Abstract
Oncogenic KRAS activation is responsible for the most common genetic subtype of lung cancer. Although many of the major downstream signaling pathways that KRAS engages have been defined, these discoveries have yet to translate into effective targeted therapy. Much of the current focus has been directed at inhibiting the activation of RAF/MAPK and PI3K/AKT signaling, but clinical trials combining multiple different agents that target these pathways have failed to show significant activity. In this article, we will discuss the evidence for RAF and PI3K as key downstream RAS effectors, as well as the RAL guanine exchange factor, which is equally essential for transformation. Furthermore, we will delineate alternative pathways, including cytokine activation and autophagy, which are co-opted by oncogenic RAS signaling and also represent attractive targets for therapy. Finally, we will present strategies for combining inhibitors of these downstream KRAS signaling pathways in a rational fashion, as multitargeted therapy will be required to achieve a cure.
Keywords: autophagy, AZD6244, CYT387, cytokines, KRAS, lung cancer, OSI-906, PI3K, RAF, RAL-GEF
Constitutively active signaling downstream of KRAS is a fundamental driver of lung tumorigenesis. KRAS is the most commonly mutated oncogene in lung adenocarcinomas, ranging in incidence from 15 to 40% across different studies [1]. The majority of oncogenic KRAS mutations are located in codons 12, 13, 61 and 146, and consist of missense transversions associated with smoking. An additional large fraction of lung adenocarcinomas engage wild-type KRAS signaling via upstream receptor tyrosine kinase activation or other means. Consistent with this observation, mutations in KRAS are generally mutually exclusive with genetic alterations in receptor tyrosine kinases such as EGFR or ALK.
Despite over 30 years of research and a detailed molecular understanding of the pathways that KRAS activates, targeted KRAS therapies that provide clinical benefit have yet to be designed. In part, this challenge relates to the fact that KRAS itself, a single-subunit small GTPase protein, has remained difficult to inhibit with small molecules. For example, despite their initial promise, farnesyltransferase inhibitors that prevent KRAS membrane anchorage have had limited activity in the clinic [2]. Although other molecules that target KRAS membrane localization [3] or inhibit the guanine nucleotide-binding site in a covalent fashion are being developed [4,5], these strategies have yet to be validated therapeutically. Instead, current attempts to target KRAS in the clinic have focused on the inhibition of downstream signaling pathways.
In this article, we will focus on the specific gene products and signaling pathways downstream of KRAS that are critical for cellular transformation and for maintenance of lung tumorigenesis. We will detail the evidence for targeting the well-established KRAS signaling effectors in the clinic and novel strategies for identifying and disrupting codependent pathways. Furthermore, given the complexity of KRAS signal transduction, it is increasingly recognized that combination therapies will be required in order to block the multiple effector pathways that KRAS utilizes to drive and sustain lung tumorigenesis. Previous reviews have discussed in depth the efforts to cotarget the MAPK and PI3K pathways, as well as early functional genomic studies designed to identify synthetic lethal targets [6]. Since clinical trials of MEK and PI3K inhibitors have yielded largely disappointing results and certain novel approaches to inhibiting the pathways that are co-opted by KRAS have shown promise, we will focus on the next generation of KRAS-targeted studies that are entering the clinic. We will also highlight how maximizing the inhibition of parallel pathways and/or those linked by feedback, while minimizing overlapping toxicities, likely represents a key feature to achieving therapeutic success.
Direct KRAS effector pathways involved in cell transformation & lung tumorigenesis
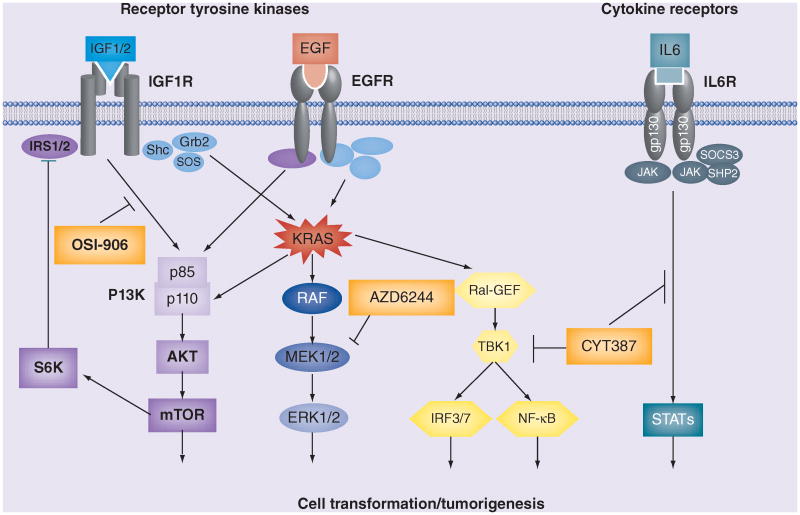
KRAS activates signaling downstream of the RAF, PI3K and RAL pathways, which have been firmly established as direct effectors of oncogenic RAS-mediated cellular transformation (Figure 1). Evidence for their key role in tumorigenesis has accumulated over the last two decades, initially from in vitro model systems and more recently in vivo using genetically engineered mouse models (GEMMs) of Kras-driven lung cancer.
Figure 1. KRAS signal transduction and selected points of therapeutic intervention.
A simplified schematic of KRAS signal transduction is shown, including upstream activation by certain receptor tyrosine kinases, the major downstream RAS effectors PI3K, RAF and RAL–GEF and cytokine signaling pathways. Examples of clinical-stage small-molecule kinase inhibitors that target each signaling axis are also depicted (orange boxes).
