 | 40 | 46 | 6 | 6 | 40 | 46 | 99 | Solitalea-like | JN236468 | ||||||||
| 4 | 1 | 28 | 2 | 12 | 3 | 1 | 17 | 12 | 3 | 1 | 2 | 3 | 7 | 30 | 21 | 27 | 99 | Kocuria sp. | AB480758.1 |
| 5 | 12 | 20 | 6 | 12 | 20 | 6 | 0 | 99 | Cardinium sp. | JX001272 | |||||||||
| 6 | 7 | 7 | 0 | 0 | 0 | 94 | Sodalis sp. | CP006569 | |||||||||||
| 7 | 1 | 1 | 0 | 0 | 0 | 2 | 99 | Staphylococcus sp. | GU451172.1 | ||||||||||
| 8 | 1 | 1 | 0 | 0 | 0 | 95 | Virgibacillus halotolerans | NR_108860 | |||||||||||
| 9 | 1 | 0 | 0 | 1 | 0 | 99 | Leuconostoc citreum | FJ716698 | |||||||||||
| 10 | 1 | 0 | 1 | 0 | 0 | 97 | Paenibacillus xylanilyticus | HQ258920 | |||||||||||
| 11 | 1 | 0 | 1 | 0 | 0 | 99 | Sphingomonas yabuuchiae | NR_028634 | |||||||||||
The data from the three samples per treatment are presented altogether.
Legend: Accession number of match in GENBANK for the most similar sequences of identified bacteria I (%) describes the similarity; A– ampicillin treated diet, C – control diet, N – neomycin treated diet, S – streptomycin treated diet (all 1 mgg−1 of diet).
Table 3. The analyses of 16S rRNA libraries from isolates from stored product mites reared on antibiotic treated and control diets.
| OTU97 | A. siro | T. putrescentiae | L. destructor | Total | GenBank match | ||||||||||||||
| C | A | N | S | C | A | N | S | C | A | N | S | C | A | N | S | I(%) | taxon | Ac. No. | |
| 1 | 15 | 6 | 8 | 8 | 8 | 3 | 9 | 5 | 8 | 4 | 5 | 5 | 31 | 13 | 22 | 18 | 99 | Bacillus sp. | KF788243.1 |
| 2 | 11 | 5 | 1 | 11 | 8 | 4 | 3 | 14 | 7 | 3 | 3 | 36 | 20 | 8 | 6 | 99 | Staphylococcus nepalensis | AB697719.1 | |
| 3 | 1 | 2 | 4 | 1 | 1 | 2 | 4 | 1 | 100 | Bacillus pumilus | KJ722435.1 | ||||||||
| 4 | 1 | 2 | 2 | 3 | 2 | 2 | 4 | 100 | Kocuria sp. | KC502897.1 | |||||||||
| 5 | 1 | 1 | 1 | 1 | 99 | Micrococcus sp. | F465977.1 | ||||||||||||
| 6 | 1 | 0 | 1 | 99 | Bacillus sp. | KJ184936 | |||||||||||||
| 7 | 1 | 1 | 99 | Oceanobacillus sp. | EU709018.1 | ||||||||||||||
| 8 | 1 | 0 | 1 | 99 | Lysinibacillus sp. | KJ191429.1 | |||||||||||||
| 9 | 1 | 0 | 1 | 99 | Acinetobacter lwoffii | KF993657.1 | |||||||||||||
| 10 | 1 | 0 | 1 | 99 | Bacillus megaterium | KJ461522.1 | |||||||||||||
Legend: Accession number of match in GENBANK for the most similar sequences of identified bacteria I (%) describes the similarity; A- ampicillin treated diet, C – control diet, N – neomycin treated diet, S – streptomycin treated diet (all 1 mgg−1 of diet).
In the cloned library, 5 OTUs97 were common in the control and antibiotic treatments (Figure 2A), while 6 OTUs97 were specific for the treatments. In the library of isolates, 3 OTUs97 were common in the control and antibiotic treatments, while (Figure 2B). The antibiotic treatments has 7 unique OTUs97.
