Abstract
Ideally antimalarial drugs can be developed which target multiple life cycle stages, thus impacting prevention, treatment and transmission of disease. Here we introduce 4-(1H)-quinolone-3-diarylethers that are selectively potent inhibitors of the parasite’s mitochondrial cytochrome bc1 complex. These compounds are highly active against the primary human malarias (falciparum and vivax), targeting the parasite at both the liver and blood stages as well as the forms that are crucial to disease transmission: gametocytes ⇒ zygotes ⇒ ookinetes ⇒ oocysts. Chosen as the preclinical candidate, ELQ-300 has good oral bioavailability at efficacious dosages in mice, is metabolically stable, and is highly active in rodent malaria models. Given a low predicted dose in patients and a long predicted half-life, ELQ-300 offers the hope of a new molecule for the treatment, prevention and, ultimately, eradication of malaria.
Malaria remains the most significant parasitic disease in the tropics where it causes ≈200 million clinical cases and is reported to claim up to 1,238,000 lives each year (1). Most of the drugs used to treat the disease target only the blood stage of infection and the efficacy of many of the historical antimalarials, such as choroquine and quinine, has been declining due to the emergence of multidrug resistant strains of Plasmodium parasites. The widespread nature of multidrug resistance has shifted the emphasis to artemisinin combination therapies (ACTs) as first-line treatments in malaria-endemic countries. While ACTs have succeeded in reducing mortality and mosquito control programs have helped to reduce the worldwide burden of disease, there are warning signs of emerging artemisinin resistance in Southeast Asia (2, 3). As a result the development of new drugs for treatment and prevention of malaria is urgently needed to circumvent resistance and to meet the challenge of malaria eradication.
To support the global malaria eradication effort there is a need for new medicines that can be given by mouth as a single dose to allow direct monitoring of administration and to ensure compliance (4, 5). Ideally such drugs will be efficacious against liver and blood stage infections and active against resistant strains. There is also a need for next generation drugs that kill gametocytes as well as the vector stages (i.e., ookinetes and oocysts) and thus can be used to prevent disease transmission. Ideally these desirable features can be incorporated into new molecules with longer half-lives to give chemoprophylaxis and to provide long-term protection against re-infection.
Several years ago the Medicines for Malaria Venture (MMV) formed a team of investigators to evaluate the potential for development of an endochin (Fig. 1) derivative for clinical use. The team used a test cascade to optimize endochin which included the design and chemical synthesis of structural analogs, in vitro tests to compare antiplasmodial activity with mammalian cytotoxicity with profiling of the life-cycle fingerprint across all parasites stages, assessment of ex vivo efficacy against P. falciparum and P. vivax clinical field isolates, the determination of metabolic stability and enzyme inhibition, assessment of in vivo efficacy against blood and liver stages of infection in murine models of malaria, pharmacokinetic and safety profiling. This paper focuses on two 4(1H)-quinolone derivatives, ELQ-300 and P4Q-391, that were identified as late lead candidates.
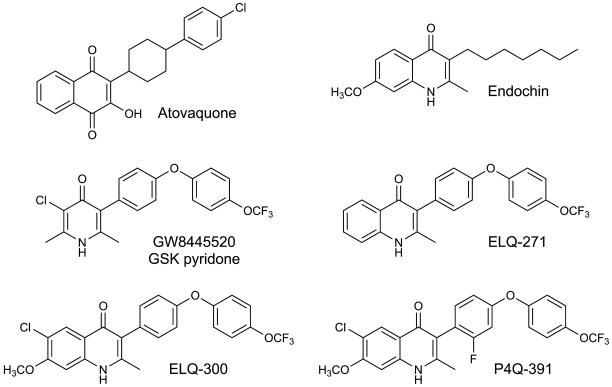
Figure 1.
Chemical structures of compounds used or referred to in this study. Atovaquone and GW8445520 are antimalarial drugs that are known to target the Plasmodium cytochrome bc1 complex. Discovery of endochin as an antimalarial dates back to the 1940’s when it was shown to be effective against several avian plasmodial species but lacked activity against mammalian species. A new structural class of antimalarial agents is represented by ELQ-271, ELQ-300 and P4Q-391, 4(1H)-quinolones bearing a diarylether side chain at the 3-position of the quinolone core. ELQ-300 and P4Q-391 were developed further and tested in a number of rodent models of malaria.
Brief history of endochin
Andersag and coworkers first described endochin over 70 years ago (6). The drug attracted interest because of its ability to prevent and treat sporozoite-induced malaria in an avian model of the disease (Plasmodium cathemerium in canaries); it failed to work in humans (7). More recent studies confirm that endochin exhibits potent antiplasmodial activity , but it is metabolically unstable in the presence of liver microsomes from all tested mammalian species, including human, and is converted to poorly active metabolites (8, 9). As a consequence we set out to design endochin derivatives with enhanced metabolic stability and potent activity against drug resistant malaria parasites.
Targeting parasite cytochrome bc1 complex
Endochin appears to target parasite mitochondrial processes since it is known to block respiration in Plasmodium infected red blood cells (9). The parasite mitochondrion has been validated as a target for malaria therapy by the clinical success of atovaquone (Fig. 1), which selectively inhibits the parasite cytochrome bc1 complex (10). Because atovaquone monotherapy leads to the rapid selection of drug resistant mutants, it is co-administered with proguanil in a synergistic combination (Malarone®). Although Malarone® is commonly prescribed for malaria prophylaxis in travel medicine it is not widely used in malaria control programs due to the high cost of production. As a result, there is increasing interest in the development of less expensive alternatives to atovaquone that target parasite respiration, are active against all known strains, and are not prone to rapid resistance selection.
The GSK pyridones
Yeates and coworkers recently described a novel series of diphenylether-substituted pyridones that, like atovaquone, are believed to target the Qo site of the Plasmodium cytochrome bc1 complex of the mitochondrial electron transport chain (11). A lead compound from this series, GW844520 (Fig. 1), was more potent than chloroquine and in vivo in treatment of P. yoelii infected mice. A structurally related pyridone (GSK932121) was progressed into clinical trials in healthy volunteers (Phase I) and low doses were delivered with success. However, the molecule was recently withdrawn from human trials due to safety concerns observed with a prodrug of GSK932121 in preclinical studies (12). The toxicity observed in rats was attributed to the inhibition of mammalian mitochondrial cytochrome bc1 complex by the parent drug GSK932121. Earlier work by our respective research teams focused on endochin derivatives with varying substituents in the benzenoid ring of the quinolone core (8, 9, 13). Endochin analogs displaying simple alkyl or aryl groups at the 3-position were made to increase our understanding of the antimalarial structure-activity profile (8, 9, 13). A decision was made to follow the lead from the GSK pyridone project by placing a diphenylether side chain at the critical 3-position (14). ELQ-300 and P4Q-391 are substituted in this manner.