Identification of downstream RAS effectors in transformation model systems
Due to the initial challenges in transforming human cells, many of the early studies that determined key roles for these effector pathways were conducted in the same rodent fibroblast cell lines that were used to identify the ras oncogene [7]. For example, the human homolog of the murine retroviral oncogene v-raf was originally found to transform NIH-3T3 cells [8]. RAF1 protein was later shown to bind directly to RAS and promote RAS-mediated transformation [9,10]. The discovery that KRAS recruits RAF to the plasma membrane provided further mechanistic insights into its activation during transformation [11, 12]. Subsequent work led to the identification of both PI3K [13] and the RAL guanine exchange factor (RAL-GEF; also known as RAL guanine nucleotide dissociation stimulator [14]) as proximal mediators of cell transformation by oncogenic RAS. Direct interaction of RAS isoforms with RAL-GEF enhanced transformation [15], whereas interference with RAL activation was sufficient to disrupt these transforming activities [15, 16]. Thus, engagement of the emerging RAF–MAPK, PI3K–AKT and RAL signaling pathways were each defined as key components of the ability of oncogenic RAS to transform cells.
The finding that oncogenic RAS could transform human cells immortalized by the expression of the hTERT catalytic subunit and the SV40 early region [17] enabled dissection of the mechanistic requirements for these downstream activities during human cell transformation. Using specific RAS effector loop mutants, a particularly essential role for RAL-GEF signaling was identified as being required for human cell transformation and tumor formation [18]. In another study, RAL-GEF engagement by RAS was universally required for transformation across different human cell types, in contrast to RAF and PI3K, which were dispensable in certain settings [19]. Taken together, these studies established the role for signaling downstream of these RAS effectors during human cell transformation, and suggested a particularly critical requirement for RAL-GEF activation in human RAS-mediated oncogenesis.
Evidence from Kras lung cancer GEMMs
The advent of mouse models of Kras-driven lung cancer [20,21], in which tumors are generated by activation of a conditional lox-stop-lox or a latent oncogenic Kras allele in the lungs, has provided a more definitive assessment of the role that these signaling pathways have in vivo during lung tumor initiation and maintenance. Several recent studies have evaluated the differential requirement for the two major Raf kinases, b-Raf and c-Raf, in this mouse model [22,23]. Homozygous loss of c-Raf, but not b-Raf, impaired tumor progression and prolonged survival during KrasG12D-induced lung tumorigenesis in mice [22]. Another more extensive analysis of the requirement for MAPK pathway signaling components in KrasG12V-driven lung cancer in mice demonstrated that c-Raf combined with Mek1/2 or Erk1/2 deletions were all sufficient for the disruption of tumor formation and prolonging survival [23]. Similarly, deletion of the Pik3ca gene that encodes the catalytic p110α subunit of PI3K that interacts with Ras was found to disrupt the ability of oncogenic Kras to promote lung tumorigenesis in mice [24] and, more recently, was also shown to impair the progression of established tumors [25]. Finally, deletion of the Ral-GEF effectors Rala and Ralb together was required to block murine Kras-driven lung tumor formation [26], revealing in vivo compensatory interactions in mice, but consistent with the general requirement for RAL-GEF activation during human RAS transformation.
Taken together, these studies have firmly established RAF, PI3K and RAL signaling as key downstream effectors of KRAS that promote lung tumorigenesis. While other direct RAS effectors have been identified, their role in RAS transformation and lung cancer remains to be clarified. Thus, we will focus primarily on the therapeutic targeting of the major RAS effector pathways, in addition to several essential co-opted pathways, as reviewed in the next section.
Co-opted pathways required by oncogenic KRAS to sustain lung tumorigenesis
Oncogenic KRAS relies on additional pathways that are engaged downstream of these effectors or are required indirectly as a consequence of RAS pathway activation. In this section, we will review several of the major co-opted pathways that represent additional tumor dependencies and signify equally tractable points of therapeutic intervention.
Inflammatory signaling pathways
A large body of work has revealed that oncogenic RAS promotes cytokine expression and dependency on specific STAT3 or NF-κB signaling components. Early studies of mutant HRAS or NRAS expression in human cell lines determined that RAS signaling induces the production of IL-1 and IL-6 [27]. Furthermore, melanomas harboring activating NRAS mutations were found to secrete IL-1 and IL-6, which was enhanced by autocrine IL-1 signaling [28]. While these studies were largely correlative in nature, subsequent work confirmed that cytokines such as IL-6 and IL-8 support RAS-mediated oncogenesis directly through cell-autonomous and non-cell-autonomous mechanisms involving the tumor microenvironment [29,30]. Recently, multiple studies in Kras GEMMs have confirmed these findings, since genetic deletion of Il-6 or Stat3 impairs lung [31] or pancreatic tumorigenesis [32]. IL-8 specifically attracts inflammatory and endothelial cells via its receptor CXCR2, and blockade of this signaling also impairs murine Kras-induced lung tumorigenesis [33,34].
Recent studies have also identified direct involvement of NF-κB signaling in KRAS-induced lung tumorigenesis, as well as specific pathway components that contribute to this inflammatory signaling network. For example, downregulation of IL-1 through GATA2 depletion [35], expression of the IκB super-repressor [36] or deletion of the NF-κB scaffolding factor p62 [37], the NF-κB subunit p65 [38] or IKK-β [39] have all been shown to impair Kras-driven lung cancer in various mouse models. Further in vitro studies have identified a particular role for NF-κB signaling downstream of the noncanonical IKKs TBK1/IKKε in promoting KRAS-mediated transformation and cell survival [40–45]. TBK1 is constitutively expressed and linked directly to KRAS pathway activation downstream of RALB [40], while IKKε expression is induced by cytokines [46]. Furthermore, TBK1/IKKε-induced cytokines such as IL-6 create feedforward signaling through JAK/STAT in order to sustain IKKe expression as part of an autocrine cytokine circuit [47]. Thus, in addition to the canonical IKKs, these kinases also represent key nodes in the inflammatory signaling pathways that promote KRAS-driven tumorigenesis (Figure 1).
