Abstract
Experimental and clinical studies have attempted to evaluate the changes in cortical activity seen after immobilization-induced longterm sensorimotor restriction, although results remain controversial. We used intracortical microstimulation (ICMS), which provides topographic movement representations of the motor areas in both hemispheres with optimal spatial characterization, combined with behavioural testing to unravel the effects of limb immobilization on movement representations in the rat primary motor cortex (M1). Unilateral forelimb immobilization in rats was achieved by casting the entire limb and leaving the cast in place for 15 or 30 days. Changes in M1 were bilateral and specific for the forelimb area, but were stronger in the contralateral-to-cast hemisphere. The threshold current required to evoke forelimb movement increased progressively over the period in cast, whereas the forelimb area size decreased and the non-excitable area size increased. Casting resulted in a redistribution of proximal/distal movement representations: proximal forelimb representation increased, whereas distal representation decreased in size. ICMS after cast removal showed a reversal of changes, which remained partial at 15 days. Local application of the GABAA-antagonist bicuculline revealed the impairment of cortical synaptic connectivity in the forelimb area during the period of cast and for up to 15 days after cast removal. Six days of rehabilitation using a rotarod performance protocol after cast removal did not advance map size normalization in the contralateral-to-cast M1 and enabled the cortical output towards the distal forelimb only in sites that had maintained their excitability. These results are relevant to our understanding of adult M1 plasticity during and after sensorimotor deprivation, and to new approaches to conditions that require longterm limb immobilization.
Introduction
Over the past 20 years, many findings from animal (Sanes & Donoghue, 2000; Fox & Wong, 2005; Petersen, 2007; Fu & Zuo, 2011) and human (Pascual-Leone & Torres 1993; Elbert et al. 1995; Sterr et al. 1998) studies have corroborated the concept of use-dependent plasticity, according to which changes in cortical activity depend on the amount of behavioural use of a specific body part. Indeed, motor mapping has revealed changes resulting from motor nerve injury (Sanes et al. 1990; Toldi et al. 1996; Franchi, 2000), loss of muscle activity (Sanes et al. 1990; Cohen et al. 1991; Franchi, 2002) and skill learning (Nudo et al. 1996; Kleim et al. 1998; Plautz et al. 2000; Coq et al. 2009). Whereas skill learning causes an increase in the area representing the trained forelimb and a decrease in the area representing the under-trained forelimb, with total forelimb area remaining constant, nerve injury and muscle inactivity both result in the expansion of the neighbouring intact representation into the disconnected cortical representation. In squirrel monkeys, for example, whereas the total forelimb area and its threshold remained constant, chronic selective cast restriction of the distal forelimb reduced motor digit representation and increased wrist and forearm representation (Milliken et al. 2013). Although an intermingling of distal and proximal forelimb-projecting neurons within the primary motor cortex (M1) (Wang et al. 2011) has been proposed to explain why selective limb joint immobilization induces a rearrangement in representation without affecting the size or excitability of the site, such a rearrangement cannot be taken as proven conclusively. Indeed, a previous study (Langlet et al. 2012) showed a reduction in overall hindlimb representation in M1 following chronic reduction in neuromuscular activity caused by hindlimb unloading, with the representation of the toes being less strongly affected than representations of the other hindlimb joints. Hence the form of M1 plasticity following limb restriction involving all joints remains unresolved. Indeed, there are few reports on cortical motor changes after immobilization in humans wearing splints for weeks (Liepert et al. 1995; Zanette et al. 2004; Clark et al. 2010), and these have yielded contradictory results. Indeed, in one case (Liepert et al. 1995), transcranial magnetic stimulation (TMS) revealed a reduction in the representation area corresponding to immobilized muscles; another study (Zanette et al. 2004) reported maps of normal area but with greater excitability to TMS, and a third (Clark et al. 2010) found no changes at rest, but increased intracortical inhibition in M1 during active movement. A recent attempt to clarify the situation using functional magnetic resonance imaging (fMRI) showed a decrease in M1 thickness after entire limb immobilization (Langer et al. 2012). We hypothesize that such changes may correspond to reductions in the size and excitability of the motor representation of a totally immobilized limb, and that such modifications will affect both hemispheres, albeit to a greater extent in the contralateral-to-cast M1. At present, however, the overt consequences of longterm forelimb disuse on M1 map organization have not been investigated. The objective of the present study was therefore to determine how the organization of M1 movement representation changes at different time-points during and after cast immobilization in both hemispheres, and to evaluate whether subsequent motor rehabilitation may affect cortical reorganization. We also investigated whether map rearrangements reflect changes in cortical synaptic connectivity by exploring the effect of local GABA receptor inhibition at different time-points.
Methods
Ethical approval
Adult male Wistar rats (n = 65; 13–15 weeks old at cast application; 300–350 g; Harlan Laboratories, Inc., San Pietro al Natisone, Italy) were kept under regular lighting conditions (12 : 12 h light : dark cycle) and given food and water ad libitum. This study was compliant with the European Council Directive of 24 November 1986 (86/609/EEC) and was approved by the Ethical Committee of the University of Ferrara and the Italian Ministry of Health. The experiments conformed to the principles of UK regulations, as described in Drummond (2009). Adequate measures were taken to minimize animal pain, as well as the number of animals used.
Experimental design
The experimental plan is illustrated in Fig.1. To analyse M1 changes that occur with unilateral forelimb immobilization, cortical output was evaluated by intracortical microstimulation (ICMS) at different time-points [i.e. after 15 and 30 days of immobilization (n = 5 each), and at 7 and 15 days after cast removal (n = 5 each); Experiment 1]. ICMS was performed at a minimum of 12 h after cast removal to ensure good forelimb reperfusion and to exclude the acute effects of reperfusion oedema. To exclude post-casting hypokinetic phenomena during the recovery period, motor activity was evaluated daily using bar and drag behavioural tests. To investigate whether cortical synaptic connectivity could account for the M1 changes recorded, the effect of local application of the GABAA-receptor antagonist bicuculline to the contralateral-to-cast hemisphere was studied after 30 days of immobilization, as well as at 7 and 15 days after cast removal (n = 5 each; Experiment 2). Finally, to evaluate the effect of enhanced limb use on the reorganization of cortical output, motor maps were assessed in two groups of rats (unconstrained and immobilized for 30 days, respectively; n = 5 each) after 6 days of daily training (Experiment 3).
Figure 1. Time sequence of experiments.
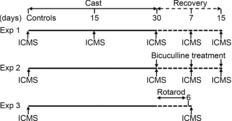
In Experiment 1, mapping was performed after 15 and 30 days of cast immobilization, and then at 7 and 15 days after cast removal. In Experiment 2, bicuculline treatment and mapping were performed after 30 days of cast immobilization, and then at 7 and 15 days after cast removal. In Experiment 3, mapping was performed at 7 days after cast removal and, in particular, after 6 days of (twice per day) rotarod training, which was begun upon cast removal following 30 days of cast immobilization. Appropriate controls were tested for all experiments (see Methods). ICMS, intracortical microstimulation.