Activity against drug resistant Plasmodium strains
Antiplasmodial activities were determined by two different assays, the fluorescence SyBr green method (15) and the 3H-hypoxanthine incorporation assay (16). ELQ-300 and P4Q-391 displayed potent activity with low nanomolar 72h EC50 values against chloroquine sensitive (D6, 3D7) and multidrug resistant P. falciparum strains (Dd2, W2 and recent isolates from SE Asia) including the atovaquone-resistant clinical isolate TM90-C2B (Tables S1 and S2). Atovaquone resistance in TM90-C2B is linked to a mutation in the parasite’s cytochrome b gene (the mutation alters the wild-type from Tyr268 to Ser268) in the ubiquinol binding region also known as the Qo site. EC50 values for ELQ-300 against the reference strains and recent isolates range from 1.3 to 13.6 nM while the range of EC50 values for P4Q-391 are slightly higher (EC50 range: 3.18 to 32.3 nM). Interestingly, cross-resistance with atovaquone in TM90-C2B was much reduced for both ELQ-300 (1.3-fold) and P4Q-391 (3.2-fold) as compared to the diarylether substituted pyridone GSK932121A (16.7-fold).
We also included the transgenic P. falciparum clone D10yDHOD and A6 clone (17) to demonstrate that the quinolone-3-diarylethers target the parasite respiratory chain. D10yDHOD parasites express the Saccharomyces cerevisiae dihydroorotate dehydrogenase, which is a cytosolic enzyme that does not require ubiquinone as an electron acceptor (18, 19). This genetic manipulation uncouples pyrimidine biosynthesis from electron transport and endows these parasites with protection against electron transport inhibitors (19). As shown in Tables S1 and S2, D10yDHOD transfectants are insensitive to ELQ-300 and P4Q-391, results that are consistent with the respiratory chain as their primary site of action.
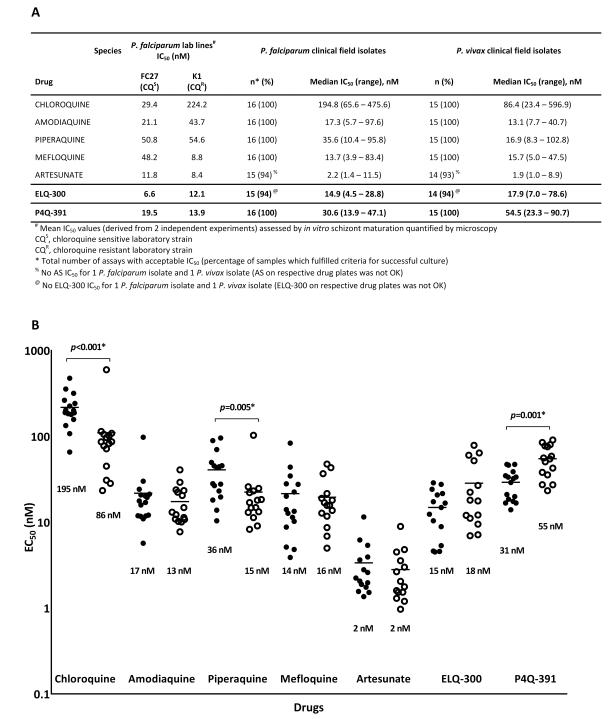
We investigated the ex vivo susceptibility profile of both compounds against clinical isolates of P. falciparum and P. vivax collected from patients attending malaria clinics in southern Papua, Indonesia, a region where multidrug-resistant strains of both P. falciparum and P. vivax are endemic. Drug susceptibility was measured using a modified WHO microtest described previously (20, 21). ELQ-300 performed well against both drug resistant P. falciparum and P. vivax field isolates with median EC50 values of 14.9 nM and 17.9 nM, respectively (Fig. 2). There was no statistical difference in ex vivo susceptibility to ELQ-300 between the two Plasmodium species. Median EC50 values of 30.6 nM (P. falciparum) and 54.5 nM (P. vivax) for P4Q-391 were somewhat higher than for ELQ-300, and there was a statistically significant (p=0.001) difference in ex vivo susceptibility to P4Q-391 between the two Plasmodium species. Taken together, our data show that ELQ-300 potently inhibits erythrocytic stages of the two most significant human malaria species, P. falciparum and P. vivax, and that it exhibits greater intrinsic antiplasmodial activity in vitro in comparison to P4Q-391.
Figure 2.
Ex vivo activity of ELQ-300 and P4Q-391 against Plasmodium clinical field isolates. (A) Ex vivo susceptibility of different Plasmodium species to ELQ-300 and P4Q-391 compared with that for other antimalarials such as chloroquine. (B) Ex vivo susceptibility (median IC50s) of P. falciparum (closed circles) and P. vivax (open circles) to ELQ-300 and P4Q-391 compared to other antimalarials. P values calculated using *Wilcoxon rank sum test.
Cytotoxicity studies
The two 4(1H)-quinolones were tested for cytotoxicity against J774 mouse macrophages, low passage (3-10) human foreskin fibroblasts (HFF), and murine bone marrow derived hematopoietic progenitors including CFU-GM (granulocyte-macrophage), BFUE (erythroid), and CFU-GEMM (pluripotent) stem cells. The 50% inhibitory concentrations for ELQ-300 and P4Q-391 in each of these systems was >10μM (Table S3). Considering that the antiplasmodial EC50 values for these drugs are in the low nanomolar range, the comparative cytotoxicity data provides an in vitro selectivity index of 400 to greater than 1,000-fold.
Selective inhibition of parasite cytochrome bc1
Drug safety is especially important in malaria since pregnant women and young children are the most vulnerable to the lethal consequences of infection and the most likely to require treatment. For this reason it was important to establish that 4-(1H)-quinolones could be modified to avoid inhibition of human cytochrome bc1. A clear structure-activity profile emerged for the 4-(1H)-quinolone series and their inhibitory activity toward human cytochrome bc1 (derived from HEK cells). The data presented in Table 1 show a correlation between inhibition and the nature of the substitution pattern on the benzenoid ring of the quinolone core. ELQ-271 (Fig. 1), with an unsubstituted benzenoid ring, inhibits the human enzyme (IC50 = 1.99 μM), while the placement of a chlorine atom and/or a methoxy group in this ring hinders interaction with human cytochrome bc1 as IC50 values for ELQ-300 and P4Q-391 are greater than 10μM (Table S4). Previously these same substitutions on the benzenoid ring were shown to enhance antimalarial potency of endochin derivatives in vitro (8, 9). We also conducted ATP depletion assays using two different mammalian cell lines to validate the degree of selectivity that was observed in the enzyme inhibitor assays. As shown in Fig. S1, ELQ-271 caused a concentration dependent decline in ATP levels (indicating that it was inhibiting host electron transport processes) with EC50 values in the range of 7.4 to 15.4 μM, whereas ELQ-300 did not have a measureable adverse effect on ATP levels in either cell line at concentrations of 10 μM and higher.
Table 1.
Inhibition profiles of human vs. P. falciparum cytochrome bc1 activity.
| Compound | IC50, nM | Selectivity Index | |
|---|---|---|---|
| human* cytochrome bc1 |
P. falciparum cytochrome bc1 |
Human/P. falciparum | |
| ELQ-271 | 1,750 | ND | ND |
| ELQ-300 | >10,000 | 0.56 | >18,000 |
| P4Q-391 | >10,000 | 1.0 | >10,000 |
| Atovaquone | 460 | 2.0 | 230 |
Human cytochrome bc1 was derived from HEK-293 cells. ND = not determined.