Autophagy/metabolic/reactive oxygen species detoxification pathways
A number of emerging studies have also identified multiple interconnected stress response pathways as being required to counterbalance the harsh cellular environment imposed by uncontrolled oncogenic KRAS activation. Macroautophagy (here referred to as autophagy), a catabolic pathway that involves the engulfment of macromolecules and certain cellular organelles, has recently been described as an adaptive response to KRAS-induced stress [48]. Over the last several years, a number of groups have shown that oncogenic RAS signaling triggers autophagic vacuole formation and that inhibition of autophagy by genetic or pharmacologic means can impair RAS-mediated transformation [49–52]. More recent studies in oncogenic Kras-induced lung GEMMs have revealed an increasingly complicated picture. Although deletion of the essential autophagy regulator Atg7 impaired KrasG12D-driven lung cancers in vivo, this effect was attenuated when Kras activation was combined with p53 deletion (Kras/p53 mutant or KP lung cancer GEMM) [53]. Similar results were also recently observed following the deletion of Atg5 in these Kras mouse models, with enhanced survival seen in wildtype p53 but not in p53-deleted mice [54]. In both instances, the authors observed impaired clearance of damaged mitochondria in the absence of autophagy, contributing to the defect in tumor progression and to impaired oxidative metabolism. Interestingly, autophagy impairment also resulted in increased inflammation [53,54], suggesting the possibility that this might also contribute to enhanced tumorigenesis through deregulated T-cell immune surveillance [54]. However, RAS-induced autophagy also appears to be required for the production of certain cytokines that promote cell-autonomous invasiveness and migration [55]. Thus, while autophagy is clearly important for Kras-driven lung tumorigenesis, its role remains complicated and incompletely understood.
The mitochondrial defects following autophagy inhibition have been associated with impaired metabolism and accumulation of reactive oxygen species (ROS) in RAS-driven tumor cells [49,51]. Several other studies have directly implicated metabolic signaling and ROS detoxification as being required for KRAS-mediated oncogenesis. For example, genetic deletion of HK2, which catalyzes the first step of glucose metabolism and is highly expressed in cancer cells, was found to be required for tumor initiation and maintenance in murine oncogenic Kras-driven lung cancers, in part by impairing ribonucleotide production as well as glutamine utilization [56]. Several studies in pancreatic cancer model systems have also highlighted a key role for the regulation of glucose and glutamine metabolism in KRAS-mediated tumorigenesis [57]. Inducible KrasG12D expression in a pancreatic GEMM stimulated glucose uptake and reprogrammed glucose metabolism by channeling intermediates into hexosamine biosynthesis and pentose phosphate pathways [58]. Enhancement of nonoxidative pentose phosphate pathways was found to support ribose biogenesis, and this metabolic reprogramming was dependent on MAPK pathway activation. Oncogenic KRAS also reprograms the metabolism of glutamine in pancreatic cancer cells [59]. Inhibition of this alternative pathway resulted in decreased glutathione levels and the accumulation of ROS, with associated growth suppression in vitro and in vivo. Murine pancreatic KrasG12D GEMMs induce the expression of Nrf2, which promotes an antioxidant transcriptional program, further supporting a direct requirement for oncogenic KRAS-driven cancers to suppress ROS production [60]. Indeed, deletion of Nrf2 impaired pancreatic intraepithelial neoplasia development in this model, revealing that ROS detoxification is essential for murine Kras-driven tumorigenesis. Consistent with this idea, Nrf2 deletion in a mouse model of urethane-induced lung carcinogenesis accelerated tumor formation, but none of the tumors with Nrf2 loss harbored activating Kras mutations, suggestive of genetic codependency and the inability of these cells to tolerate excessive ROS accumulation [61]. Finally, the induction of endoplasmic reticulum stress in aggressive KP lung tumors by combined HSP90 and mTOR inhibition was linked to ROS generation and glutathione depletion, further identifying the need for the detoxification of ROS as a therapeutically targetable vulnerability in oncogenic KRAS-driven lung cancer cells [62].
Cell cycle regulators
Genetic screens for oncogenic KRAS synthetic lethal targets have also unveiled a particular requirement for kinases that regulate key components of cell cycle transitions. For example, genome-wide RNAi screening in isogenic colorectal cancer cell lines with or without activating KRAS alleles identified the enrichment of genes with mitotic function, particularly PLK1, as being selectively required in oncogenic KRAS-dependent cells [63]. Similarly, a specific requirement for the G1/S-phase regulatory cyclin-dependent kinase CDK4 was observed in lung cancer cells expressing oncogenic KRAS, with CDK4 inhibition resulting in cellular senescence and therapeutic benefit in a Kras lung cancer GEMM [64]. As discussed below, given the general requirement for these kinases in cell cycle control, the therapeutic window for their pharmacologic inhibition alone or in combination with other agents remains uncertain, but nonetheless, oncogenic KRAS-driven lung cancer cells appear to be particularly susceptible to the perturbation of cell cycle regulation.
Therapeutic targeting of downstream KRAS signaling pathways
In contrast to KRAS itself, many of the aforementioned downstream signaling molecules consist of kinases that are more readily druggable targets. Indeed, a great deal of effort over the past several years has been devoted to the development of kinase inhibitors directed against some of the most well-validated members of KRAS downstream pathways, with a major focus on components of the RAF–MAPK and PI3K–AKT signaling cascades [6]. As reviewed below, although founded upon a sound principle and solid preclinical data, combinations of inhibitors targeting these pathways have had limited activity in the clinic. The failure of this strategy to date may relate to overlapping toxicities, incomplete dual-pathway suppression at the maximum tolerated dose or the rapid emergence of resistance associated with an impaired apoptotic response [65]. Indeed, more generally, it is likely that inhibition of two downstream signals alone will be insufficient to overcome the heterogeneity and adaptability of most KRAS-driven lung cancers. Instead, effective KRAS-directed therapies will likely require the inhibition of additional targets, the alternative targets described above or those that are yet to be discovered.