Limb immobilization
Unilateral immobilization of the forelimb and forepaw was induced in ketamine-anaesthetized rats in a manner similar to that described in previous studies (Jones & Schallert, 1994; Coq & Xerri, 1999). Briefly, the selected forelimb (alternating right and left) was first wrapped in a thin layer of cotton to prevent compression and subsequently blocked with a plaster bandage (Platrix; BSN Medical SRL, Milan, Italy). During this procedure, the digits were maintained in full extension in order to prevent the obstruction of normal blood circulation. The limb was cast against the chest, with the joints in a natural position, and the bandage was used to form a one-holed vest around the upper trunk. The limited movement of the confined limb imposed increased use of the contralateral (unconstrained) forelimb to improve posture, gait, grooming, and holding and eating activity. During the period of immobilization, all rats were brushed and swabbed with a wet pad twice per day. Although it was beyond the scope of this investigation to discover whether induced forelimb immobility influences behaviour, chronic immobilization is known to induce stress in rodents (Ghosh et al. 2013). Indeed, as a stress indicator, we observed an increase in the use of the ipsilateral-to-cast hindlimb to scratch the portion of the cast closest to the neck in immobilized animals. However, overall exploratory behaviour and food intake did not seem to be affected at any time during immobilization.
Bicuculline treatment
Local GABA receptor blockade was induced, as previously described (Viaro et al. 2011), in control conditions and in animals at 30 days of immobilization, as well as at 7 and 15 days after cast removal (n = 5 each). Under surgical stereomicroscopy, the dura at the M1 contralateral to the forelimb cast was removed to allow drug diffusion through the cortical layers, and a 30 μl solution of 50 μm bicuculline methochloride (Sigma Chemical Co., St Louis, MO, USA) was applied to the cortical surface using a Gilson micropipette. The drug was freshly dissolved in isosmotic saline solution just before use. The temperature of the solution was kept constant at 36–38°C, and one or two supplementary applications were performed to keep the cortex moist. This bicuculline application protocol has been reported to provide effective GABAA receptor blockade in all cortical layers (Stojic et al. 2000). The cortical region between 1.75 mm and 4.25 mm [anteroposterior (AP)] and 1.75 mm and 4.25 mm [mediolateral (ML)] from the bregma (representing the rostral forelimb and, partially, the vibrissa and caudal forelimb areas) was mapped in each animal before and after bicuculline application in order to limit interindividual variability. The second mapping was begun 10 min after pharmacological treatment.
Behavioural tests
Motor activity (akinesia, bradykinesia and asymmetry) was evaluated daily, beginning 12 h after cast removal, by means of two different behavioural tests, which provide complementary information on different motor parameters: (i) the ‘bar test’ (Sanberg et al. 1988), which measures the ability of the rat to respond to an externally imposed static posture, and (ii) the ‘drag test’ (Marti et al. 2005), which measures the ability of the rat to balance its body posture using its forelimbs in response to an externally imposed dynamic stimulus (backward dragging).
Bar test
Each rat was placed gently on a table and each forepaw was placed alternately on blocks of increasing height (3 cm, 6 cm and 9 cm). The total time (in seconds) spent by each paw on the blocks (defining akinesia) was recorded (cut-off time of 20 s).
Drag test
Each rat was gently lifted by the tail (keeping the forepaws on the table) and dragged backwards at a constant speed (∼20 cm s−1) over a fixed distance (100 cm). The numbers of steps made by each paw were counted by two separate observers.
Motor rehabilitation
A fixed-speed rotarod performance protocol (Rozas et al. 1997) was employed to impose greater limb use after cast removal. A rotating cylinder with a diameter of 8 cm was used according to a procedure derived from previously described protocols (Viaro et al. 2010). After 30 days of immobilization, beginning 12 h after cast removal, rats were trained twice per day for 6 days at increasing speeds (5–30 rpm, 180 s each) for the first 18 min, after which the speed was maintained at a constant 30 rpm for a further 12 min. This protocol allowed all animals to be trained in the same way and excluded any differences in performance between individuals (i.e. inability to run at relatively high speeds). ICMS was performed 7 days after cast removal at precisely 12 h after the last session of motor training in order to avoid any influence of acute motor effects on motor maps.
ICMS
Anaesthesia was induced by ketamine hydrochloride (80 mg kg−1 i.p.) and maintained throughout the experiment by supplementary ketamine injections (25 mg kg−1 i.m.) so that long-latency, sluggish hindlimb withdrawal could be provoked by pinching the hindfoot. This level of anaesthesia was light [stage III 1–2 (Friedberg et al. 1999)] and allowed comparable patterns of movements to be evoked in all animals without undue influence on ICMS results. Body temperature was maintained at 36–38°C using a heat lamp. Each animal was placed in a Kopf stereotaxic apparatus, and a large craniotomy was performed over the frontal cortex. The dura was left intact (except in bicuculline experiments, in which it was removed by surgical stereomicroscopy) and kept moist with saline solution.
Mapping procedure
Cortical mapping to define the extent of movement representations and the current threshold required to elicit such movements was performed according to a procedure similar to that described elsewhere (Sanes et al. 1990; Franchi, 2000). Briefly, electrode penetrations were regularly spaced out over a 500-μm grid. Adjustments to the coordinate grid were sometimes necessary to keep the electrode from penetrating a blood vessel. These adjustments were not reported in the reconstructed maps because when the necessary adjustment exceeded 50 μm, penetration was not performed at this site. Glass-insulated tungsten microelectrodes (0.6–1.0 MΩ impedance at 1 kHz), mounted on an electrical microadvancer (Burleigh Inchworm System; Burleigh Instruments, Inc., Fishers, NY, USA), were used for stimulation. The electrode was lowered perpendicularly into the cortex to 1500 μm below the cortical surface (1300 μm without dura), a depth corresponding to layer V of the frontal agranular cortex (Franchi, 2000). It was then adjusted by ±200 μm to find the lowest threshold at which movement was evoked. Monophasic cathodal pulses (30 ms train duration at 300 Hz, 200 μsec pulse duration) of a maximum of 60 μA were passed through the electrode at minimum intervals of 2.5 s. Starting at a current of 60 μA, intensity was decreased in 5 μA steps until movement was no longer evoked; it was then increased to a level at which approximately 50% of the stimulations elicited movement. This level defined the current threshold. Two separate observers were involved in the experiment: one researcher adjusted current levels, and the other, who was blind to the current intensity and treatment, detected movements. We began the exploration at each site with the current at its maximum level (60 μA) because the initial robust multisynaptic recruitment of remote neurons around the electrode tip optimized the detection of movements in our 500 μm grid map. When movement was detected, the current was rapidly decreased until the movement was no longer detectable. This procedure was performed quickly to ensure that no more than 10–15 trains of pulses were delivered at a single site because excess stimulation can alter the integrity of cortical representation size and border (Nudo et al. 1990; Young et al. 2011). If no movements or twitches were evoked at 60 μA, the site was considered non-excitable. When movement was observed in two or more different body parts, current thresholds were determined for each component. Body parts activated by microstimulation were identified by visual inspection and/or muscle palpation. When eye movement was observed, the threshold current was determined with the aid of a surgical microscope. The areas of eye, eyelid and miosis sites in each hemisphere were so small that these sites are collectively referred to as ‘eye sites’.