The ability of ELQ-300 and P4Q-391 to inhibit cytochrome bc1 activity from P. falciparum mitochondria was assessed. As shown in Table 1, both compounds are potent inhibitors of the P. falciparum cytochrome bc1 with IC50 values of 0.58 nM and 1.06 nM, respectively (Fig. S2). Mather et al. have previously reported an IC50 value of 2.0 nM for atovaquone vs. P. falciparum cytochrome bc1 (22). Thus while it is apparent that ELQ-300 and P4Q-391 target the same parasite enzyme complex as atovaquone, each retains significant potency against an atovaquone resistant clinical isolate harboring a mutation in the Qo region of cytochrome b (see Tables S1 and S2). While additional biochemical studies are required to fully elucidate the antimalarial mechanism of these quinolones it is interesting to speculate that they may target the Q i site of the enzyme complex. Doggett et al. (23) recently reported that a mutation in the Qi site region of the yeast cytochrome b gene greatly diminished the growth inhibitory activity of ELQ-271. Together our data show that ELQ-300 and P4Q-391 are highly selective inhibitors of plasmodial cytochrome bc1 complexes with selectivity indices that are ≥10,000. This high level of selectivity suggests a low potential for side effects in humans due to inhibition of the host enzyme.
Parasite reduction ratio
Speed-of-action is an important determinant of antimalarial efficacy and a crucial parameter guiding clinical utility. Antimalarial endoperoxides like artesunate are rapid-acting drugs that typically clear parasitemia in the first 48 h of treatment. In contrast, atovaquone displays an initial lag phase of cytostatic action over the first 48 h after which the drug exerts cidal activity (24). We used an in vitro assay to measure the effect of ELQ-300 on parasite viability over time by exposing P. falciparum to the drug for up to 120 hr (24). Our results show that ELQ-300 is a slow-acting drug with a delayed parasite reduction ratio similar to atovaquone. The time required to kill >99.9% of parasites treated with 10x EC50 concentration of ELQ-300 is estimated to be ≈100 h (Fig. S3).
Resistance propensity
Drug resistance is inevitable for antimalarial agents and consequently it is prudent to evaluate new candidates to ensure a low propensity for resistance development. Experiments were performed with 108 Dd2 parasites exposed to 10x EC50 of either atovaquone (10nM) or ELQ-300 (150nM) (Table S5). Cultures were continued under drug pressure for 8 weeks. Atovaquone-resistant parasites were observed on day 30 in each of three atovaquone treatment flasks. In contrast no parasites could be detected after 8 weeks of treatment in the ELQ-300 containing flasks. Under the same culture conditions, seeding of 10 parasites with no drug treatment resulted in detectable parasites in 14-15 days. Thus the lack of resistant parasites emerging within the 8-week period predicts a resistance frequency of <1 in 108 parasites for ELQ-300. This experiment was repeated and the results were the same, i.e., atovaquone resistant parasites emerged in culture flasks after ≈16 days while resistant parasites were not detected in any of the ELQ-300 treatment flasks. Similar experiments with P4Q-391 (300 nM) also failed to select mutants (Table S5). Our data suggest the propensity for quinolone-3-diarylether resistance in vitro is significantly less than for atovaquone.
Synergism studies
Although our studies showed that the propensity for resistance to both 4(1H)-quinolones is quite low relative to atovaquone we were interested to evaluate the potential for synergism with proguanil, the clinical partner of atovaquone in Malarone®. For in vitro isobolar testing of ELQ-300 and proguanil we used fixed combination ratios of drug mixtures (25). As shown in Fig. S4 the antiplasmodial effect was greater at every dose combination than expected for a simple additive response. On the basis of isobolar testing against 4 different P. falciparum strains (D6, Dd2, TM90-C2B, and V1/S) we conclude that a strong pharmacological synergism exists between ELQ-300 and proguanil.
In vivo efficacy – blood stage activity
Efficacy against erythrocytic stages was determined in several standard rodent malaria models. In the Thompson test model (26) with P. berghei infected ICR mice (Fig. S5), ELQ-300 and P4Q-391 were highly potent, with fifty percent effective doses (ED50) of 0.016 and 0.27 mg/kg/day, respectively (Table S6). Cures were produced with doses as low as 0.1 mg/kg/day and the lowest dose group that resulted in no recrudescence within 30 days received 1.0 mg/kg/day.
The therapeutic efficacy of ELQ-300 against P. yoelii and its pharmacokinetic parameters after oral administration in CD1 Swiss mice were assessed. The antimalarial activity of ELQ-300 was measured in direct comparison to atovaquone using a standard Peters 4-day test and blood parasitemia was quantified by FACS analysis. ELQ-300 proved highly efficacious against P. yoelii; the dose that suppressed parasite proliferation by 90% compared to vehicle (PEG400)-treated mice (ED90) was 0.15mg/kg/day (Fig. 3). By comparison, the ED90 value for atovaquone was 0.04mg/kg/day. The drug exposure (AUC0-24 h) of ELQ-300 in peripheral blood of infected mice after single oral administration at the ED90 was 1.4μg·h·mL−1. Importantly, while atovaquone’s ED50 and ED90 values were superior, ELQ-300 was more powerful as a curative agent in this murine model of acute malaria infection in that the non-recrudescence dose was 30 times lower than that of atovaquone (Table S6).
Figure 3.
Comparative effect of ELQ-271 and ELQ-300 on the intracellular level of ATP in two different mammalian cell lines. The ability of ELQ-271 and ELQ-300 to deplete ATP levels in two rat cell lines in galactose medium was evaluated with antimycin A and cycloheximide as positive and negative controls, respectively. As shown in the figure while ELQ-271 caused a concentration dependent decline in ATP levels (indicating that it was inhibiting host electron transport processes), ELQ-300 did not have a measureable adverse effect on ATP levels in either cell line with an IC50 level above 100µM.