Therapeutic RAF & PI3K pathway inhibition
As discussed above, RAF–MAPK and PI3K–AKT signaling clearly contribute to RAS transformation and are distinctly required in mouse models of oncogenic Kras-driven lung tumorigenesis. However, the effects of pharmacologic inhibition differ from genetic deletion, and murine drug trials are susceptible to limitations based upon the pharmacokinetic and pharmaco-dynamic properties of the specific small molecules. In addition, whereas most genetic studies have involved the conditional inactivation of drug targets specifically in lung tissue, the exposure of the entire animal to pharmacologic inhibition limits the maximum doses that can be used and depends upon the therapeutic window of the targeted pathway. Since both RAF and PI3K are involved in signal transduction and the proliferation of normal cells, the relative dependence of KRAS-driven tumor cells on the activation of these pathways is a particularly important issue.
Despite these limitations, combined inhibition of RAF and PI3K pathway signaling using the MEK inhibitor ARRY-142886 (renamed AZD6244/selumetinib) and the pan-PI3K/mTOR inhibitor NVPBEZ235 resulted in marked synergy in the KrasG12D lung cancer GEMM [66]. Although single-agent NVPBEZ235 had activity in another model of PIK3CAH1047R-induced lung cancer, it failed to inhibit the growth of KrasG12D-driven lung tumors by itself, in contrast to MEK inhibition, which had single-agent activity [66]. Studies in non-small-cell lung cancer cell lines have also supported the cooperativity between MEK and PI3K inhibition in the setting of oncogenic KRAS activation [67]. However, the durability of response and the activity in more aggressive models, such as the KP lung cancer GEMM, is less clear, and recent work has suggested that the failure of combined MEK and PI3K inhibition to induce apoptosis may explain the lack of robust efficacy for this therapy [65]. Nevertheless, the modest single-agent activity of MEK inhibition in KRAS-mutant tumors, coupled with the development of improved MEK inhibitors that also inhibit feedback MEK phosphorylation [68], suggests that maintenance of ERK signaling in particular represents a key requirement for KRAS-driven lung tumorigenesis and is an important pharmacologic target.
Indeed, several other studies in mouse models of oncogenic KrasG12D murine lung cancer have focused on MEK inhibition as a backbone for other combination therapies. Although treatment of these mice with the standard second-line chemotherapeutic agent docetaxel had minimal activity on its own, the addition of selumetinib resulted in significant tumor regression [69]. Despite its toxicity, docetaxel/selumetinib therapy also showed efficacy in the aggressive KP lung cancer GEMM, although resistance developed rapidly and activity was not observed in a Kras–Lkb1-mutated lung cancer GEMM [69]. Others have explored systematic screens for the identification of modifiers of MEK inhibitor sensitivity or alternative methods of suppressing PI3K/AKT pathway activity. For example, in contrast to PI3K inhibitors, BCL-XL antagonism cooperated with selumetinib to induce apoptosis, inhibiting the survival of multiple KRAS-driven human cancer cell lines and causing tumor regression in oncogenic Kras lung cancer GEMMs, including the aggressive KP model [70]. Another strategy has been to develop multitargeted agents against PI3K/mTOR and PIM kinases, the inhibition of which synergizes to impair KRAS-driven tumorigenesis in preclinical models [71]. Prevention of feedback IGF1R signaling was also recently found to be a particularly effective way of suppressing PI3K/AKT activity in KRAS-dependent lung cancer cell lines, and the IGF1R inhibitor OSI906 (linsitinib) cooperated with MEK inhibition to impair tumor growth in the KP lung cancer GEMM [72]. Thus, chemotherapy and novel approaches to inducing apoptosis or targeting PI3K signaling are being incorporated into MEK inhibitor-based regimens as alternative strategies for targeting these well-established pathways.
Although multiple different clinical trials are underway in order to examine the efficacy of combined MEK and PI3K inhibition across different KRAS-mutated tumor types (Table 1), initial results have been disappointing, which is probably due to the aforementioned issues [65]. By contrast, the combination of docetaxel with selumetinib was shown to prolong progression-free survival in a Phase II study in KRAS-mutant lung cancer, although these results await confirmation in a larger Phase III clinical trial and this regimen requires growth factor support, given the elevated risk of febrile neutropenia with this combination [73]. More targeted alternative strategies of enhancing apoptosis (MEK + BCL2/BCL-XL inhibition) or targeting PI3K upstream (MEK + IGF1R inhibition) have also entered the clinic. The results of these and other studies that incorporate novel MEK inhibitors with the capability to impair feedback MEK reactivation by RAF [68,74] are eagerly anticipated.
Table 1.
Clinical trials of combination therapies that target KRAS signaling pathways.
| Targets | Drug combination | NCT ID† |
|---|---|---|
| MEK/PI3K | MEK162 (MEKi) + BKM120 (PI3Ki/mTORi) | NCT01363232 |
| MEK/PI3K | MSC1936369B (MEKi) + SAR245409 (PI3Ki/mTORi) | NCT01390818 |
| MEK/PI3K | GDC-0973 (MEKi) + GDC-0941 (PI3Ki) | NCT00996892 |
| MEK/BCL-XL | Trametinib (MEKi) + navitoclax (BCL-XLi) | NCT02079740 |
| MEK/IGF1R | AZD6244 (MEKi) + cixutumumab (IGF1Ri) | NCT01061749 |
| MEK/IGF1R | MEK162 (MEKi) + AMG 379 (IGF1Ri) | NCT01562899 |
| MEK/TBK1 | Trametinib (MEKi) + momelotinib (JAK/TBK1i) | Pending |
| MEK/CDK4 | PD-0325901 (MEKi) + palbociclib (CDK4i) | NCT02022982 |
| MEK/CDK4 | Trametinib (MEKi) + palbociclib (CDK4i) | NCT02065063 |
Representative KRAS-directed combination therapy trials are shown that have completed accrual, are currently open or are awaiting activation.