Map construction
Representation maps at threshold current were generated from the pattern of electrode penetrations. In each hemisphere, movements were mapped in order to determine the location and extent of vibrissa and forelimb movement representations. Other cortical areas (e.g. those evoking eye, neck, jaw/tongue and hindlimb movements) were so small that these values were not considered in the quantitative analysis. Each stimulated site was taken to represent 0.25 mm2 of cortical surface (0.5 × 0.5 mm); cortical area was calculated as the number of sites × 0.25 mm2. Map borders were defined as the mid-point between sites with different movement thresholds. If a site eliciting movement was flanked by a site that showed no movement upon stimulation of up to 60 μA, the border of the represented movement was set at 250 μm from the movement site. In our analysis, a non-excitable area was calculated from the number of unexcitable sites in the regions between 0.75 mm and 4.25 mm (AP) and 1.25 mm and 4.25 mm (ML) from the bregma. This part of the frontal cortex corresponded to the vibrissa and forelimb site distribution. Hindlimb representation delimited the posterior boundary of the vibrissa and forelimb representations, and no-response sites formed the basis for delineations of the rostral M1 border. Based on physiological and anatomical evidence (Neafsey & Sievert, 1982; Neafsey et al. 1986; Rouiller et al. 1993), we distinguished two subregions within the forelimb area, namely the caudal and rostral areas [3.25 mm posterior and anterior to AP, respectively (Viaro et al. 2011)]. Forelimb movements were classified as either distal (wrist/digit) or proximal (elbow/shoulder). In addition, to characterize the spatial distribution across animals of movements in the cortex, a two-dimensional distribution of movement-responsive sites at the coordinates relative to the bregma was generated. Each movement-related site was taken to represent a 0.25 mm2 square of cortical surface (0.5 × 0.5 mm) and was assigned a probability. The probability was calculated as the percentage of animals in the same group showing the forelimb movement at the same coordinates and thus 100% probability at a given site was achieved when a movement was observed at that site in all animals. Penetrations were divided into 0.5 mm-wide bins, into which all sites were grouped, irrespective of their AP coordinates. For each bin, starting from 1 mm from the mid-line and extending 4.5 mm laterally, numbers of unresponsive, vibrissa and forelimb sites were tallied and assigned a probability. The probability was expressed as a percentage of the total number of sites and was calculated as the percentage of sites with the same coordinates in which movement was evoked, considering all animals in the same group. In this way, 100% probability is achieved when a movement at that ML coordinate is observed in all animals in a group.
Data presentation and statistical analysis
Data are expressed as the mean ± s.e.m. of five determinations per group. As not all values displayed normal distribution (Kolmogorov–Smirnov test), statistical analysis was performed using the Kruskal–Wallis non-parametric test, followed by Dunn's test. In addition, the Mann–Whitney non-parametric test was performed to detect differences at each time-point. In the bicuculline experiments, statistical analysis was performed by two-way repeated-measures (RM) ANOVA, followed by contrast analysis and the sequentially rejective Bonferroni test for multiple comparisons. Statistical results (F- and P-values) are presented in figure legends. P-values of <0.05 were considered to indicate statistical significance.
Results
Behavioural observations
During the first few days after casting, each animal periodically attempted to free its immobilized forelimb. The animals with casts moved less than control animals, but this effect disappeared within a few days. After this time, the animals with casts recovered their normal exploratory activity, using the free forelimb for postural support during locomotion, grooming and self-cleaning. By contrast, activities that required bilateral forelimb use (i.e. fine holding and manipulation) were clearly compromised throughout the entire limb immobilization period. Immediately after cast removal, the paw and limb showed reperfusion oedema, but no injury. Within 12 h, all animals had gradually recovered limb use for posture, gait, grooming, and holding and eating. Beginning 12 h after the removal of the forelimb cast, motor performance was evaluated using the bar and drag tests and daily rotarod training. The baseline activity of control rats was 2.7 ± 0.1 s (immobility time in the bar test) and 10.2 ± 0.2 steps (drag test). No changes in immobility time (2.0 ± 0.4 s and 2.3 ± 0.3 s, respectively) or number of steps (10.3 ± 0.1 and 9.8 ± 0.3, respectively) were detected at 12 h after cast removal in either of the experimental groups (15 days and 30 days of immobilization, respectively). No statistically significant differences between the right and left forelimbs were recorded. Similarly, no major differences with respect to baseline values were detected throughout the recovery period.
Cortical changes during and after casting
Examination of M1 maps produced after immobilization (Fig.2A–E) revealed bilateral changes in movement representations. A progressive decrease in excitability within M1 was observed consistently during the immobilization period, and cortical output was not fully restored at the end of the subsequent recovery period.
Figure 2. Unilateral forelimb casting affected M1 output.
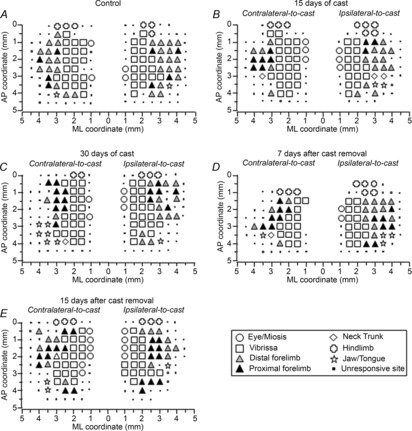
Representative bilateral M1 maps of movements evoked at threshold current levels in control rats (A), and after 15 days (B) and 30 days (C) of cast immobilization, and at 7 days (D) and 15 days (E) after cast removal (following 30 days of cast immobilization). Microelectrodes were sequentially introduced to a depth of 1500 μm. Interpenetration distances were 500 μm. In these mapping schemes, frontal poles are at the bottom. Zero corresponds to the bregma and numbers indicate rostral distance from the bregma or lateral distance from the mid-line. Movement evoked at one point is indicated by symbols, and forelimb movement type by grey scale. Absence of a symbol (within or at the border of the maps) indicates that the presence of a large blood vessel prevented penetration.
Representation size
During the immobilization period, rats exhibited a widening of the non-excitable area in the contralateral M1 compared with that in control animals (Fig.3A). The effect was present after 30 days of immobilization, and still evident at 7 days after cast removal. Normalization was observed at 15 days after cast removal. Limb immobilization did not affect the size of the vibrissa area (Fig.3B). However, in both ipsilateral and contralateral sides, caudal forelimb area was strongly reduced (Fig.3C) after 15 and 30 days of immobilization. This reduction was also present 7 days after cast removal, when a larger effect was registered on the contralateral side. Caudal forelimb area size returned to normal at 15 days after cast removal. The rostral forelimb area (Fig.3D) was also found to be reduced in the contralateral-to-cast M1; this effect was present after 30 days of immobilization and remained evident at 7 days into the recovery period. Consistent with previous parameters, its size was restored at 15 days after cast removal.
Figure 3. Unilateral forelimb casting changed M1 excitability.