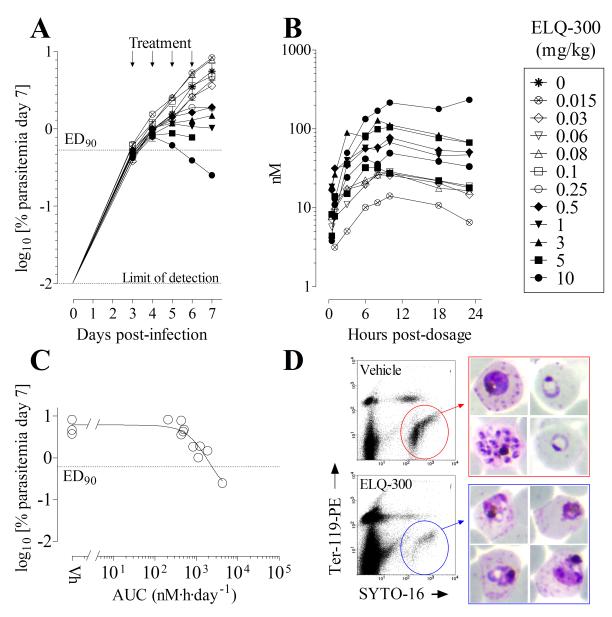
Efficacy of ELQ-300 was also tested in an immuno-deficient mouse model engrafted with human erythrocytes to support P. falciparum infection (27). Therapeutic efficacy was assessed by administering an oral dose of ELQ-300 daily for four consecutive days (see supplemental information) and measuring the effect on blood parasitemia by fluorescence-activated cell sorter analysis (27, 28). Blood concentrations of ELQ-300 were determined in all mice over a 23 h period following the first dose. Because of the sensitivity of the SCID mice engrafted with human erythrocytes to PEG400 (the vehicle used in the P. yoelii studies), a non-optimized suspension formulation was used in these studies and exposure levels in plasma for a given dose of ELQ-300 were lower than what had been observed in other mouse models using solubilizing formulations. The efficacy study was primarily aimed at estimating the 24 h exposure of ELQ-300 (AUCED90) necessary to suppress P. falciparum-parasitemia at day 7 in drug-treated mice by 90% with respect to vehicle-treated mice as a measure of in vivo potency, which does not depend on formulation. Data shown in Fig. 4 indicate that blood exposure is not dose proportional in humanized mice. The dose that reduced parasitemia by 90% at day 7 after infection with respect to vehicle-treated mice was 5.9 mg/kg/day (Fig. 4). The estimated exposure of ELQ-300 at the ED90 dose in the SCID mouse model was AUCED90 = 1.1 μg·h/mL/day, which corresponds very closely to the potency in vivo observed vs. P. yoelii (AUCED90 = 1.4 μg·h/mL/day and ED90 = 0.15 mg/kg/day) in the 4-day test described above. Collectively, our in vivo data shows that ELQ-300 and P4Q-391 are highly active against two different species of murine malaria and that ELQ-300 is equally potent against the human parasite P. falciparum in SCID mice.
Figure 4.
EC50 curves for ELQ-300 and P4Q-391 vs. P. falciparum cytochrome bc1. Cytochrome c reductase activity was monitored by spectroscopic analysis with a dual wavelength spectrophotometer in dual mode (550 nm – 541 nm). The assay was performed at 35°C in a stirred cuvette with 2,3-dimethoxy-5-methyl-6-decyl-1,4 benzohydroquinone (decylubiquinol) and horse heart cytochrome c in a buffered solution. Reactions were initiated by addition of hemozoin-free mitochondrial preparations.
Liver stage activity
ELQ-300 and P4Q-391 were assessed for activity against exoerythrocytic stages of P. berghei in vitro. In this model sporozoites of luciferase expressing P. berghei were added to a monolayer of HEPG2 hepatoma cells and the infection was allowed to proceed for an hour. After this period the drugs were diluted across the plate and the cultures were incubated at 37 °C. Luciferase expression was determined at 44 h post infection and the data were fit to a nonlinear logistic dose response function. ELQ-300 and P4Q-391 were highly efficacious in this model with EC50 values of 1.02 and 5.11 nM, respectively (Table S7).
Next we were interested in evaluating the quinolone-3-diphenylethers against P. cynomolgi because this non-human primate malaria species resembles the human malaria P. vivax in that the liver stage involves both replicating and non-dividing forms. ELQ-300 and P4Q-391 were tested in an in vitro liver stage assay involving the infection of primary rhesus hepatocytes with sporozoites from the M strain of P. cynomolgi essentially as published (29), except for a 6 day exposure period. Compounds were evaluated in duplicate in a 6-point 3-fold dilution series from 0.04 to 10 μM (Table S8, Fig. S6). The parasiticidal effects were assessed by high content fluorescence imaging to quantify the number of developing liver schizonts (large forms) and the small uni-nucleate parasite forms believed to be latent hypnozoites (29). After a 6-day incubation both compounds showed remarkable activity against P. cynomolgi liver schizonts (IC50 ≈0.1 μM for both compounds, as compared to 0.04 μM for primaquine, the positive control). Assessing the effect of the compounds on the small hypnozoite-like forms is complex because the number of small forms increases dramatically in the presence of both of the quinolones. We interpret these results as indicative that schizont development is blocked at an early stage so the parasites remain small (or are killed and disappear from the culture). This same phenomenon has been observed for atovaquone and other antirespiratory compounds (30). An in vivo evaluation in the gold standard P. cynomolgi-infected rhesus monkey relapse model (31) will be used to address this issue in the future.
The in vitro activity against liver stages of malaria prompted further evaluation in vivo with P. berghei (luciferase) sporozoite infections of BALB/c mice. In this model five mice per group were infected and then dosed with ELQ-300 or P4Q-391 per os one hour post infection. Efficacy was determined by bioluminescence imaging; at 44 h and days 6 and 13 post infection. At each interval mice were injected with D-luciferin, anesthetized, and imaged to monitor progression of liver and blood stage parasitemia. As shown in Fig. 5, both ELQ-300 and P4Q-391 were highly effective. No luminescence was observed in the liver at doses as low as 0.03 mg/kg and subsequent follow up confirmed the infections were completely blocked by single doses of ≥0.03 mg/kg ELQ-300 and ≥0.1 mg/kg P4Q-391. These data highlight the potential of 3-diarylether-4(1H)-quinolones for single dose prophylaxis and cure.
Figure 5.

ELQ-300 activity compared with atovaquone activity against P. yoelii murine malaria. (A) Dose response for ELQ-300 versus atovaquone. Groups of n=4 mice/group were infected with 6.4×106 P. yoelii-infected erythrocytes. Drug treatment started 1 hour after infection and was administered on days 1-4. (B) Whole blood and plasma concentrations of ELQ-300 (nM) after oral gavage administration (target dose = 0.1 mg/kg) to P. yoelii infected CD-1 mice (n = 4 for each time point). Blood samples were taken after the last dose (day 4).
Effects on transmission stages
Antimalarials that prevent the transmission of malaria to the mosquito vector have an important role to play in malaria control programs and may help to contain the spread of drug resistance (32). To explore the potential transmission blocking activity of the 4(1H)-quinolone-3-diarylether chemotype we investigated their ability to prevent development of stage V gametocytes, to kill late stage gametocytes, and to prevent the development of viable ookinetes and oocysts in vitro and in vivo.
Inhibition of gametocyte development by quinolone-3-diarylethers was evaluated in vitro. P. falciparum (NF54) gametocyte cultures were initiated by using standard methods and early stage gametocytes (Stage I-II) were exposed to 0.1, 1, and 10 μM ELQ-300 and P4Q-391 for three days. Drug pressure was then removed and further development was assessed by microscopic examination of Giemsa-stained blood smears on days 3 and 7 following initiation of drug treatment (Day 0). As shown in Fig. S7 both drugs significantly inhibited development of early stage gametocytes. ELQ-300 treated gametocytes did not develop past stage III, even at 0.1 μM, the lowest dose tested. P4Q-391 was slightly less potent with 1 μM exposure being the lowest dose to prevent development to Stage IV. Development to Stage IV was observed with 10 μM exposure of P4Q-391, which we attribute to the limited solubility of this compound.