Clinicaltrials.gov identifier.
i: Inhibitor.
Pharmacological inhibition of RAL signaling & cytokines
Despite the strong evidence that RAL-GEF activation is critical for RAS-mediated cell transformation and tumorigenesis, pharmacological inhibition of RAL signaling has been more difficult to achieve and has remained a secondary focus. In part, this has been related to the fact that RALA and RALB, similar to KRAS, are small GTPases that represent challenging drug targets themselves. Similarly, attempts to target cytokines have been hampered by the fact that multiple different cytokines contribute to KRAS-driven tumorigenesis. However, in contrast to MEK and PI3K, which promote physiological signal transduction and cell proliferation, activation of cytokines such as IL-1, IL-6 and IL-8 instead cause senescence in normal cells with intact checkpoints [75,76]. Thus, while inhibition of cytokine signaling might be associated with certain immunologic toxicities, these findings in general suggest the potential for a wider therapeutic window compared with MEK and PI3K inhibition.
Although inhibition of RAL-GEF or its direct targets RALA and RALB has currently remained intractable, pathways further downstream of RALA or RALB may provide additional opportunities for therapeutic intervention. For example, RALB induces autophagy [77], which is susceptible to pharmacological inhibition at multiple levels. The engagement of TBK1 by RALB provides a specific drug target for KRAS-driven lung cancers [40,42]. Indeed, activation of TBK1 and its related homolog IKKε links oncogenic KRAS to the expression of the cytokines CCL5 and IL-6, which directly promote tumorigenesis [47]. CYT387 (renamed GS-0387/momelotinib) was recently identified as a potent JAK/TBK1/IKKε inhibitor that disrupts this autocrine cytokine production and has significant therapeutic efficacy in murine KrasG12D-induced lung cancer [47]. Furthermore, the combination of momelotinib with the MEK inhibitor selumetinib resulted in potent synergy in the aggressive KP lung cancer GEMM, indicating that combination therapy targeting the RAF, RAL and cytokine signaling axes holds significant promise for the treatment of oncogenic KRAS-driven lung cancer. Since momelotinib has already progressed into advanced-phase human clinical trials for myelofibrosis [78], these findings are rapidly being incorporated into a clinical trial for KRAS-mutant lung cancer (Table 1).
Although additional therapeutic strategies to target RAL signaling and cytokines are likely to emerge, in general, inhibitors of these pathways remain underdeveloped. While neutralizing antibodies that target individual cytokines have entered clinical use for several rheumatologic and autoimmune disorders, adapting these for KRAS-targeted cancer therapy remains a challenge due in part to the redundancy described above. Specific NF-κB inhibitors have yet to enter the clinic, although a proof-of-principal study utilizing the drug BAY-117082 also demonstrated response and resistance in aggressive KP murine lung cancer [79]. Interestingly, similar to momelotinib, this inhibitor has a broad spectrum of activity and inhibits TBK1/IKKε as well as the canonical IKKs through its suppression of activating ubiquitin conjugation and the linear ubiquitin assembly complex [80]. Further development of this class of agents or other NF-κB inhibitors may also hold promise as components of KRAS-targeted therapy.
Pharmacological targeting of other co-opted pathways
Pharmacological approaches to co-opted signaling pathways, such as those involved in autophagy, metabolism and ROS suppression, largely remain in pre-clinical stages. Although the drug chloroquine inhibits autophagy and impairs murine oncogenic Kras-induced pancreatic tumorigenesis [49] and a number of clinical trials evaluating its efficacy in cancer are underway, its pharmacological properties are not ideal. Similarly, the mitochondrial inhibitor phenformin has activity specifically in the Kras–Lkb1-mutant murine lung cancer model [81], but phenformin causes lactic acidosis in humans, and it is unclear whether its analog, the diabetes drug metformin, has adequate potency in order to achieve desirable effects. Metformin has also been shown to reduce the production of certain cytokines, such as IL-6, in lung cancer cell lines [82], and is currently undergoing evaluation in multiple clinical trials alone or in a combination therapy for lung cancer.
Currently, the most clinically advanced therapies directed against pathways that are codependent with oncogenic KRAS are those that target the cell cycle. Although chemotherapeutics such as taxanes, which target mitosis, have long been in the clinic, it is not clear whether these provide particular benefits for KRAS-mutant lung cancers, and the effects of more specific Polo-like kinase or Aurora kinase inhibitors remain to be determined. However, several newer selective CDK4/6 inhibitors, including palbociclib and LY2835219, have entered clinical trials. Encouragingly, a Phase I study of LY2835219 was associated with responses in KRAS-mutant lung cancer [83]. Combination therapy between palbociclib and the MEK inhibitor PD-0325901 is also underway in a Phase I/II study for this population (Table 1). Such studies will help us to define the clinical activity and the tolerability of agents that also inhibit the cell cycle and suppress proliferation in normal cells, but potentially with a window that differs from KRAS-mutant lung cancer cells that are addicted to these pathways.
Conclusion: reflections on therapy
As reviewed above, much has been learned over the past 20 years regarding the pathways that KRAS engages and requires during lung tumorigenesis, and specific inhibitors of these downstream signaling molecules continue to undergo clinical evaluation. Developing effective targeted therapeutic regimens for KRAS-driven lung cancer will probably require the key principle that shaped the formation of combination chemotherapy regimens that have been successful in curing childhood leukemias and certain lymphomas: integration of multiple agents with differing mechanisms of action and nonoverlapping toxicities. It is clear from these regimens, and from the effective therapy of TB and HIV, that probably at least triple-combination drug therapy will be required in order to eradicate the disease and prevent resistance. We conclude this article with theoretical considerations on how to build such regimens in order to target oncogenic KRAS in a rational fashion.