Antibiotics differed in their effect on bacterial community further supporting their species specific effects on mites. In A. siro, bacterial numbers decreased 13 to 16 times on antibiotic treated diets in comparison to the control (Figure 3). The effect was significant (F(3,42) = 22; and P<0.001) and all antibiotic treatments differed from the control. Similar high decrease 5 to 30 times on antibiotic treated diet in comparison to the control was observed in T. putrescentiae. The effect was significant (F(3,32) = 10; P<0.001) and all antibiotic treatments differed from the control. No decrease was (F(3,32) = 1.7; P = 0.18) observed on L. destructor (Figure 3).
Figure 3. Quantitative real-time PCR of total bacteria from the DNA extracted from stored product mites reared on control and antibiotic treated diets.
The numbers of copies were recalculated per one specimen, averages with respective standard deviations. The Dunnet significant differences from the control are marked by asterisk; CON - untreated control, AMP –ampicillin, NEO – neomycin, STR – streptomycin.
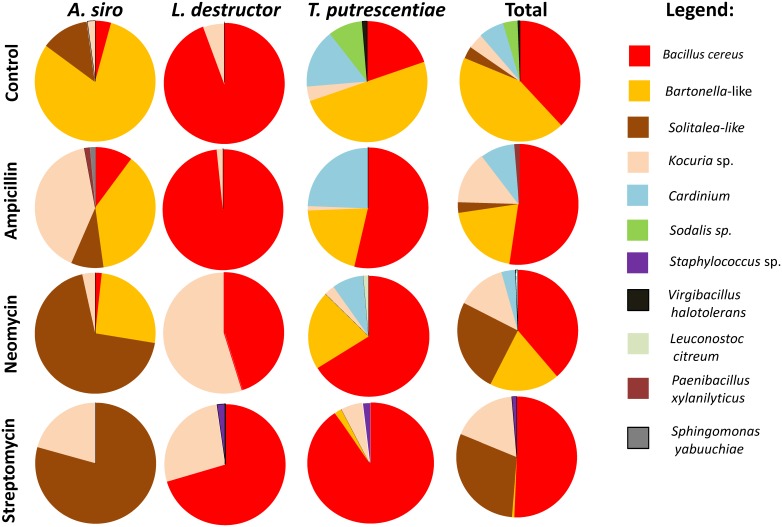
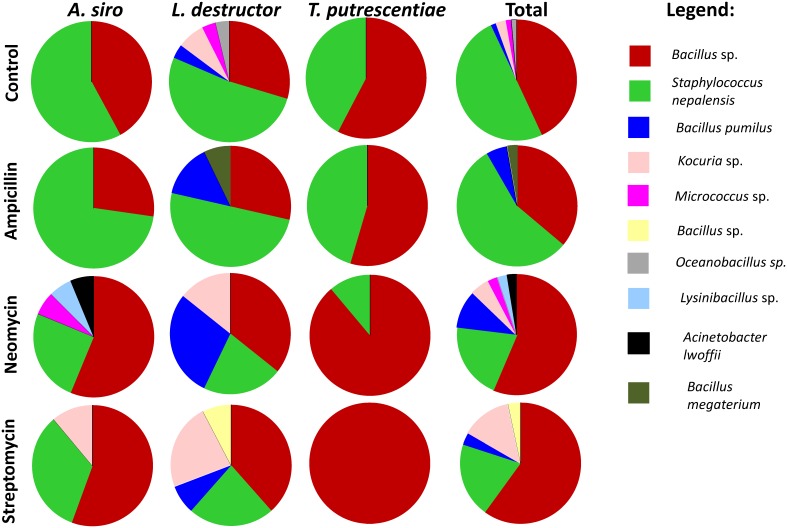
In the clone library of A. siro, Bartonella-like OTU97 proportion decreased in ampicillin and neomycin treatment and was eliminated completely with streptomycin treatment (Figure 4). Kocuria OTU97 increased in ampicillin and streptomycin treatments. Solitalea-like OTU97 predominated in neomycin and streptomycin treatments (Figure 4). The isolate library did not differ between the control and ampicillin treatment (Figure 5). The proportion of Bacillus OTU97 was higher than Staphylococcus nepalensis OTU97 in neomycin and streptomycin treatments.