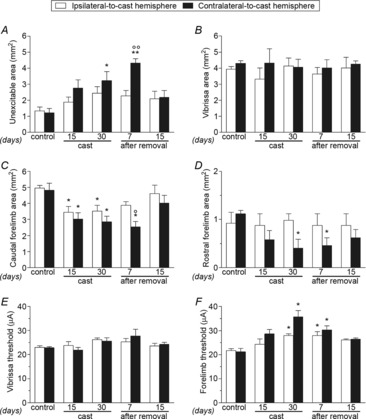
Effects of cast immobilization on the size (in mm2) of unexcitable (A), vibrissa (B), caudal (C) and rostral (D) forelimb areas, and on threshold currents (in μA) in vibrissa (E) and forelimb (F) areas in the ipsilateral- and contralateral-to-cast hemispheres. All measurements were obtained in control rats, and in experimental rats after 15 and 30 days of cast immobilization, and at 7 and 15 days after cast removal (following 30 days of cast immobilization). Data are expressed as the mean ± s.e.m. of five measurements per group. Note that during and after casting the excitability of the forelimb movement representation decreased in both hemispheres. This effect was more pronounced at 30 days of cast immobilization and at 7 days after cast removal. Statistical results: A, ipsilateral: H = 5.06, P = 0.28; contralateral: H = 14.82, P = 0.0051; B, ipsilateral: H = 1.35, P = 0.85; contralateral: H = 3.22, P = 0.52; C, ipsilateral: H = 10.08, P = 0.0391; contralateral: H = 13.03, P = 0.0111; D, ipsilateral: H = 1.08, P = 0.90; contralateral: H = 9.97, P = 0.0409; E, ipsilateral: H = 5.35, P = 0.25; contralateral: H = 7.13, P = 0.13. *P < 0.05, **P < 0.01 different from control (Kruskal–Wallis test followed by Dunn's test); °P < 0.05, °°P < 0.01 different from the ipsilateral-to-cast hemisphere (Mann–Whitney test).
Movement thresholds
Unilateral limb immobilization did not cause any change in threshold currents in the vibrissa area (Fig.3E). However, in the forelimb area a bilateral increase in threshold values was found after 30 days of immobilization (Fig.3F), with the increase being greater on the contralateral-to-cast side. Increases in threshold values on both sides were also observed at 7 days after cast removal. No differences in thresholds between the caudal and rostral regions were detected (data not shown).
Site distribution
To characterize changes in the spatial distribution of forelimb sites across groups of animals, a 2-D bregma-relative frequency distribution of forelimb-responsive sites was generated, in which cumulative sites were coded according to their rate. With respect to control animals (Fig.4A), a progressive shrinkage of the sites in which movement was frequently evoked (i.e. ≥50% of animals; 100% probability is achieved when movement at that site is observed in all animals in a group) was noted after 30 days of immobilization (Fig.4B). This reduction in the frequency of the number of sites remained evident at 7 days after cast removal (Fig.4C). The shrinkage was bilateral, but was more pronounced in the contralateral-to-cast hemisphere. Note that the decrease in frequency was not homogeneous throughout the area, but that more and less affected regions could be distinguished. Indeed, at this time-point, sites in which movement was frequently evoked were located only at caudal coordinates of the forelimb area, within the region 1.0–2.5 mm anterior to the bregma. The shrinkage of forelimb representations seems to occur as a result of the loss of excitability of the sites located more laterally in the map, without affecting the vibrissa–forelimb border. At 15 days after cast removal, expansion of the forelimb representation in the cortical regions corresponding to the anterolateral part of the normal forelimb representation was seen (Fig.4D). This indicates that the changes induced by immobilization were reversible.
Figure 4. Unilateral forelimb casting affected distribution of forelimb sites across the cortical surface.
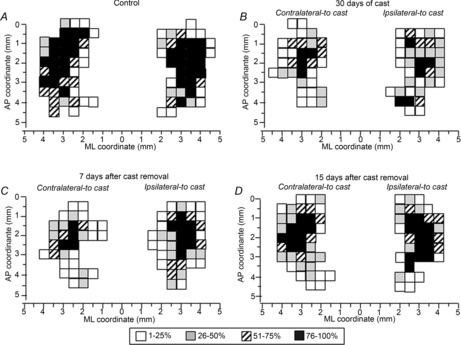
Descriptions of forelimb movements evoked in all animals at threshold current levels in control rats (A), and in experimental rats after 30 days of cast immobilization (B), and at 7 days (C) and 15 days (D) after cast removal (following 30 days of cast immobilization). The frequency of movement at each site is coded on a grey scale; 100% probability is achieved when a movement at that site is observed in all animals in a group. Conventions are as in Fig.2.
Forelimb movement
To assess the type of muscles recruited during movement, frequencies of proximal and distal forelimb movements in response to M1 stimulation were evaluated in both hemispheres. Under control conditions, M1 stimulation evoked almost totally distal movements at threshold current, whereas limb immobilization caused a strong increase in the recruitment of proximal movements, which predominated over distal movements, on the contralateral-to-cast side (Fig.5B). This effect was seen throughout immobilization and persisted up to 15 days after cast removal, when the distal and proximal frequencies were similar. Surprisingly, a large increase in the area eliciting proximal movements was also found on the ipsilateral-to-cast side (Fig.5A) at all time-points, although this increase was smaller than that in the opposite hemisphere.
Figure 5. Unilateral forelimb casting changed the type of intracortical microstimulation-evoked movements.
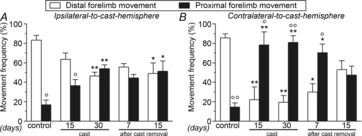
Effects of cast immobilization on the frequency (percentage of total forelimb movements) of distal and proximal forelimb movements in the ipsilateral-to-cast (A) and contralateral-to-cast (B) hemispheres. All measurements were obtained in control rats, and in experimental rats after 15 and 30 days of cast immobilization, and at 7 and 15 days after cast removal (following 30 days of cast immobilization). Data are expressed as the mean ± s.e.m. of five measurements per group. Note that in both hemispheres, during and after casting, the recruitment of proximal movements increased at the expense of distal movements. Statistical results: A, distal: H = 11.24, P = 0.0240; proximal: H = 11.24, P = 0.0240; B, distal: H = 15.51, P = 0.0038; proximal: H = 15.51, P = 0.0038. *P < 0.05, **P < 0.01 different from control (Kruskal–Wallis test followed by Dunn's test); °P < 0.05, °°P < 0.01 different from distal forelimb movement (Mann–Whitney test).
Bicuculline treatment after casting
In order to verify whether cortical synaptic connectivity contributed to the changes in the forelimb map seen at different time-points, we compared cortical maps generated before and after local application of bicuculline (50 μm). Bicuculline-induced M1 changes in the contralateral-to-cast hemisphere were investigated after 30 days of immobilization, and at 7 and 15 days after cast removal. Immediately after cast removal, bicuculline displayed very different effects than those evoked by its application under control conditions (Fig.6A–D). No effect was evident when saline was applied (data not shown).
Figure 6. Bicuculline affected M1 output after unilateral forelimb casting.