As highlighted above, the key issues facing combination MEK/PI3K inhibitor therapy have been ascribed to the narrow therapeutic window or the failure to induce apoptosis [65]. At the same time, it is clear from numerous studies that inhibiting RAF and PI3K activity in KRAS-driven tumors should be effective at impairing tumorigenesis. The finding that combined inhibition of MEK and IGF1R also accomplishes this goal and has activity in the KP lung cancer model is significant [72], and suggests that alternative routes to PI3K pathway inhibition may be less toxic and disrupt specific MEK/ERK-regulated feedback loops (Figure 2A). Similarly, inhibition of autocrine cytokine signaling by momelotinib in KRAS-dependent cells is associated with feedback induction of MEK/ERK signaling (Figure 2B), and combined inhibition of both STAT3 and ERK activation through the addition of selumetinib results in impressive synergy in the KP lung cancer GEMM [47]. Furthermore, IGF1R inhibitors such as linsitinib (OSI-906), MEK inhibitors such as selumetinib (AZD6244) or trametinib (GSK1120212) and JAK/TBK1 inhibitors such as momelotinib (CYT387) each have a unique side effect profile and sets of nonoverlapping toxicities. Specifically, dose-limiting toxicities of OSI-906 involve hyperglycemia and MEK inhibitors cause rashes, whereas CYT387 induces cytokine suppression and may predispose individuals to immune compromise. Thus, in contrast to PI3K and MEK inhibitors, which both induce rashes, it is possible that this alternative combination of pathway-targeting agents may achieve potent inhibition of KRAS downstream signaling at doses that could be tolerated together in humans. A particularly rational and appealing strategy would be to evaluate this triple-combination therapy that incorporates suppression of all three major RAS effectors (Figure 1) in preclinical models and, ultimately, in patients.
Figure 2. Feedback loops that limit the activity of individual pathway-targeted therapies.
(A) MEK inhibition results in feedback activation of IGF1R and downstream PI3K/AKT signaling, with an increase in pAKT levels. Combination therapy with IGF1R inhibitors such as linsitinib (OSI-906) suppresses this feedback and synergizes to inhibit KRAS-driven lung tumorigenesis. (B) TBK1 inhibition leads to induction of MEK/ERK signaling and increased pERK levels through an unclear mechanism. Cotreatment of momelotinib (CYT387) with the MEK inhibitor selumetinib (AZD6244) blocks this compensatory ERK activation and also leads to synergistic tumor regression in aggressive Kras/p53 mutant murine lung cancer.
pAKT: Phosphorylated AKT; pERK: Phosphorylated ERK.
Another important consideration is the heterogeneity of KRAS-driven lung cancers and the fact that specific subsets may be particularly sensitive or resistant to certain therapies. For example, Kras–Lkb1-mutant murine lung cancer was less sensitive to the docetaxel/selumetinib combination [69], but might be more sensitive to targeted inhibition of other pathways, particularly in light of its known propensity for inducing cytokine expression [84]. Similarly, the type of oncogenic KRAS mutation could influence the pattern of downstream effector engagement. For example, one study suggested that KRASG12C and KRASG12V preferentially induce RAL signaling and are associated with a worse prognosis [85]. However, large numbers of patients and samples will be needed in order to separate the potential confounding of concurrent p53 and LKB1 tumor-suppressor mutations with oncogenic KRAS mutation type when distinguishing the effectiveness of any particular targeted therapy within these subgroups. Nevertheless, the expansion of clinical genotyping will enable the discovery of subgroups with enhanced responses or resistance to these novel targeted therapeutic approaches that are entering clinical trials.
It is also likely that intratumoral heterogeneity will affect the outcomes of ongoing and future KRAS-targeted therapy trials. For example, two drug regimens that are more effective against a certain subgroup may cause transient tumor regressions, only to be subverted by the outgrowth of clones that cause resistance. However, the identification of patients that respond to a particular drug combination but subsequently become resistant may provide an unbiased way of determining the optimal route to triple-combination therapy by uncovering specific compensatory pathways. Thus, the expansion of novel targeted therapeutic trials directed against downstream KRAS signaling pathways, while hopefully successful, will nevertheless provide a wealth of information and guide the next generation of studies that may bring us closer to the ultimate goal of a cure.
Future perspective
In 5–10 years, data from the second generation of clinical trials targeting KRAS downstream signaling pathways will have emerged, incorporating novel therapeutic approaches such as the inhibition of RAL-GEF and cytokine signaling. The safety and efficacy observed in these studies will determine the ability to move beyond pairwise combinations of targeted therapeutics and into triple-combination therapy, which would be necessary in order to suppress all three major KRAS effector pathways. In addition, targeting KRAS-codependent pathways will emerge as an equally tractable option for incorporation into these multidrug regimens, which will comprise the third generation of KRAS-directed clinical trials.
Supplementary Material
Executive Summary.
Direct KRAS effector pathways involved in cell transformation & lung tumorigenesis
RAF, PI3K and the RAL guanine exchange factor (RAL-GEF) were initially identified as essential components of RAS-mediated transformation in rodent cells.
Human cell transformation systems revealed that RAL-GEF signaling is particularly required for oncogenic RAS function in human cancer.
Murine Kras-driven lung cancer models have confirmed the key requirements for RAF, PI3K and RAL-GEF in vivo.
Co-opted pathways required by oncogenic KRAS in order to sustain lung tumorigenesis
Cytokine signaling and its associated STAT3 and NF-κB activation are required for KRAS-induced tumorigenesis.
Autophagy counterbalances cellular stress associated with oncogenic KRAS activation, as do specific metabolic and reactive oxygen species detoxifying adaptions that represent acquired vulnerabilities.