Figure 4. The comparison of cloned 16S rRNA sequences of stored product mites (Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae) on control and antibiotic supplementation (1 mg g−1 of diet) diets.
Figure 5. The comparison of 16S rRNA sequences from isolated bacteria from of stored product mites (Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae) on control and antibiotic supplementation (1 mg g−1 of diet) diets.
In the clone library of L. destructor, the bacterial community after ampicillin treatment was similar to the control. The proportion of Kocuria OTU97 increased and Bacillus spp OTU97 decreased in neomycin and streptomycin treatments. The isolate libraries showed small difference between control and ampicillin treatments. While in neomycin and streptomycin treated specimens the proportion Staphylococcus nepalensis and Bacillus spp OTUs97 increased. Bacillus pumilis OTU97 and Kocuria spp OTU97 proportions increased on neomycin and streptomycin, respectively (Figure 5).
In the clone library T. putrescentiae, Bartonella-like OTU97 proportion decreased after all antibiotic treatments, while the proportion of Bacillus cereus OTU97 increased (Figure 4). Cardinium OTU97 was reduced on streptomycin and partly on neomycin. The isolate libraries showed small difference between the control and ampicillin treatment. The proportion of Bacillus OTU97 to the proportion of Staphylococcus nepalensis OTU97 significantly increased in neomycin treatment, while Bacillus sp. OTU97 formed exclusively the library in streptomycin treatment (Figure 5)..
Discussion
The size of bacterial communities of stored product mites treated by selected antibiotics decreased in all studied mite species confirming the negative effect of ingested antibiotics on the community of mite associated bacteria. The selected broad spectrum antibiotics have a different spectrum of target taxa [20], and consequently, the decrease in bacterial numbers was specific to mite species, i.e. differed by the taxonomic composition of associated bacterial communities. That might also result from a different gene pool of resistance. The antibiotic effect was suppressive to the dominant species and shifted the community to newly established species leading to the disruption of symbiotic interaction [17]. Some of the detrimental effect of the antibiotic treatment can be a result of eliminating beneficial bacteria. The decrease of some predominant bacterial taxa means an increase of proportion of bacteria resistant to the particular antibiotic [9].
Generally in our experiments, the reduction of population growth seemed to be caused by antibiotic toxicity. All tested antibiotics showed negative effect on mite growth (A. siro, T. putrescentiae, and L. destructor) confirming the previous results [27], [28]. Effective antibiotic doses (EC50) for mite suppression were rather low (up to 3 mg g−1) in comparison with other compounds, which were tested in feeding biotests. For example, plant volatiles, (2E)-hexenal, (2E, 6Z)-nonadienal and (2E)-nonenal had EC50 in range from 4 to 35 mg g−1 [29].
In this study, the pre-treatments of diets by neomycin and streptomycin positively affected the growth of A. siro and neomycin that of L. destructor. The bacterial communities of A. siro on neomycin and streptomycin treated diets were characterized by low numbers of Bartonella-like bacteria. The speculation is that Bartonella-like bacteria have an antagonistic effect to A. siro. In L. destructor, the neomycin treatment strongly decreased the proportion of Bacillus in the bacterial community. The Bacillus bacteria may have both positive and negative effects. The acaropathogenic Bacillus thuringiensis var. tenebrionis suppressed population growth of L. destructor in laboratory experiments [30] and Bacillus sphaericus caused higher mortality and prolonged development of Dermatophagoides pteronyssinus [31]. Other Bacillus species can interact with nutrient utilization or serve as food sources in mites [13], [14]. The stored product mites (e.g. A. siro, Aleuroglyphus ovatus, Carpoglyphus lactis, L. destructor and T. putrescentiae) are used in mass production of mite predators in the system of arthropod pest biological control in the grain stores (Cheyletus eruditus, C. malaccenis) [32], [33] or greenhouses (e.g. Neoseiulus cucumeris, N. barkeri, N. californicus and Amblyseius swirskii) [34], [35]. For such production the “good fitness” of prey mites is necessary to reach high predator numbers. The positive effect of antibiotic pre-treatment can improve the fitness of the prey mites resulting in higher population growth, and consequently in improved the predatory mite production. However the use of antibiotics for these reasons remains questionable due to the risk of spreading resistant bacterial strains.