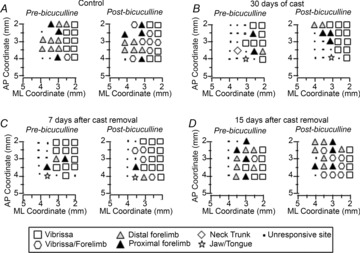
Representative contralateral-to-cast M1 maps of movements evoked at threshold current levels before and after cortical application of bicuculline (50 μm) in control rats (A), and in experimental rats after 30 days of cast immobilization (B), and at 7 days (C) and 15 days (D) after cast removal (following 30 days of cast immobilization). Conventions are as in Fig.2.
Representation size
In control rats, bicuculline greatly increased the size of the cortical area in which both vibrissa and forelimb movements were evoked at the same threshold current (vibrissa–forelimb area; Fig.7D), resulting in a substantial overlap between vibrissa and forelimb representations. In immobilized rats, however, bicuculline failed to trigger changes in the forelimb areas immediately after cast removal (Fig.7C, D), but strongly increased the size of the cortical area in which vibrissa movements were evoked (Fig.7B). This expansion involved cortical sites at which no response was detectable before bicuculline application (Fig.7A). By contrast, at 7 days and, in most cases, 15 days after cast removal, bicuculline showed an effect similar to that observed in control rats in all areas considered.
Figure 7. Bicuculline effects on M1 output after unilateral forelimb casting are time-dependent.
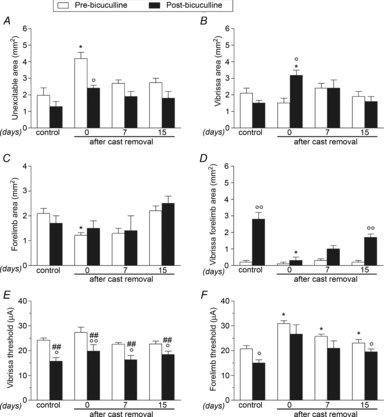
Effects of cortical application of bicuculline (50 μm) on the size (in mm2) of unexcitable (A), vibrissa (B), forelimb (C) and vibrissa–forelimb (D) areas, and on threshold currents (in μA) in vibrissa (E) and forelimb (F) areas in the contralateral-to-cast hemisphere. All measurements were obtained in control rats, and in experimental rats after 30 days of cast immobilization, and at 7 and 15 days after cast removal (following 30 days of cast immobilization). Data are the mean ± s.e.m. of five determinations per group. Note that bicuculline applied after 30 days of immobilization showed an effect on forelimb movement representations that is not comparable with that observed under control conditions, gradually revealing its effect at subsequent time-points. Statistical results: A, effect of treatment (F1,4 = 11.81, P = 0.0264), time (F3,24 = 10.53, P = 0.0001), but not time × treatment interaction (F3,24 = 1.57, P = 0.22); B, no effect of treatment (F1,4 = 1.39, P = 0.30) or time (F3,24 = 2.53, P = 0.08), but significant time × treatment interaction (F3,24 = 5.32, P = 0.0059); C, effect of time (F3,24 = 4.77, P = 0.0095), but not treatment (F1,4 = 0.51, P = 0.52) or time × treatment interaction (F3,24 = 0.34, P = 0.79); D, effect of treatment (F1,4 = 62.27, P = 0.0014), time (F3,24 = 13.67, P < 0.0001) and time × treatment interaction (F3,24 = 12.05, P = 0.0001); E, effect of treatment (F1,4 = 37.10, P = 0.0037), time (F3,24 = 3.42, P = 0.0334), but not time × treatment interaction (F3,24 = 0.89, P = 0.46), and F, effect of treatment (F1,4 = 9.65, P = 0.0360), time (F3,24 = 13.11, P < 0.0001), but not time × treatment interaction (F3,24 = 0.07, P = 0.97). *P < 0.05 different from respective control; °P < 0.05, °°P < 0.01 different from pre-bicuculline condition; #P < 0.05, ##P < 0.01 different from pre-bicuculline in control (repeated-measures ANOVA followed by contrast analysis and the sequentially rejective Bonferroni test for multiple comparisons).
Threshold current
Bicuculline produced a robust reduction in threshold currents in the vibrissa area of controls, as well as in experimental rats at all time-points after cast removal (Fig.7E). However, in the forelimb area, bicuculline reduced threshold currents only in control rats and in rats tested 15 days after cast removal (Fig.7F).
Motor rehabilitation after cast removal
In an attempt to rehabilitate the cast forelimb and to normalize cortical output, rats were trained daily (for 6 days after 30 days of immobilization) on the rotarod, and the number of falls occurring during each exercise session was recorded. Control rats showed a progressive increase in motor performance, approaching maximum performance levels after 3 days (Fig.8). By contrast, immediately after cast removal, test rats displayed a significant motor deficit, evidenced by a reduced performance in comparison with control rats over the first 4 days of rehabilitation. However, after 5 days of training, their performance was similar to that of controls.
Figure 8. Rotarod performance transiently decreased after unilateral forelimb casting.
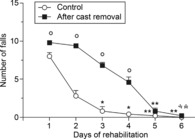
Rats were trained twice per day at increasing speeds (5–30 rpm, 180 s each) for the first 18 min and at a constant speed (30 rpm) for a further 12 min. All measurements were obtained for 6 days in control rats and in experimental rats after 30 days of cast immobilization (beginning 12 h after cast removal). Daily performance was expressed as the mean of two daily sessions. Data are expressed as the mean ± s.e.m. of five measurements per group. Note that in the first 4 days after cast removal, test rats displayed a higher number of falls than control rats. Statistical results: control: H = 22.52, P = 0.0004; after cast removal: H = 27.15, P < 0.0001. *P < 0.05, **P < 0.01 different from day 1 (Kruskal–Wallis test followed by Dunn's test); °P < 0.05 different from control (Mann–Whitney test).
To test whether a return to normal performance was accompanied by a return to a normal motor map, ICMS was performed 12 h after the last rotarod session (i.e. 7 days after cast removal). Surprisingly, although motor rehabilitation led to a substantial increase in distal forelimb movement representations, it did not fully restore the cortical map size. No major effects were observed in the vibrissa area (data not shown).
Representation size
Examination of M1 maps after rotarod training revealed that this training did not affect the size of either caudal or rostral areas in control rats. After cast removal, rotarod training failed to affect the size of the caudal forelimb area in the contralateral-to-cast hemisphere (Fig.9A), its only effect being size normalization in the ipsilateral-to-cast hemisphere. Likewise, rotarod exercise failed to restore the normal size of the rostral forelimb area (Fig.9B).
Figure 9. Rotarod rehabilitation affected the type of intracortical microstimulation-evoked movements, but not M1 excitability, after unilateral forelimb casting.