We also studied the effect of ELQ-300 on late stage gametocyte viability. High content fluorescence-based image analysis was used to measure the activity of ELQ-300 against the late stage IV - V P. falciparum gametocytes (4, 33). The presence of ELQ-300 inhibited stage IV-V gametocyte viability throughout a 72 hr period, with an IC50 value of 71.9 nM (Fig. S8). Although the use of Mitotracker Red for viability assessment may be confounded by the mechanism of action of ELQ-300, the level of activity observed was comparable to the internal control drug for this assay, puromycin (IC50 = 75.3 nM), and indicates a high level of activity against late stage gametocyte viability.
Both compounds were also evaluated in an in vitro P. berghei ookinete assay which focuses on the first 24 h of parasite development in the mosquito from gamete production, through fertilization, zygote formation and finally, to the development of the mature ookinete . Production of ookinetes was reported by expression of GFP controlled by an ookinete-specific promoter using a fluorescence endpoint (34, 35). ELQ-300 demonstrated high potency with an IC50 of 56 nM, similar to the atovaquone control (67 nM) and P4Q-391 (43 nM) providing further evidence of the transmission blocking potential of these molecules.
We evaluated the effect of ELQ-300 on in vivo P. berghei oocyst formation in Anopheles stephensi mosquitoes (36). Briefly, mouse blood infected with GFP-expressing P. berghei parasites was mixed with ELQ-300 and immediately placed into an array of membrane feeders at 39°C and offered to ≈75 overnight-starved mosquitoes. After 7 days fluorescent oocysts were counted on the dissected midguts using a semi-automated image-capture/analysis procedure with DMSO as a negative control (37). In three separate experiments the presence of ELQ-300 in the infectious blood feed had a significant impact on the mean oocyst numbers per mosquito. In one exemplary experiment (see Fig. S9) the prevalence of oocyst infections in the control group was 88.3%, which ranged from 0 to 496 oocysts/mosquito, yielding a geometric mean of 188.8 oocysts/mosquito. ELQ-300 demonstrated potent activity, i.e. no oocysts formed in mosquitoes fed with infected blood containing 1 μM drug, and there was a 99.4% reduction of the total oocyst count relative to controls with blood containing 100 nM ELQ-300. The lowest concentration tested, 10 nM, reduced oocyst burden in mosquito midguts by 19.2%.
Next we added ELQ-300 to the bloodmeal of mosquitoes in the standard membrane feeding assay (SMFA) with infected blood containing gametocytes of P. falciparum strain 3D7. The results from two independent experiments are presented in Fig. S10. In one of the studies ELQ-300 completely blocked P. falciparum oocyst formation in blood-fed mosquitoes at concentrations as low as 10 nM while in the other study a 95.9% reduction in oocyst formation was observed. These findings indicate that ELQ-300 is more effective against P. falciparum transmission stages than in the P. berghei rodent model. It is worth noting that the antimalarial spiroindolone NITD609 required concentrations of 500 nM to completely inhibit oocyst formation in a comparable SMFA (38). Taken together these results demonstrate that ELQ-300 has the potential to significantly impact malaria transmission by reducing the prevalence of infected mosquitoes and intensity of infection.
The promising transmission-blocking activities of ELQ-300 and P4Q-391 in vitro prompted further evaluation in an in vivo model (Fig. S11). In these studies, mice with approximately 3% parasitemia (P. berghei) were treated with a single oral dose of ELQ-300, P4Q-391, or vehicle control at doses ranging from 0.1 – 10 mg/kg. Transmission-blocking efficacy was then determined by feeding female Anopheles stephensi mosquitoes on the P. berghei-infected mice one hour after treatment. On day 10 post-infection the mosquitoes were dissected and midguts were examined for the presence of oocysts. Remarkably, doses as low as 0.1 mg/kg of ELQ-300 and P4Q-391 completely prevented transmission as measured by the development of oocysts. Positive controls for this experiment included 10mg/kg dihydroartemisinin which inhibited oocyst production by 63.7% and primaquine (10 mg/kg) which inhibited oocyst development by 95.4%. Taken together, these data from in vitro and in vivo models demonstrate that ELQ-300 and P4Q-391 are promising drugs for malaria elimination and control efforts.
Physiochemical and pharmacokinetic properties
The lead optimization testing strategy included an assessment of the physicochemical, metabolic and pharmacokinetic properties to identify features that would limit oral bioavailability and result in rapid clearance and poor exposure profiles. Early in the program it was recognized that one of the most significant limitations of the series was very poor aqueous solubility, most likely due to the highly crystalline properties. Unfortunately, all structural modifications that resulted in improved solubility also led to an unacceptable loss of in vivo efficacy. Solubility measurements (see supplemental information) were conducted using two different methods: a kinetic method that was more reflective of the procedures used in many of the in vitro test assays (i.e., dilution of a DMSO stock solution, albeit into differing media), and a thermodynamic equilibrium method under physiologically-relevant conditions (i.e., with respect to media and incubation time). Both methods suggested poor aqueous solubility (Table S9), however solubility results with the kinetic method were 10-50-fold higher than the thermodynamic measurements which is consistent with the high crystallinity being a significant barrier to aqueous solubility. Given these results, particular attention was paid to all of the in vitro measurements to ensure that compound was in solution under the conditions used in the individual assays.
Even though ELQ-300 and P4Q-391 both have low aqueous solubility, the remaining physicochemical properties (MW, polar surface area, Log P, H-bond donors/acceptors) suggested that membrane permeability would likely not limit oral absorption and could help to attenuate the effect of poor solubility (Table S9). The compounds also were found to be highly stable to metabolic degradation by cytochrome P450 enzymes during in vitro incubation with liver microsomes from multiple species, and they did not inhibit the major cytochrome P450 enzymes at concentrations needed for efficacy (Table S10).
Following IV administration to mice, both compounds were found to have low clearance (~0.7 mL/min/kg) and moderate volumes of distribution (~1.2 L/kg), with half-lives exceeding 15-20 h (Figure 6). These parameters are most likely influenced by the high plasma protein binding as shown in Table S9. At an efficacious dose of 0.3 mg/kg with the PEG400 vehicle used in the P. yoelii efficacy experiments, the bioavailability of both compounds in mice was good (~100%) following oral administration. Under these dosing conditions, plasma concentrations were maintained at concentrations well above the in vitro P. falciparum EC50 for more than 48 h post-dosing. As the dose in mice was increased, the bioavailability decreased in line with solubility-limited absorption. Studies in rats resulted in similar pharmacokinetic properties, although even at low dose, the oral bioavailability in rats was lower than that at low dose in mice (Figure S12 and Table S11). Formulation approaches are currently in progress to address the solubility limitation and the lack of dose proportionality.
Figure 6.