The observed bacterial communities in analyzed stored product mites (A. siro, L. destructor and T. putrescentiae) reared on the control diet corresponded to the previous descriptions in these mite species [22], [36], [37], [38]. Bacterial communities of A. siro and T. putrescentiae containing symbiotic/parasitic bacteria (Bartonella-like, Cardinium and Solitalea-like) were 10 times more abundant than in L. destructor, whose bacterial community was formed mainly by Bacillus spp. Our previous observation of the bacterial community of T. putrescentiae feeding on different Fusarium spp. fungi indicated a possible association to some members of Bacillus [36]. However, both findings were at a population, not individual level, so high variability of outcomes at individual level may change our conclusions.
Bacterial communities of the three mite species differed from the bacterial community described in another stored product mite Rhizoglyphus robin [12]. The bacterial communities can be different due to feeding on a different diet, also [39]. The bacterial community of T. putrescentiae feeding on various fungal strains is well documented showing switches in bacterial community to chitinolytic strains Pseudomonas stutzeri, Brevundimonas vesicularis, Stenotrophomonas maltophilia, Serratia liquefaciens and Serratia marcescens [11].
The specific effects of various antibiotics on internal bacterial communities were demonstrated previously. In vitro cultivation test of cells with Cytophaga-like symbionts (i.e. Cardinium) showed that ampicillin was highly effective compared to streptomycin [40]. In the Buchnera-Serratia and aphid symbiotic system, the ampicillin treatment eliminated Serratia in a dose-dependent manner, while rifampicin treatment eliminated Buchnera in dose-independent manner [41]. Here, the treatment of mites resulted in decrease of Gram-negative Bartonella-like bacteria by all antibiotics. Consequently, all used antibiotics can be applied to reduce or eliminate Bartonella-like species, which might be useful since the genus Bartonella has been suggested as horizontally transferred group in the ticks [42]. The Cardinium in T. putrescentiae was sensitive to neomycin and streptomycin, but not ampicillin. Cardinium belongs to the known arthropod symbionts transmitted vertically, and it can be eliminated from mites by antibiotics or heat treatment [43], [44], [45], [46].
The last group of bacteria suppressed by antibiotics was Bacillus cereus and similar taxa in L. destructor. However, Bacillus-cereus-like sequences formed the same OTU97 with those in T. putrescentiae, where the proportion increased. A closer identification of Bacillus using 16S rRNA gene is limited [47], so different Bacillus species could inhabit T. putrescentiae and L. destructor mites.
The decrease of above mentioned taxa lead to the proportional increase (not absolute) of taxa such as Solitalea-like bacteria and Kocuria. Solitalea-like bacteria were not influenced by antibiotic treatment, therefore their proportion increased in the communities of neomycin and streptomycin treated A. siro. Our suggestion is that Kocuria originates from the diet of mites, since Kocuria were also reported from the insects [2], [48] and related species of house dust mites [49].
In conclusion, the treatment of stored product mite by broad-spectrum antibiotics decreased the bacterial numbers in tested mites. All tested antibiotics suppressed the Bartonella-like bacteria, and neomycin and streptomycin suppressed Cardinium. This findings should be useful in production of mites for medical diagnostics (purified natural allergens) and immunomodulations [50], [51]. However, Solitalea-like associated group was not influenced by treatment with the antibiotics used here. In A. siro and L. destructor, the antibiotic pre-treatment (streptomycin and neomycin) can improve fitness levels, which is beneficial for mass production.