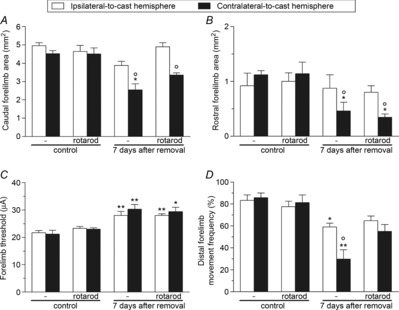
Effects of rotarod rehabilitation on the size (in mm2) of caudal (A) and rostral (B) forelimb areas, threshold currents (in μA) in forelimb areas (C), and the frequency (percentage of total forelimb movements) of distal forelimb movements (D) in the contralateral- and ipsilateral-to-cast hemispheres. All measurements were obtained in control rats, and in experimental rats at 7 days after cast removal (following 30 days of cast immobilization). Data are expressed as the mean ± s.e.m. of five measurements per group. Note that motor rehabilitation did not affect cortical size or thresholds, but did increase the distal forelimb movement representation. Statistical results: A, ipsilateral: H = 7.70, P = 0.0526; contralateral: H = 14.10, P = 0.0028; B, ipsilateral: H = 0.77, P = 0.86; contralateral: H = 13.60, P = 0.0035; C, ipsilateral: H = 15.19, P = 0.0017; contralateral: H = 14.14, P = 0.0027; D, ipsilateral: H = 10.64, P = 0.0138; contralateral: H = 14.45, P = 0.0023. *P < 0.05, **P < 0.01 different from control (Kruskal–Wallis test followed by Dunn's test); °P < 0.05 different from the ipsilateral-to-cast hemisphere (Mann–Whitney test).
Threshold current
No significant changes in threshold current values were observed after rotarod training in control rats. Moreover, rotarod exercise also failed to restore normal threshold current values 7 days after cast removal (Fig.9C).
Forelimb movement
Rotarod training did not change the frequency of distal movements in control rats. However, a bilateral but slight increase in the frequency of distal movements (with a concomitant decrease in proximal movements) was seen at 7 days after cast removal in the trained rats (Fig.9D).
Discussion
Immobilization caused a marked decrease in cortical excitability. Overall, the increases in threshold current and concomitant size reductions appear to be specific (for forelimb area), progressive and bilateral, albeit greater in the contralateral-to-cast hemisphere. Accompanying the shrinkage of the forelimb area, we noted an internal reorganization of the forelimb map, specifically a reduction in distal movement representations and a concomitant expansion in proximal movement representations. Blockade of intracortical GABAergic transmission, by local treatment with bicuculline, had an effect similar to that observed under control conditions when performed at 15 days after cast removal. By contrast, the effect of bicuculline application performed before this time-point was not comparable with that observed under control conditions. Furthermore, motor rehabilitation did not restore normal forelimb map expression, but did increase the representation of distal movements.
Cortical plasticity following unilateral forelimb immobilization
Several human studies have evidenced neural adaptations in cortical structure after limb immobilization. However, any comparison of the present findings with those of human studies is problematic because there are several important differences in sensorimotor cortex organization, as well as in experimental conditions and methods. Nevertheless, changes in cortical area, in the form of restrictions in the cortical representations of immobilized joints, have been demonstrated in humans by both TMS (Liepert et al. 1995) and, more recently, fMRI (Lissek et al. 2009; Roll et al. 2012). The findings of another TMS study, performed after forearm immobilization, suggest that threshold changes may be state-dependent because no change in motor threshold at rest but a transient reduction during muscle contraction was observed (Clark et al. 2008).
The present ICMS study in rats allows us to discern immobilization-specific changes in motor maps at a level of spatial detail that is not easily obtainable using alternative methods. That said, the increase in the ‘resting’ motor threshold registered in the present study is in line with observations in a previous TMS study (Zanette et al. 2004), although it should be noted that other TMS studies have demonstrated relatively stable motor thresholds after immobilization (Clark et al. 2010). Nonetheless, in our study, whole-limb sensorimotor restriction profoundly increased thresholds and shrunk the forelimb representation area, without compensation by the neighbouring cortex, leaving the lateral part of the forelimb representation unresponsive to electrical stimulation. Thus, considering the entire forelimb motor representation, we can distinguish different functional (sub)regions, characterized by a different sensibility for the movement restriction. The more sensible regions appear to be those located in the rostral and lateral part of M1. Consequently, no changes in the border between forelimb and vibrissa representations were detected, a finding very different from those observed in previous studies on forelimb cortex plasticity (Sanes et al. 1990; Teskey et al. 2003; Maggiolini et al. 2008). This suggests that this form of cortical plasticity is specifically related to whole forelimb movement restriction (i.e. by the application of a cast), and our observations were consistent with the cortical changes induced by adult rat hindlimb movement restriction after a 14 day period of limb unloading (Langlet et al. 2012; Trinel et al. 2013). Indeed, hindlimb unloading induced a shrinkage of the hindlimb representation of the M1, and increased current thresholds for eliciting hindlimb movement. Nonetheless, the M1 remodelling brought about by hindlimb unloading primarily affected the representation of proximal joints and representations of the toes were less affected. This contrasts with our findings of an increase in the relative area representing proximal forelimb movements and a drastic under-representation of distal movements. However, this difference in motor cortical map remodelling may be correlated to the function of the limb in question. Indeed, the forelimbs are involved in manipulative behaviour, whereas the hindlimbs are mainly tasked with maintaining body stability. This interpretation is supported by the forelimb map remodelling seen in both hemispheres and agrees with a general principle according to which over- or under-trained limb parts are over- or under-represented on motor maps (Kleim et al. 1998; Milliken et al. 2013). In all likelihood, the bilateral rearrangement of proximal and distal movement representations seen in the present work arose as a result of the new motor adjustments developed, which favoured greater proximal representation. Thus, wrist/forearm stabilization for postural support, which requires substantial coordination with increased elbow/shoulder activity, may explain the imbalance in the frequencies of distal and proximal movements in the forelimb maps of both hemispheres.
However, these findings do not provide a direct explanation for why the forelimb area displayed selective shrinkage. One possibility is that the most lateroposterior part of the motor map includes the forelimb somatosensory cortex (S1), which is involved in a reduction of excitability in parallel with the proper motor region (Coq & Xerri, 1999; Lissek et al. 2009). Moreover, the lateral region of the forelimb area selectively receives input from the proper forelimb S1, whereas the medial region is selectively connected with the dysgranular zone located lateral to the proper forelimb S1 (Kim & Lee, 2013). Thus, a loss of excitability in the forelimb S1 and its connection with the lateral part of the forelimb area may also explain this directional shrinkage. Moreover, our motor maps reflect the efferent organization of the corticospinal tract, but do not provide details of complex features of M1 organization such as movement patterns, spatial topography (Bonazzi et al. 2013) and spatiotemporal dynamics of neuron clusters (Dombeck et al. 2009; Hira et al. 2013). It is possible that changes in these complex features may also help to explain the impaired cognitive representation of limb movement after immobilization (Toussaint & Meugnot, 2013).