Efficacy of ELQ-300 against the human malaria parasite in the immune-deficient (SCID) humanized mouse model of P. falciparum. A group of 3 mice treated with vehicle and another group of 11 mice treated with different doses of ELQ-300 were analyzed to estimate ED90 and AUCED90 as parameters of efficacy. (A) Parasitemia in individual mice treated with different doses of ELQ-300 during the efficacy assay. Each symbol represents an individual mouse. (B) Concentrations of ELQ-300 in the blood of each individual mouse in the efficacy study measured for 24 h after the initial oral dose administration. Each symbol represents an individual mouse. (C) Graphic estimation of AUCED90 for ELQ-300. Data are the area under the curve for ELQ-300 in blood during the first 24 h after first oral dose administration (AUC0→23h) vs log10 [parasitemia at day 7] for each individual mouse in the efficacy study. (D) Microscopic and flow cytometry analysis of P. falciparum present in peripheral blood of mice treated with vehicle or ELQ-300. Samples were taken 48 h after the start of treatment (i.e., 1 cycle of exposure to drug). Flow cytometry dot plots from samples of peripheral blood show P. falciparum infected human erythrocytes inside of the polygonal regions. Images in the right hand panels show Giemsa-stained bloodstream parasites present at the 48 h time point. Blood films from control untreated animals show normal staining and appearance while the parasites in ELQ-300 treated animals stain poorly and exhibit altered morphology.
Selectivity and safety profile
On the basis of its superior intrinsic antiplasmodial activity in vitro and in vivo against blood and liver stages of malarial parasites, outstanding transmission blocking activity, target selectivity, low propensity for resistance, ease of synthesis, and lack of cross resistance against multidrug resistant strains, ELQ-300 was chosen as the preclinical candidate from an impressive series of novel 3-diarylether substituted 4-(1H)-quinolones. To support further progression, additional safety profiling of ELQ-300 was performed. It is known that several antimalarial drugs, including halofantrine, block the hERG (human-Ether-a-go-go-Related Gene) channel and produce a QT interval prolongation, which is potentially life threatening. hERG patch clamp assays with ELQ-300 yielded IC50 values >11μM, consistent with a low risk of cardiotoxicity (Table S12). We also established that ELQ-300 lacks mutagenic potential in a 5-strain Ames bacterial assay, and the compound was negative for genotoxicity in an in vitro micronucleus assay. Finally, we did not observe any significant inhibition in a screen of 100 neurotransmitter receptors, peptide receptors, ion channels, reuptake sites, and enzymes of therapeutically relevant targets (Table S13). On the basis of these studies it appears that ELQ-300 has no significant off-target pharmacological activities that are a concern for human safety. Formulation studies are currently in progress to support the assessment of the safety margin in preclinical animal species.
Conclusion
Quinolone-3-diarylethers represent a novel antimalarial series active on a clinically validated pathway that overcome the shortcomings of atovaquone, a clinical drug with the same enzyme target. From this potent series ELQ-300 was selected as a preclinical candidate. ELQ-300 is potent in vitro against blood stage P. falciparum strains including a clinical isolate with a mutation in the cytochrome b gene that is associated with atovaquone resistance. Whilst the compound rapidly inhibits oxygen respiration, the killing profile of ELQ-300 resembles atovaquone – with rapid killing coming only after an initial cytostatic phase. Repeated attempts to generate resistant mutants using single step methodology have failed, thus ELQ-300 has a demonstrated resistance frequency far improved over atovaquone. The in vitro potency, combined with high metabolic stability, results in excellent oral efficacy with a curative blood stage dose of 1 mg/kg in the Thompson or Peter’s tests and activity in the falciparum SCID mouse model that is consistent with these values based on blood exposure to the drug. Interestingly, the molecule is also highly potent against exo-erythrocytic stages and impacts not only liver schizonts and gametocytes, but also inhibits the formation of ookinetes and oocysts in the mosquito midgut. Although poorly soluble in aqueous media, ELQ-300 exhibits good oral bioavailability at therapeutically relevant doses with extended half-lives in rodents. Impressively, in vivo doses lower than the ED90 result in causal prophylaxis and killing of all P. berghei primary liver schizonts; furthermore such a dose also results in complete inhibition of P. berghei oocyst formation in a mouse feeding study thus totally inhibiting sporogony and demonstrating a 100% block of transmission. Unlike the structurally related pyridone GW844520 (12) from GSK and 4(1H)-quinolones recently reported by Biagini et al. (30), ELQ-300 has high in vitro selectivity for P. falciparum bc1 complex over the human enzyme complex and various mammalian cell types including hematopoietic stem cells. It does not inhibit a large panel of receptors and enzymes, including the hERG channel, nor is the compound genotoxic. In line with a low predicted dose in humans, and an expected long human half-life, ELQ-300 offers the potential as a combination partner aimed at a single dose cure. Furthermore, the effects on liver and mosquito stages offers not only the hope of a new molecule to help protect and cure patients, but also to block transmission, thus breaking the parasite lifecycle. An exploratory formulation investigation is underway to support preclinical safety and toxicity studies prior to initiation of human clinical trials. ELQ-300 offers hope towards the elimination and eradication of malaria.
Supplementary Material
Figure 7.
Whole animal bioluminescence imaging of mice infected with luciferase transfected P. berghei sporozoites. Mice were treated with different doses of ELQ-300, P4Q-391 or atovaquone. Animals (n=5 per group) received a single dose by gavage one hour following inoculation with sporozoites. Representative images taken at 44 h after infection are shown. Bioluminescent signal was detected in control untreated animals with the highest intensity noted in the area overlaying the liver, consistent with the presence of liver-stage parasites. Notice that while all three drugs exhibited a dose-dependent effect on liver stages, ELQ-300 fully protected mice against P. berghei liver stage infection at doses as low as 0.03 mg/kg.
Figure 8.
ELQ-300 (filled circles) and P4Q-391 (open triangles) were administered intravenously and orally to non-fasted male Swiss outbred mice. The intravenous dose (0.1 mg/kg) was formulated in mouse plasma to facilitate solubilization and to avoid precipitation upon administration via the tail vein. The oral dose was 0.3 mg/kg and was administered via gavage (0.1 mL) as a solution in undiluted PEG400. Following intravenous administration (Panel A), both of the drugs exhibited low clearance and a low volume of distribution, with a long half-life of about 15 to 18 h. The low clearance is consistent with in vitro data showing their high metabolic stability in the presence of hepatic microsomes (see supplemental materials). Following oral administration, the terminal half-life of each compound was similar to that after intravenous dosing, and absorption appeared to be slow with Tmax values of approximately 7.5 h. The oral bioavailability of both ELQ-300 and P4Q-391 at 0.3 mg/kg was approximately 100%.
Acknowledgements
We wish to acknowledge Grover C. Bagby and R. Keaney Rathbun for cytotoxicity experiments involving hematopoietic stem cells and Sovitj Pou and Cynthia Lichorowic (recipient of a Genshaft Family Doctoral Fellowship from the University of South Florida) for assistance with large-scale synthesis of ELQ-300 and P4Q-391, respectively. We thank Ms. April Pershing for her help in isolation of parasite mitochondria. We are also grateful to María Santos Martínez, Ph.D., (DMPK); Maria Jesus Almela, Sonia Lozano (Parasitology and Cell Biology) and the Therapeutic Efficacy, Pharmacology and Laboratory Animal Science groups at GSK-Tres Cantos Medicines Development Campus for assistance. We thank Dr. Leonard D. Shultz and The Jackson Laboratory for providing access to NOD scid IL2Rγc null mice through their collaboration with GlaxoSmithKline-Tres Cantos Medicines Development Campus.