Acknowledgments
The authors are obligated to anonymous referee for valuable comments, to Jessica Guy for comments and improving of language and Tomas Erban for antibiotic diet preparation.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the Czech Science Foundation (http://www.gacr.cz/en/) grant No. GA525/09/1872 to JK, Ministrz of education youth and sport of the Czech Republic (www.msmt.cz/index.php?lang=2) grant No. LD13052/COST FA1105 action to JH and Ministry of Agriculture of the Czech Republic (http://eagri.cz/public/web/en/mze/) grant NO. RO0414 to MMS and MN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moran NA (2007) Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl. Acad. Sci. U S A. 15 104 Suppl 1 8627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morales-Jiménez J, Zúñiga G, Ramírez-Saad HC, Hernández-Rodríguez C (2012) Gut-associated bacteria throughout the life cycle of the bark beetle Dendroctonus rhizophagus Thomas and Bright (Curculionidae: Scolytinae) and their cellulolytic activities. Microb Ecol. 64: 268–78. [DOI] [PubMed] [Google Scholar]
- 3. Breznak JA (1982) Intestinal microbiota of termites and other xylophagous insects. Annu Rev Microbiol. 36: 323–43. [DOI] [PubMed] [Google Scholar]
- 4. Asselt- van L (1999) Interactions between domestic mites and fungi. Indoor and Built Env. 8: 216–220. [Google Scholar]
- 5. Douglas AE (2009) The microbial dimension in insect nutritional ecology. Functional Ecol. 23: 38–47. [Google Scholar]
- 6. Hay DB, Hart B J, Douglas AE (1993) Effects of the fungus Aspergillus penicillioides on the house dust mite Dermatophagoides pteronyssinus: an experimental re-evaluation. Med. Vet. Entomol. 7: 271–274. [DOI] [PubMed] [Google Scholar]
- 7. Genta FA, Dillon RJ, Terra WR, Ferreira C (2006) Potential role for gut microbiota in cell wall digestion and glucoside detoxification in Tenebrio molitor larvae. J. Insect Physiol. 52: 593–601. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AM 1976. The mites of stored food and houses. Technical Bulletin of the UK Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationery Office, London. 400 p.
- 9. Lundgren JG, Lehman RM (2010) Bacterial gut symbionts contribute to seed digestion in an omnivorous beetle. PLoS One 5: e10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smrz J (2003) Microanatomical and biological aspects of bacterial associations in Tyrophagus putrescentiae (Acari: Acaridida). Exp Appl Acarol 31: 105–113. [DOI] [PubMed] [Google Scholar]
- 11. Smrz J, Catska V (2010) Mycophagous mites and their internal associated bacteria cooperate to digest chitin in soil. Symbiosis 52: 33–40. [Google Scholar]
- 12. Zindel R, Ofek M, Minz D, Palevsky E, Zchori-Fein E, et al. (2013) The role of the bacterial community in the nutritional ecology of the bulb mite Rhizoglyphus robini (Acari: Astigmata: Acaridae). FASEB J. 27: 1488–97. [DOI] [PubMed] [Google Scholar]
- 13. Childs M, Bowman CE (1981) Lysozyme activity in 6 species of economically important astigmatid mites. Comp. Biochem. Physiol. 70B: 615–617. [Google Scholar]
- 14. Erban T, Hubert J (2008) Digestive function of lysozyme in synanthropic acaridid mites enables utilization of bacteria as a food source. Exp Appl Acarol 44: 199–212. [DOI] [PubMed] [Google Scholar]
- 15. Geest-van LP, Elliot SL, Breeuwer JA, Beerling EA (2000) Diseases of mites. Exp. Appl. Acarol. 24: 497–560. [DOI] [PubMed] [Google Scholar]
- 16. Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J (2010) Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb. Ecol. 59: 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosengaus RB, Zecher CN, Schultheis KF, Brucker RM, Bordenstein SR (2011) Disruption of the termite gut microbiota and its prolonged consequences for fitness. Appl Environ Microbiol. 77: 4303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kafil M, Bandani AR, Kaltenpoth M, Goldansaz SH, Alavi SM (2013) Role of symbiotic bacteria in the growth and development of the Sunn pest, Eurygaster integriceps. J. Insect. Sci. 13: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edlund A, Ek K, Breitholtz M, Gorokhova E (2012) Antibiotic-induced change of bacterial communities associated with the copepod Nitocra spinipes. PLoS One. 7(3): e33107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greewood D, Whitley R (2003). Mode of action. In Finch R, Greenwood D, Norrby SR, Whitley RJ (ed), Antibiotic and Chemotherapy: Anti-infective Agents and Their Use in Therapy, 8th ed, Churchill Livingstone, Edinburgh, UK., p11–22.