The normalization in size of the forelimb motor map seen 15 days after cast removal (which returned to roughly the values found in control animals) suggests a progressive adaptive cortical recovery as a consequence of restored overall motor activity (i.e. forelimb use in natural behaviour). This normalization of the forelimb map size correlated to an increase in the number of sites at the more lateral coordinates of the forelimb cortex. However, the persistence of proximal versus distal movement activation pattern discrepancies indicates that cortical circuits had not yet fully recovered their basic activities at 2 weeks after cast removal. This finding suggests that, within cortical networks engaged in novel motor behaviour, the distal-projecting forelimb neurons recovered their excitability later than their proximal-projecting counterparts. A difference in the timing of recovery between the distal- and proximal-projecting forelimb neurons intermingled within the motor cortex (Wang et al. 2011) may reflect a time difference in forelimb movement recovery after cast removal. Indeed, whereas elbow and shoulder movements are required in postural activity immediately after cast removal, skilled motor behaviour involving distal forelimb joints may be re-learned at a later stage.
Involvement of intracortical synaptic transmission in cortical plasticity
Several TMS studies suggest that immobilization results in an imbalance between intracortical facilitation and inhibition circuitries, but results remain controversial. For example, in one experiment (Zanette et al. 2004), intracortical facilitation was found to be enhanced and intracortical inhibition reduced, whereas a more recent study (Clark et al. 2010) showed no changes at rest, but an increased inhibition during active contraction. In ICMS studies, the contribution of intrinsic cortical circuitry to movement representations can be evidenced by rapid modifications in evoked responses to pharmacological manipulation (Jacobs & Donoghue, 1991; Viaro et al. 2011). Indeed, bicuculline application at subconvulsive concentrations (Stojic et al. 2000) depresses GABAergic function, revealing a transynaptic excitatory activity that facilitates the activation of corticospinal neurons (Jacobs & Donoghue, 1991; Schneider et al. 2002). The expansion of representations seen after bicuculline application in control rats is consistent with previous findings (Jacobs & Donoghue, 1991; Schneider et al. 2002) of expanded motor maps with lower movement thresholds after M1 disinhibition (Young et al. 2012). Bicuculline applied immediately after cast removal did not increase the size of the forelimb area, suggesting an important loss of intracortical synaptic connectivity, upon which the drug relies to exert its effects. The cortical effect of the forelimb cast may reflect an inability to maintain pre-established intracortical connections that support the physiological motor map (Leingärtner et al. 2007; Henderson et al. 2012; Trinel et al. 2013). This may be ascribable to a reduced convergence of thalamocortical inputs, which relay sensory information to sensorimotor cortical neurons. This input restriction may have major effects on intra- and inter-columnar circuitry, resulting in suboptimal size and excitability in M1, as confirmed in clinical studies (Lissek et al. 2009; Roll et al. 2012). However, as we did not assess the effects at spinal or muscular levels, we cannot exclude other subcortical contributions along the corticospinal system.
Effect of motor rehabilitation on cortical plasticity
Previous works have shown that when animals undertake some form of exercise, changes in the organization of the motor cortex are detectable under both intact and injured conditions (Nudo et al. 1996; Ramanathan et al. 2006; Giszter et al. 2008; Kao et al. 2011). Indeed, in the present experiments, a week of rotarod training after cast removal led to a redistribution of movement representation in M1. This was seen as an expansion of distal movement representation in both hemispheres, but no significant effects on map size or current thresholds were seen in the contralateral-to-cast hemisphere.
The fact that rotarod training mainly affected the ipsilateral-to-cast hemisphere provides evidence that this hemisphere showed none of the perturbations hypothesized for the contralateral-to-cast hemisphere. This difference in motor map reorganization between hemispheres may reflect different stages of map plasticity (Molina-Luna et al. 2008; Reed et al. 2011) and prompts two considerations. The first of these is that only the part of the motor cortex that has preserved its excitability appears to be sensitive to post-cast training; the second is that preserving motor cortex excitability during cast immobilization may promote post-cast recovery. If this is indeed the case, proprioceptive stimulation during immobilization, combined with after-cast exercise, might enhance recovery.
Glossary
- fMRI
functional magnetic resonance imaging
- ICMS
intracortical microstimulation
- M1
primary motor cortex
- S1
somatosensory cortex
- TMS
transcranial magnetic stimulation
Key points
To shed light on the controversial issue of how chronic immobilization affects cortical output, adult rats were subjected to intracortical microstimulation at different time-points during and after unilateral forelimb casting.
After cast application, cortical hypoexcitability appeared bilateral, specific for forelimb area, but stronger in the contralateral-to-cast hemisphere. Cortical excitability progressively decreased over 30 days of immobilization and, after cast removal, steadily increased, but remained partial at 15 days.
Cortical application of the GABAA-receptor antagonist bicuculline revealed an impairment of intracortical synaptic connectivity in the forelimb area during the cast period and for up to 15 days after cast removal.
Rehabilitation using a rotarod performance protocol did not advance the normalization of normal forelimb map extension and enabled cortical output towards the distal forelimb only in sites that had maintained their excitability.
Cortical hypoexcitability following immobilization is caused by reversible impairment of intracortical synaptic connectivity. This may suggest new approaches in conditions that require longterm limb immobilization.
Additional information
Competing interests
None declared.
Author contributions
R.V. and G.F. contributed to the conception and design of the experiments. All authors contributed to the collection, analysis and interpretation of data. R.V. and G.F. contributed to the drafting and critical revision of the article for important intellectual content. All authors approved the final manuscript for submission.
Funding
This work was supported by a local grant from the University of Ferrara to G.F.