Funding: Supported by the Medicines for Malaria Venture, and by the National Institutes of Health #AI29398 (M.W.M and A.B.V.), # R01 GM097118-01 (R.M. and D.E.K.) and #’s AI079182 and AI100569 (M.K.R.). C.K. receives additional support from the Wellcome Trust.
Dedication: The authors wish to dedicate this work to the memory of Dr. Ian Bathurst (1949 - 2011).
Footnotes
Supplementary information is available in the supplemental information provided with the online version of the paper at www.science.com.
Author contributions: Drug design: A.N., R.W. D.E.K., R.M.C., R.M., M.K.R., and J.B.; A.N. and R.W. synthesized ELQ-300; R.M.C. and R.M. synthesized P4Q-391; T.M., D.E.K., Y.L., and J.X.K. conducted in vitro susceptibility against laboratory adapted strains and cytotoxicity assays; J.M., G.W., and B.S. conducted the in vitro studies of field isolates, with program supervision from R.P. and R.N.; Y.L. and J.X.K. performed in vitro drug synergy studies; L.S. and F-J.G. determined the PRR for ELQ-300; A-M.Z. and C.H.M.K. evaluated drugs against P. cynomolgi; E.H. conducted in vitro ATP depletion assays; A.N.L., F.E.S., D.E.K evaluated drugs for therapeutic efficacy against blood stage infections with P. berghei in mice; I, A-B., M.B.J-D. evaluated drugs for in vivo efficacy against P. falciparum and P. yoelii blood stage infections in mice and S.F. determined the pharmacokinetics of each drug in these efficacy experiments; Y.L. and J.X.K. assessed drugs for in vivo efficacy against blood stage infections (P. yoelii) in mice; A.N.L., T.M., and D.E.K. conducted in vitro and in vivo liver stage determinations; J.M.M. and A.B.V. conducted propensity for resistance experiments; I.P.F., M.W.M., A.B.V., and M.K.R. conducted enzyme inhibitor assays; K.L.W., D.M.S., J.S., E.R., and S.A.C. determined the pharmacokinetics of each drug in this investigation in uninfected preclinical animals species; A.N.L., F.E.S., D.E.K. evaluated drugs for inhibition of gametocyte development (in vitro); S.D. and V.M.A. conducted late stage gametocyte viability assays; M.J.D. and R.E.S. evaluated ELQ-300 in ookinete, oocyst, and SMF assays; A.N.L., F.E.S., and D.E.K. carried out in vivo transmission blocking assays including bioluminescence imaging; P.S. provided guidance and oversight on issues relating to in vitro safety assessment; A.N., A.N.L., S.A.C., A.B.V., D.E.K., J.N.B., R.M., and M.K.R. wrote the paper; M.K.R., R.M., S.A.C., A.B.V., D.E.K., R.W., R.K.G., and J.N.B. provided overall management and oversight of the project and collaboratively designed studies, analyzed data, and led the candidate selection process; M.K.R. and R.M. were project leaders for their respective teams while J.N.B. and I.B. (deceased) served as overall project directors.
Competing interests: M.K.R., J.X.K., R.W.W., A.N., J.N.B., D.E.K., R.M., and R.M.C. are named as co-inventors on the following patent applications related to this work: WO/2010/065905 and WO/2012/167237. I.A-B., M.B.J-D., S.F., E.H., L.M.S., F-J.G. are employees of GlaxoSmithKline, and the experimental work by them has been carried out in the GSK Drugs for the Developing World Department. The other authors declare that they have no competing interests.
References and Notes
- 1.Murray CJ, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012 Feb 4;379:413. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, et al. The threat of artemisinin-resistant malaria. N Engl J Med. 2011 Sep 22;365:1073. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phyo AP, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012 May 26;379:1960. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucantoni L, Avery V. Whole-cell in vitro screening for gametocytocidal compounds. Future medicinal chemistry. 2012 Dec;4:2337. doi: 10.4155/fmc.12.188. [DOI] [PubMed] [Google Scholar]
- 5.A research agenda for malaria eradication: drugs. PLoS Med. 2011;8:e1000402. doi: 10.1371/journal.pmed.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzer W, Timmler H, Andersag H. Über einen neuen, gegen Vogelmalaria wirksamen Verbindungstypus. Chem. Ber. 1948;81:12. [Google Scholar]
- 7.Kikuth W, Mudrow-Reichenow L. Über kausalprophylaktisch bei Vogelmalaria wirksame Substanzen. Zeitschrift für Hygiene und Infektionskrankheiten, medizinische Mikrobiologie. Immunologie und Virologie. 1947;127:151. [PubMed] [Google Scholar]
- 8.Cross RM, et al. Endochin optimization: structure-activity and structure-property relationship studies of 3-substituted 2-methyl-4(1H)-quinolones with antimalarial activity. J Med Chem. 2010 Oct 14;53:7076. doi: 10.1021/jm1007903. [DOI] [PubMed] [Google Scholar]
- 9.Winter R, et al. Optimization of endochin-like quinolones for antimalarial activity. Exp Parasitol. 2011 Feb;127:545. doi: 10.1016/j.exppara.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80) Biochem Pharmacol. 1992;43:1545. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 11.Yeates CL, et al. Synthesis and structure-activity relationships of 4-pyridones as potential antimalarials. J Med Chem. 2008 May 8;51:2845. doi: 10.1021/jm0705760. [DOI] [PubMed] [Google Scholar]
- 12.Bueno JM, et al. Exploration of 4(1H)-pyridones as a novel family of potent antimalarial inhibitors of the plasmodial cytochrome bc1. Future medicinal chemistry. 2012 Dec;4:2311. doi: 10.4155/fmc.12.177. [DOI] [PubMed] [Google Scholar]
- 13.Winter RW, et al. Antimalarial quinolones: synthesis, potency, and mechanistic studies. Exp Parasitol. 2008 Apr;118:487. doi: 10.1016/j.exppara.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riscoe MK, et al. In: W. I. P. O. (WIPO) # WO/2010/065905, editor. Oregon Health & Sciences University, The US Department of Veterans Affairs, Medicines for Malaria Venture, and the University of South Florida; 2010. [Google Scholar]
- 15.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004 May;48:1803. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16:710. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smilkstein MJ, et al. A drug-selected Plasmodium falciparum lacking the need for conventional electron transport. Mol Biochem Parasitol. 2008 May;159:64. doi: 10.1016/j.molbiopara.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke H, et al. Variation among Plasmodium falciparum strains in their reliance on mitochondrial electron transport chain function. Eukaryot Cell. 2011 Aug;10:1053. doi: 10.1128/EC.05049-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007 Mar 1;446:88. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 20.Marfurt J, et al. Ex vivo activity of histone deacetylase inhibitors against multidrug-resistant clinical isolates of Plasmodium falciparum and P. vivax. Antimicrob Agents Chemother. 2011 Mar;55:961. doi: 10.1128/AAC.01220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell B, et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008 Mar;52:1040. doi: 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mather MW, Morrisey JM, Vaidya AB. Hemozoin-free Plasmodium falciparum mitochondria for physiological and drug susceptibility studies. Mol Biochem Parasitol. 2010 Dec;174:150. doi: 10.1016/j.molbiopara.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doggett JS, et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A. 2012 Sep 25;109:15936. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz LM, et al. P. falciparum in vitro killing rates allow to discriminate between different antimalarial mode-of-action. PLoS ONE. 2012;7:e30949. doi: 10.1371/journal.pone.0030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fivelman QL, Adagu IS, Warhurst DC. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob Agents Chemother. 2004 Nov;48:4097. doi: 10.1128/AAC.48.11.4097-4102.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ager AJ, editor. Rodent malaria models. Vol. 68. Springer-Verlag; New York, N.Y.: 1984. pp. 225–264. vol. 68. [Google Scholar]