- 21. Hubert J, Dolecková-Maresová L, Hýblová J, Kudlíková I, Stejskal V, et al. (2005) In vitro and in vivo inhibition of alpha-amylases of stored-product mite Acarus siro. Exp. Appl. Acarol. 35: 281–291. [DOI] [PubMed] [Google Scholar]
- 22. Hubert J, Kopecký J, Perotti AM, Nesvorna M, Braig HR, et al. (2012) Detection and identification of species-specific bacteria associated with synanthropic mites. Microbial Ecol 63: 919–928. [DOI] [PubMed] [Google Scholar]
- 23. Barbieri E, Paster BJ, Hughes D, Zurek L, Moser DP, et al. (2001) Phylogenetic characterization of epibiotic bacteria in the accessory nidamental gland and egg capsules of the squid Loligo pealei (Cephalopoda:Loliginidae). Environ. Microbiol 3: 151–67. [DOI] [PubMed] [Google Scholar]
- 24. Wang Q, Garrity GM, Tiedje JM, Cole J R (2007) Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl Environ Microbiol 73: 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 75: 7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sagova-Mareckova M, Omelka M, Cermak L, Kamenik Z, Olsovska J, et al. (2011) Microbial communities show parallels at sites with distinct litter and soil characteristics. Appl Environ Microbiol. 77: 7560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boczek J (1965) Effect of antimicrobial agnets and antibiotics on some stored product mites. Boll. Zool. Agr. Bachic. 7: 299–300. [Google Scholar]
- 28. Boczek J, Czajkowska B (1968) The effect of antimicrobial agents and antibiotics on some stored product mites (Acaroidea). Rocz. Nauk. Roln. 93: 579–612 (in Polish, English abstract).. [Google Scholar]
- 29. Hubert J, Münzbergová Z, Nesvorná M, Poltronieri P, Santino A (2008) Acaricidal effects of natural six-carbon and nine-carbon aldehydes on stored-product mites. Exp Appl Acarol. 44: 315–321. [DOI] [PubMed] [Google Scholar]
- 30. Erban T, Nesvorna M, Erbanova M, Hubert J (2009) Bacillus thuringiensis var. tenebrionis control of synanthropic mites (Acari: Acaridida) under laboratory conditions. Exp. Appl. Acarol. 49: 339–346. [DOI] [PubMed] [Google Scholar]
- 31. Saleh SM, Keladan NL, Shaker N (1991) Control of European house dust mite Dermatophagoides pteronyssinus (Trouessart) with Bacillus spp. Acarologia (Acarologia) 32: 257–260. [Google Scholar]
- 32. Palyvos NE, Emmanouel NG (2011) Reproduction, survival, and life table parameters of the predatory mite Cheyletus malaccensis (Acari: Cheyletidae) at various constant temperatures. Exp. Appl. Acarol. 54: 139–50. [DOI] [PubMed] [Google Scholar]
- 33. Zdarkova E (1998) Biological control of storage mites by Cheyletus eruditus. Integrated Pest Manag. Rev. 3: 111–116. [Google Scholar]
- 34. Castagnoli M, Nannelli R, Tarchi F, Simoni S (2006) Screening of astigmatid mites for mass-rearing Neoseiulus californicus (McGregor) (Acari: Phytoseiidae). Redia 89: 55–58. [Google Scholar]
- 35. Xia B, Zou Z, P Lin (2012) Effect of temperature on development and reproduction of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Aleuroglyphus ovatus. Exp. Appl. Acarol. 56: 33–41. [DOI] [PubMed] [Google Scholar]