Kinases that regulate mitotic and G1/S-phase transitions are also potential therapeutic targets.
Therapeutic targeting of downstream KRAS signaling pathways
Combined MEK and PI3K inhibition has had limited success in the clinic, although alternative methods of targeting these pathways may be effective.
MEK inhibitors form the backbone of multiple regimens entering clinical trials and are being combined with BCL-XL, JAK/TBK1 and CDK4 inhibitors.
Rationally designed triple-therapeutic regimens will most likely be required in order to obtain durable responses.
Acknowledgments
DA Barbie is supported by the NIH grant K08 CA138918-01A1, Uniting against Lung Cancer, and the GTM Fund for Lung Cancer Research. Z Zhu has been supported by a Uniting against Lung Cancer Award. DA Barbie is a consultant for NofOne.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript
References
Papers of special note have been highlighted as:
• of interest
- 1.Porta M, Crous-Bou M, Wark PA, et al. Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: etiopathogenic similarities, differences and paradoxes. Mutat Res. 2009;682(2–3):83–93. doi: 10.1016/j.mrrev.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Holstein SA, Hohl RJ. Is there a future for prenyltransferase inhibitors in cancer therapy? Curr Opin Pharmacol. 2012;12(6):704–709. doi: 10.1016/j.coph.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann G, Papke B, Ismail S, et al. Small molecule inhibition of the KRAS–PDEdelta interaction impairs oncogenic KRAS signalling. Nature. 2013;497(7451):638–642. doi: 10.1038/nature12205. [DOI] [PubMed] [Google Scholar]
- 4.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SM, Westover KD, Ficarro SB, et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew Chem Int Ed Engl. 2014;53(1):199–204. doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines AT, Xu D, Der CJ. Inhibition of Ras for cancer treatment: the search continues. Future Med Chem. 2011;3(14):1787–1808. doi: 10.4155/fmc.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg RA. Hunting the elusive oncogene: a stroke of good luck. Nat Cell Biol. 2011;13(8):876. doi: 10.1038/ncb2302. [DOI] [PubMed] [Google Scholar]
- 8.Bonner TI, Kerby SB, Sutrave P, Gunnell MA, Mark G, Rapp UR. Structure and biological activity of human homologs of the raf/mil oncogene. Mol Cell Biol. 1985;5(6):1400–1407. doi: 10.1128/mcb.5.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moodie SA, Willumsen BM, Weber MJ, Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 10.White MA, Nicolette C, Minden A, et al. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80(4):533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 11.Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 12.Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Viciana P, Warne PH, Khwaja A, et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89(3):457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 14.White MA, Vale T, Camonis JH, Schaefer E, Wigler MH. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271(28):16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 15.Urano T, Emkey R, Feig LA. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15(4):810–816. [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki M, Kishida S, Murai H, Hinoi T, Kikuchi A. Ras-interacting domain of Ral GDP dissociation stimulator like (RGL) reverses v-Ras-induced transformation and Raf-1 activation in NIH3T3 cells. Cancer Res. 1996;56(10):2387–2392. [PubMed] [Google Scholar]
- 17.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400(6743):464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 18.Hamad NM, Elconin JH, Karnoub AE, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16(16):2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangarajan A, Hong SJ, Gifford A, Weinberg RA. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6(2):171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra C, Mijimolle N, Dhawahir A, et al. Tumor induction by an endogenous K-Ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4(2):111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 22.Karreth FA, Frese KK, Denicola GM, Baccarini M, Tuveson DA. C-Raf is required for the initiation of lung cancer by K-Ras(G12D) Cancer Discov. 2011;1(2):128–136. doi: 10.1158/2159-8290.CD-10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Blasco RB, Francoz S, Santamaria D, et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell. 2011;19(5):652–663. doi: 10.1016/j.ccr.2011.04.002. Elegant dissection of the genetic requirement for RAF signaling components in murine KRAS-induced lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Ramjaun AR, Haiko P, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129(5):957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Castellano E, Sheridan C, Thin MZ, et al. Requirement for interaction of PI3-kinase p110alpha with RAS in lung tumor maintenance. Cancer Cell. 2013;24(5):617–630. doi: 10.1016/j.ccr.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peschard P, Mccarthy A, Leblanc-Dominguez V, et al. Genetic deletion of RALA and RALB small GTPases reveals redundant functions in development and tumorigenesis. Curr Biol. 2012;22(21):2063–2068. doi: 10.1016/j.cub.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Demetri GD, Ernst TJ, Pratt ES, 2nd, Zenzie BW, Rheinwald JG, Griffin JD. Expression of Ras oncogenes in cultured human cells alters the transcriptional and posttranscriptional regulation of cytokine genes. J Clin Invest. 1990;86(4):1261–1269. doi: 10.1172/JCI114833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castelli C, Sensi M, Lupetti R, et al. Expression of interleukin 1 alpha, interleukin 6, and tumor necrosis factor alpha genes in human melanoma clones is associated with that of mutated N-RAS oncogene. Cancer Res. 1994;54(17):4785–4790. [PubMed] [Google Scholar]
- 29.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21(14):1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X, Carretero J, Chen Z, et al. Loss of p53 attenuates the contribution of IL-6 deletion on suppressed tumor progression and extended survival in Kras-driven murine lung cancer. PLoS ONE. 2013;8(11):e80885. doi: 10.1371/journal.pone.0080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Ancrile BB, O'Hayer KM, Counter CM. Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Mol Interv. 2008;8(1):22–27. doi: 10.1124/mi.8.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong L, Cumpian AC, Caetano MS, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013;12(1):154. doi: 10.1186/1476-4598-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar MS, Hancock DC, Molina-Arcas M, et al. The GATA2 transcriptional network is requisite for Ras oncogene-driven non-small cell lung. cancer Cell. 2012;149(3):642–655. doi: 10.1016/j.cell.