- 27.Jimenez-Diaz MB, et al. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rgammanull mice engrafted with human erythrocytes. Antimicrob Agents Chemother. 2009 Oct;53:4533. doi: 10.1128/AAC.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Diaz MB, et al. Improvement of detection specificity of Plasmodium-infected murine erythrocytes by flow cytometry using autofluorescence and YOYO-1. Cytometry A. 2005 Sep;67:27. doi: 10.1002/cyto.a.20169. [DOI] [PubMed] [Google Scholar]
- 29.Dembele L, et al. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS ONE. 2011;6:e18162. doi: 10.1371/journal.pone.0018162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biagini GA, et al. Generation of quinolone antimalarials targeting the Plasmodium falciparum mitochondrial respiratory chain for the treatment and prophylaxis of malaria. Proc Natl Acad Sci U S A. 2012 May 22;109:8298. doi: 10.1073/pnas.1205651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt LH. Relationships between chemical structures of 8-aminoquinolines and their capacities for radical cure of infections with Plasmodium cynomolgi in rhesus monkeys. Antimicrob Agents Chemother. 1983 Nov;24:615. doi: 10.1128/aac.24.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters W. In: Epidemiology, chemotherapy, morphology, and metabolism. Kreier JP, editor. Academic Press; New York, New York: 1980. pp. 214–283. [Google Scholar]
- 33.Duffy S, Avery VM. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am J Trop Med Hyg. 2012 Jan;86:84. doi: 10.4269/ajtmh.2012.11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delves M, et al. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012 Feb;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delves MJ, et al. A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. Int J Parasitol. 2012 Oct;42:999. doi: 10.1016/j.ijpara.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nature protocols. 2006;1:346. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 37.Delves MJ, Sinden RE. A semi-automated method for counting fluorescent malaria oocysts increases the throughput of transmission blocking studies. Malar J. 2010;9:35. doi: 10.1186/1475-2875-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Pelt-Koops JC, et al. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother. 2012 Jul;56:3544. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart ME, et al. Trace Amine-Associated Receptor Agonists: Synthesis and Evaluation of Thyronamines and Related Analogues. Journal of Medicinal Chemistry. 2006;49:1101. doi: 10.1021/jm0505718. [DOI] [PubMed] [Google Scholar]
- 40.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 41.Marfurt J, et al. Ex vivo drug susceptibility of ferroquine against chloroquine-resistant isolates of Plasmodium falciparum and P. vivax. Antimicrob Agents Chemother. 2011 Sep;55:4461. doi: 10.1128/AAC.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eling W. Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull World Health Organ. 1977;55:105. [PMC free article] [PubMed] [Google Scholar]
- 43.Krungkrai J, Krungkrai SR, Suraveratum N, Prapunwattana P. Mitochondrial ubiquinol-cytochrome c reductase and cytochrome c oxidase: chemotherapeutic targets in malarial parasites. Biochem Mol Biol Int. 1997 Aug;42:1007. doi: 10.1080/15216549700203461. [DOI] [PubMed] [Google Scholar]
- 44.Krungkrai J. The multiple roles of the mitochondrion of the malarial parasite. Parasitology. 2004 Nov;129:511. doi: 10.1017/s0031182004005888. [DOI] [PubMed] [Google Scholar]
- 45.Guarino RD, et al. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol Bioeng. 2004 Jun 30;86:775. doi: 10.1002/bit.20072. [DOI] [PubMed] [Google Scholar]
- 46.Trumpower BL, Edwards CA. Purification of a reconstitutively active iron-sulfur protein (oxidation factor) from succinate . cytochrome c reductase complex of bovine heart mitochondria. J Biol Chem. 1979 Sep 10;254:8697. [PubMed] [Google Scholar]
- 47.Ager AJ, editor. Rodent malaria models. 68/I. Springer-Verlag; Berlin: 1984. pp. 225–264. vol. 68/I. [Google Scholar]
- 48.Peters W, Robinson BL. In: Handbook of animal models of infection. Zak O, Sande MA, editors. Academic Press; London: 1999. pp. 757–773. [Google Scholar]
- 49.Angulo-Barturen I, et al. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS ONE. 2008;3:e2252. doi: 10.1371/journal.pone.0002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jimenez-Diaz MB, et al. Quantitative measurement of Plasmodium-infected erythrocytes in murine models of malaria by flow cytometry using bidimensional assessment of SYTO-16 fluorescence. Cytometry A. 2009 Mar;75:225. doi: 10.1002/cyto.a.20647. [DOI] [PubMed] [Google Scholar]
- 51.Ploemen IH, et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One. 2009;4:e7881. doi: 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaCrue AN, et al. 4(1H)-Quinolones with Liver Stage Activity against Plasmodium berghei. Antimicrob Agents Chemother. 2013 Jan;57:417. doi: 10.1128/AAC.00793-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cross RM, et al. Optimization of 1,2,3,4-tetrahydroacridin-9(10H)-ones as antimalarials utilizing structure-activity and structure-property relationships. J Med Chem. 2011 Jul 14;54:4399. doi: 10.1021/jm200015a. [DOI] [PubMed] [Google Scholar]
- 54.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999 Nov;27:1350. [PubMed] [Google Scholar]
- 55.Broudy VC, Zuckerman KS, Jetmalani S, Fitchen JH, Bagby GC., Jr. Monocytes stimulate fibroblastoid bone marrow stromal cells to produce multilineage hematopoietic growth factors. Blood. 1986 Aug;68:530. [PubMed] [Google Scholar]
- 56.Rathbun RK, et al. Interferon-gamma-induced apoptotic responses of Fanconi anemia group C hematopoietic progenitor cells involve caspase 8-dependent activation of caspase 3 family members. Blood. 2000 Dec 15;96:4204. [PubMed] [Google Scholar]
- 57.Li Y, et al. The relationship between diphenylamine structure and NSAIDs-induced hepatocytes injury. Toxicol Lett. 2009 Apr 25;186:111. doi: 10.1016/j.toxlet.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Dykens JA, Marroquin LD, Will Y. Strategies to reduce late-stage drug attrition due to mitochondrial toxicity. Expert Rev Mol Diagn. 2007 Mar;7:161. doi: 10.1586/14737159.7.2.161. [DOI] [PubMed] [Google Scholar]
- 59.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007 Jun;97:539. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.