References
- Bonazzi L, Viaro R, Lodi E, Canto R, Bonifazzi C. Franchi G. Complex movement topography and extrinsic space representation in the rat forelimb motor cortex as defined by long-duration intracortical microstimulation. J Neurosci. 2013;33:2097–2107. doi: 10.1523/JNEUROSCI.3454-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Issac LC, Lane JL, Damron LA. Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol. 2008;105:868–878. doi: 10.1152/japplphysiol.90530.2008. [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL, Hoffman RL, Dearth DJ. Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve. 2010;42:363–372. doi: 10.1002/mus.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW. Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Coq JO, Barr AE, Strata F, Russier M, Kietrys DM, Merzenich MM, Byl NN. Barbe MF. Peripheral and central changes combine to induce motor behavioral deficits in a moderate repetition task. Exp Neurol. 2009;220:234–245. doi: 10.1016/j.expneurol.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coq JO. Xerri C. Tactile impoverishment and sensorimotor restriction deteriorate the forepaw cutaneous map in the primary somatosensory cortex of adult rats. Exp Brain Res. 1999;129:518–531. doi: 10.1007/s002210050922. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Graziano MS. Tank DW. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J Neurosci. 2009;29:13751–13760. doi: 10.1523/JNEUROSCI.2985-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B. Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Fox K. Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–477. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Franchi G. Reorganization of vibrissal motor representation following severing and repair of the facial nerve in adult rats. Exp Brain Res. 2000;131:33–43. doi: 10.1007/s002219900297. [DOI] [PubMed] [Google Scholar]
- Franchi G. Time course of motor cortex reorganization following botulinum toxin injection into the vibrissal pad of the adult rat. Eur J Neurosci. 2002;16:1333–1348. doi: 10.1046/j.1460-9568.2002.02195.x. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM. Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anaesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Fu M. Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter S, Davies MR, Ramakrishnan A, Udoekwere UI. Kargo WJ. Trunk sensorimotor cortex is essential for autonomous weight-supported locomotion in adult rats spinalized as P1/P2 neonates. J Neurophysiol. 2008;100:839–851. doi: 10.1152/jn.00866.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Laxmi TR. Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J Neurosci. 2013;33:7234–7244. doi: 10.1523/JNEUROSCI.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AK, Pittman QJ. Teskey GC. High frequency stimulation alters motor maps, impairs skilled reaching performance and is accompanied by an upregulation of specific GABA, glutamate and NMDA receptor subunits. Neuroscience. 2012;215:98–113. doi: 10.1016/j.neuroscience.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Hira R, Ohkubo F, Tanaka YR, Masamizu Y, Augustine GJ, Kasai H. Matsuzaki M. In vivo optogenetic tracing of functional corticocortical connections between motor forelimb areas. Front Neural Circuits. 2013;7:55. doi: 10.3389/fncir.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM. Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–945. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jones TA. Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Knudsen EB, Murray M. Moxon KA. Functional role of exercise-induced cortical organization of sensorimotor cortex after spinal transection. J Neurophysiol. 2011;106:2662–2674. doi: 10.1152/jn.01017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U. Lee T. Intra-areal and corticocortical circuits arising in the disgranular zone of rat primary somatosensory cortex that processes deep input. J Comp Neurol. 2013;521:2585–2601. doi: 10.1002/cne.23300. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S. Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Langer N, Hänggi J, Müller NA, Simmen HP. Jäncke L. Effects of limb immobilization on brain plasticity. Neurology. 2012;78:182–188. doi: 10.1212/WNL.0b013e31823fcd9c. [DOI] [PubMed] [Google Scholar]
- Langlet C, Bastide B. Canu MH. Hindlimb unloading affects cortical motor maps and decreases corticospinal excitability. Exp Neurol. 2012;237:211–217. doi: 10.1016/j.expneurol.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Leingärtner A, Thuret S, Kroll TT, Chou SJ, Leasure JL, Gage FH. O'Leary DD. Cortical area size dictates performance at modality-specific behaviors. Proc Natl Acad Sci U S A. 2007;104:4153–4158. doi: 10.1073/pnas.0611723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M. Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, Peters SA, Nicolas V, Tegenthoff M. Dinse HR. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol. 2009;19:837–842. doi: 10.1016/j.cub.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Maggiolini E, Viaro R. Franchi G. Suppression of activity in the forelimb motor cortex temporarily enlarges forelimb representation in the homotopic cortex in adult rats. Eur J Neurosci. 2008;27:2733–2746. doi: 10.1111/j.1460-9568.2008.06248.x. [DOI] [PubMed] [Google Scholar]
- Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S, Guerrini R, Romualdi P, Candeletti S, Simonato M, Cox BM. Morari M. Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson's disease. J Neurosci. 2005;25:9591–9601. doi: 10.1523/JNEUROSCI.2546-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken GW, Plautz EJ. Nudo RJ. Distal forelimb representations in primary motor cortex are redistributed after forelimb restriction: a longitudinal study in adult squirrel monkeys. J Neurophysiol. 2013;109:1268–1282. doi: 10.1152/jn.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Luna K, Hertler B, Buitrago MM. Luft AR. Motor learning transiently changes cortical somatotopy. Neuroimage. 2008;40:1748–1754. doi: 10.1016/j.neuroimage.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ. Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res. 1982;232:151–156. doi: 10.1016/0006-8993(82)90617-5. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF. Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM. Merzenich MM. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens Mot Res. 1990;7:463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM. Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A. Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW. Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM. Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci U S A. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V. Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Roll R, Kavounoudias A, Albert F, Legré R, Gay A, Fabre B. Roll JP. Illusory movements prevent cortical disruption caused by immobilization. Neuroimage. 2012;62:510–519. doi: 10.1016/j.neuroimage.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V. Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Rozas G, Guerra MJ. Labandeira-Garcia JL. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Brain Res Protoc. 1997;2:75–84. doi: 10.1016/s1385-299x(97)00034-2. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M. Norman AB. The catalepsy test: its ups and downs. Behav Neurosci. 1988;102:748–759. doi: 10.1037//0735-7044.102.5.748. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S. Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term pattern of reorganization following motor or mixed peripheral nerve lesion. Exp Brain Res. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Sanes JN. Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schneider C, Devanne H, Lavoie BA. Capaday C. Neural mechanisms involved in the functional linking of motor cortical points. Exp Brain Res. 2002;146:86–94. doi: 10.1007/s00221-002-1137-2. [DOI] [PubMed] [Google Scholar]
- Sterr A, Müller MM, Elbert T, Rockstroh B, Pantev C. Taub E. Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. J Neurosci. 1998;18:4417–4423. doi: 10.1523/JNEUROSCI.18-11-04417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojic AS, Lane RD, Killackey HP. Rhoades RW. Suppression of hindlimb inputs to S-I forelimb-stump representation of rats with neonatal forelimb removal: GABA receptor blockade and single-cell responses. J Neurophysiol. 2000;83:3377–3387. doi: 10.1152/jn.2000.83.6.3377. [DOI] [PubMed] [Google Scholar]
- Teskey GC, Flynn C, Goertzen CD, Monfils MH. Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25:794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- Toldi J, Laskawi R, Landgrebe M. Wolff JR. Biphasic reorganization of somatotopy in the primary motor cortex follows facial nerve lesions in adult rats. Neurosci Lett. 1996;203:179–182. doi: 10.1016/0304-3940(95)12295-8. [DOI] [PubMed] [Google Scholar]
- Toussaint L. Meugnot A. Short-term limb immobilization affects cognitive motor processes. J Exp Psychol Learn Mem Cogn. 2013;39:623–632. doi: 10.1037/a0028942. [DOI] [PubMed] [Google Scholar]
- Trinel D, Picquet F, Bastide B. Canu MH. Dendritic spine remodeling induced by hindlimb unloading in adult rat sensorimotor cortex. Behav Brain Res. 2013;249:1–7. doi: 10.1016/j.bbr.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Viaro R, Marti M. Morari M. Dual motor response to l-dopa and nociceptin/orphanin FQ receptor antagonists in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) treated mice: paradoxical inhibition is relieved by D2/D3 receptor blockade. Exp Neurol. 2010;223:473–484. doi: 10.1016/j.expneurol.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Viaro R, Morari M. Franchi G. Progressive motor cortex functional reorganization following 6-hydroxydopamine lesioning in rats. J Neurosci. 2011;31:4544–4554. doi: 10.1523/JNEUROSCI.5394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Conner JM, Rickert J. Tuszynski MH. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A. 2011;108:2545–2550. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NA, Vuong J, Flynn C. Teskey GC. Optimal parameters for microstimulation derived forelimb movement thresholds and motor maps in rats and mice. J Neurosci Methods. 2011;196:60–69. doi: 10.1016/j.jneumeth.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Young NA, Vuong J. Teskey GC. Development of motor maps in rats and their modulation by experience. J Neurophysiol. 2012;108:1309–1317. doi: 10.1152/jn.01045.2011. [DOI] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A. Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115:1264–1275. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]