- 36. Hubert J, Nesvorná M, Ságová-Marečková M, Kopecký J (2012) Shift of bacterial community in synanthropic mite Tyrophagus putrescentiae induced by Fusarium fungal diet. PLoS One 7(10): e48429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kopecky J, Perotti MA, Nesvorna M, Erban T, Hubert J (2013) Cardinium endosymbionts are widespread in synanthropic mite species (Acari: Astigmata). J. Invertebr. Pathol. 112: 20–3. [DOI] [PubMed] [Google Scholar]
- 38. Kopecký J, Nesvorná M, Hubert J (2014) Bartonella-like bacteria carried by domestic mite species. Exp. Appl. Acarol. 64: 21–32. [DOI] [PubMed] [Google Scholar]
- 39. Díaz A, Okabe K, Eckenrode CJ, Villani MG, Oconnor BM (2000) Biology, ecology, and management of the bulb mites of the genus Rhizoglyphus (Acari: Acaridae). Exp Appl Acarol. 24: 85–113. [DOI] [PubMed] [Google Scholar]
- 40. Morimoto S, Kurtti TJ, Noda H (2006) In vitro cultivation and antibiotic susceptibility of a Cytophaga-like intracellular symbiote isolated from the tick Ixodes scapularis . Curr Microbiol 52: 324–9. [DOI] [PubMed] [Google Scholar]
- 41. Koga R, Tsuchida T, Sakurai M, Fukatsu T (2007) Selective elimination of aphid endosymbionts: effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol Ecol. 60: 229–39. [DOI] [PubMed] [Google Scholar]
- 42. Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB (2008) Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med. Vet. Entomol. 22: 1–15. [DOI] [PubMed] [Google Scholar]
- 43. Chigita A, Miura K (2005) Detection of ‘Candidatus Cardinium’ bacteria from the haploid host Brevipalpus californicus (Acari: Tenuipalpidae) and effect on the host. Exp Appl Acarol 37: 107–116. [DOI] [PubMed] [Google Scholar]
- 44. Gotoh T, Noda H, Ito S (2007) Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98: 13–20. [DOI] [PubMed] [Google Scholar]
- 45. Morag N, Mullens BA, Gottlieb Y (2013) Assessment of survival and body size variation of Culicoides imicola (Diptera: Ceratopogonidae) as functions of “Candidatus Cardinium” (Bacteroidetes) infection status. Appl Environ Microbiol. 79: 6260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang JJ, Dong P, Xiao LS, Dou W (2008) Effects of removal of Cardinium infection on fitness of the stored-product pest Liposcelis bostrychophila (Psocoptera: Liposcelididae). J. Econ. Entomol. 101: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 47. Oliwa-Stasiak K, Molnar CI, Arshak K, Bartoszcze M, Adley CC (2010) Development of a PCR assay for identification of the Bacillus cereus group species. J Appl Microbiol. 108: 266–73. [DOI] [PubMed] [Google Scholar]
- 48. Wenzel M, Schönig I, Berchtold M, Kämpfer P, König H (2002) Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J Appl Microbiol. 92: 32–40. [DOI] [PubMed] [Google Scholar]
- 49. Tang VH, Chang BJ, Srinivasan A, Mathaba LT, Harnett GB, et al. (2013) Skin-associated Bacillus, staphylococcal and micrococcal species from the house dust mite, Dermatophagoides pteronyssinus and bacteriolytic enzymes. Exp. Appl. Acarol. 61: 431–47. [DOI] [PubMed] [Google Scholar]
- 50. Fernández-Caldas E, Iraola V, Boquete M, Nieto A, Casanovas M (2006) Mite immunotherapy. Curr Allergy Asthma Rep 6: 413–9. [DOI] [PubMed] [Google Scholar]
- 51. Arlian LG, Morgan MS (2011) Immunomodulation of skin cytokine secretion by house dust mite extracts. Int Arch Allergy Immunol. 156: 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.