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 36•.Meylan E, Dooley AL, Feldser DM, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462(7269):104–107. doi: 10.1038/nature08462. First definitive demonstration of the essential role of NF-κB in KRAS-induced lung tumorigenesis in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duran A, Linares JF, Galvez AS, et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13(4):343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Basseres DS, Ebbs A, Levantini E, Baldwin AS. Requirement of the NF-kappaB subunit p65/RelA for K-Ras-induced lung tumorigenesis. Cancer Res. 2010;70(9):3537–3546. doi: 10.1158/0008-5472.CAN-09-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y, Yeddula N, Leblanc M, et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat Cell Biol. 2012;14(3):257–265. doi: 10.1038/ncb2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chien Y, Kim S, Bumeister R, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127(1):157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 41.Boehm JS, Zhao JJ, Yao J, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 42.Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou YH, Torres M, Ram R, et al. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41(4):458–470. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajurkar M, De Jesus-Monge WE, Driscoll DR, et al. The activity of Gli transcription factors is essential for Kras-induced pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2012;109(17):E1038–E1047. doi: 10.1073/pnas.1114168109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman AC, Scholefield CL, Kemp AJ, et al. TBK1 kinase addiction in lung cancer cells is mediated via autophagy of Tax1bp1/Ndp52 and non-canonical NF-kappaB signalling. PLoS ONE. 2012;7(11):e50672. doi: 10.1371/journal.pone.0050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo J, Kim D, Gao J, et al. IKBKE is induced by STAT3 and tobacco carcinogen and determines chemosensitivity in non-small cell lung cancer. Oncogene. 2013;32(2):151–159. doi: 10.1038/onc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47•.Zhu Z, Aref AR, Cohoon TJ, et al. Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov. 2014;4(4):452–465. doi: 10.1158/2159-8290.CD-13-0646. Evidence that targeting the cytokine circuitry of KRAS-driven lung cancers with novel small-molecule inhibitors may be clinically effective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev. 2011;25(19):1999–2010. doi: 10.1101/gad.17558811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717–729. doi: 10.1101/gad.2016111. One of the first papers to demonstrate that oncogenic KRAS induces and requires autophagy in order to maintain tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Guo JY, Chen HY, Mathew R, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–470. doi: 10.1101/gad.2016311. Another key initial paper that revealed the essential role of autophagy and, specifically, mitochondrial maintenance downstream of KRAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lock R, Roy S, Kenific CM, et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22(2):165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim MJ, Woo SJ, Yoon CH, et al. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem. 2011;286(15):12924–12932. doi: 10.1074/jbc.M110.138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo JY, Karsli-Uzunbas G, Mathew R, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27(13):1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao S, Tortola L, Perlot T, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. doi: 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- 55.Lock R, Kenific CM, Leidal AM, Salas E, Debnath J. Autophagy-dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov. 2014;4(4):466–479. doi: 10.1158/2159-8290.CD-13-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39(2):91–100. doi: 10.1016/j.tibs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic KRAS maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73(13):4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 62.De Raedt T, Walton Z, Yecies JL, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20(3):400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo J, Emanuele MJ, Li D, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137(5):835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puyol M, Martin A, Dubus P, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18(1):63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 65.Hata AN, Yeo A, Faber AC, et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS mutant lung cancers. Cancer Res. 2014;74(11):3146–3156. doi: 10.1158/0008-5472.CAN-13-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant KRAS G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sos ML, Fischer S, Ullrich R, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA. 2009;106(43):18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatzivassiliou G, Haling JR, Chen H, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501(7466):232–236. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- 69•.Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483(7391):613–617. doi: 10.1038/nature10937. Demonstration that co-clinical trials between mouse models and humans can inform translation of novel therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corcoran RB, Cheng KA, Hata AN, et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23(1):121–128. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aparicio CB, Renner O, Gomez-Casero E, et al. Co-targeting PIM and PI3K/mTOR pathways with a single molecule: novel orally available combined PIM/Pi3K and PIM/PI3K/ mTOR kinase inhibitors. Mol Cancer Ther. 2013;12(11 Suppl.):A275. [Google Scholar]
- 72•.Molina-Arcas M, Hancock DC, Sheridan C, Kumar MS, Downward J. Coordinate direct input of both KRAS and IGF1 receptor to activation of PI3 kinase in KRAS-mutant lung cancer. Cancer Discov. 2013;3(5):548–563. doi: 10.1158/2159-8290.CD-12-0446. Identification of IGF1R signaling as a key input to AKT activation in KRAS-dependent lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, Phase 2 study. Lancet Oncol. 2013;14(1):38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 74.Lito P, Saborowski A, Yue J, et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 2014;25(5):697–710. doi: 10.1016/j.ccr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106(40):17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 77.Bodemann BO, Orvedahl A, Cheng T, et al. RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell. 2011;144(2):253–267. doi: 10.1016/j.cell.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue W, Meylan E, Oliver TG, et al. Response and resistance to NF-kappaB inhibitors in mouse models of lung adenocarcinoma. Cancer Discov. 2011;1(3):236–247. doi: 10.1158/2159-8290.CD-11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strickson S, Campbell DG, Emmerich CH, et al. The anti-inflammatory drug BAY 11–7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem J. 2013;451(3):427–437. doi: 10.1042/BJ20121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23(2):143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li L, Han R, Xiao H, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014;20(10):2714–2726. doi: 10.1158/1078-0432.CCR-13-2613. [DOI] [PubMed] [Google Scholar]
- 83.Shapiro GI, Rosen LS, Tolcher AW, et al. First-in-human Phase 1 study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol. 2013;31(Suppl.) Abstract 2500. [Google Scholar]
- 84.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 85.Ihle NT, Byers LA, Kim ES, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104(3):228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.