Abstract
In mammals, eupnoeic breathing is periodically interrupted by spontaneous augmented breaths (sighs) that include a larger-amplitude inspiratory effort, typically followed by a post-sigh apnoea. Previous in vitro studies in newborn rodents have demonstrated that the respiratory oscillator of the pre-Bötzinger complex (preBötC) can generate the distinct inspiratory motor patterns for both eupnoea- and sigh-related behaviour. During mouse embryonic development, the preBötC begins to generate eupnoeic rhythmicity at embryonic day (E) 15.5, but the network's ability to also generate sigh-like activity remains unexplored at prenatal stages. Using transverse brainstem slice preparations we monitored the neuronal population activity of the preBötC at different embryonic ages. Spontaneous sigh-like rhythmicity was found to emerge progressively, being expressed in 0/32 slices at E15.5, 7/30 at E16.5, 9/22 at E17.5 and 23/26 at E18.5. Calcium imaging showed that the preBötC cell population that participates in eupnoeic-like discharge was also active during fictive sighs. However, patch-clamp recordings revealed the existence of an additional small subset of neurons that fired exclusively during sigh activity. Changes in glycinergic inhibitory synaptic signalling, either by pharmacological blockade, functional perturbation or natural maturation of the chloride co-transporters KCC2 or NKCC1 selectively, and in an age-dependent manner, altered the bi-phasic nature of sigh bursts and their coordination with eupnoeic bursting, leading to the generation of an atypical monophasic sigh-related event. Together our results demonstrate that the developmental emergence of a sigh-generating capability occurs after the onset of eupnoeic rhythmogenesis and requires the proper maturation of chloride-mediated glycinergic synaptic transmission.
Introduction
Respiratory rhythmogenesis arises from oscillatory neuronal assemblages, so-called central pattern generators or CPGs (Marder & Calabrese, 1996; Stein et al. 1997; Grillner, 2006) located within the hindbrain (Feldman & Del Negro, 2006; Feldman et al. 2013). One of the respiratory oscillators, the pre-Bötzinger complex (preBötC), which is essential for normal breathing (Gray et al. 2001; McKay et al. 2005; Tan et al. 2008), comprises neural circuitry thought to control different inspiratory-related activities, including eupnoea and sigh production in normoxic conditions or gasping in a hypoxic environment (Smith et al. 1991; Lieske et al. 2000; Peña et al. 2004; Paton et al. 2006). This capability presumably arises from reconfiguration processes occurring within the preBötC network (Lieske et al. 2000), which can be influenced by central inputs from neighbouring brainstem regions, neuromodulatory influences and sensory feedback from the periphery (Lieske et al. 2000; Peña et al. 2004; Paton et al. 2006; Ruangkittisakul et al. 2008; Tryba et al. 2008; Bell et al. 2009).
Sighs (or augmented breaths) are normal components of mammalian respiratory behaviour that are generated spontaneously and harmonically phase-coupled with activity of the faster eupnoeic breathing rhythm. Sigh production is important for effective lung function, since it augments residual volume, increases lung compliance and recruits atelectatic alveoli (Nicholas et al. 1982; Davis & Moscato, 1994; Poets et al. 1997; Patroniti et al. 2002; Qureshi et al. 2009). Sighs can also be involved in triggering arousal responses, failure of which is suspected to be the cause of premature or unexplained death, such as in cases of sudden infant death syndrome (SIDS; Thach & Lijowska, 1996; Lijowska et al. 1997; McNamara et al. 2002; Franco et al. 2003). A sigh is generally defined as a biphasic sequence of breathing activity, the first component being comparable to a typical eupnoeic respiratory event while the second phase is larger in amplitude, occurs at low frequency (∼1 min−1), and is followed by a short pause or ‘post-sigh apnoea’ (Cherniack et al. 1981; Orem & Trotter, 1993; Takeda & Matsumoto, 1997). Neural activity expressing these features has also been reported in newborn rodent in vitro medullary slices containing the preBötC oscillator in physical isolation (Lieske et al. 2000; Ruangkittisakul et al. 2008; Tryba et al. 2008) thereby suggesting that sigh generation relies on mechanisms intrinsic to this network.
In addition to the hypothesized role of sighs in arousal and SIDS affecting newborn babies between one month and one year, sighs might also have clinical relevance for premature babies often suffering from acute respiratory distress syndrome (ARDS; Hummler et al. 1997; Qureshi et al. 2009; Sürmeli-Onay et al. 2012). However, the mechanisms underlying the developmental onset and the production of sighs at prenatal stages remain largely unexplored. In rodents, it has been shown that the preBötC respiratory network becomes active during the last third of gestation (Pagliardini et al. 2003; Thoby-Brisson et al. 2005). However, an ability of preBötC circuitry to generate distinct inspiratory-related activities, and specifically eupnoea and sighs, remains unknown at embryonic stages. In the present study using transverse brainstem slices obtained from mouse embryos, we show that the prenatal preBötC is indeed capable of generating sigh-like activity, albeit beginning at a later stage than that when eupnoeic bursting is first established. We also provide evidence for the cellular substrates and synaptic maturational processes that contribute to this novel activity's emergence in the embryonic preBötC network.
Methods
Ethical approval
All experiments were performed in accordance with the guidelines of the European and French National legislation on animal experimentation and the local ethics committee of the University of Bordeaux (permit number 5012031A). They also conformed to UK regulations, as described by Drummond (2009).
Animals
Pregnant OF1 outbred (Swiss) mice were obtained from a commercial supplier (Janvier, Le Genest Saint Isle, France) and from a local animal facility. KCC2 knock-down animals were kindly provided by Dr Laurent Vinay (Marseille, France). In these animals the expression of the KCC2 protein is reduced by more than 95% (Woo et al. 2002).
In vitro slice preparations
Embryonic brainstem transverse slices containing the preBötC were obtained as previously described (Thoby-Brisson et al. 2005; Bouvier et al. 2010). Briefly, pregnant mice were killed by cervical dislocation at the desired stage of gestation (from embryonic day (E) 15.5 until E18.5; the day following the vaginal plug being considered as embryonic day 0.5). Embryos (of either sex) were removed from their uterine sac and placed in artificial cerebrospinal fluid (aCSF) at 20°C under continuous oxygenation (by bubbling carbogen (95% O2/5% CO2)) until their experimental use. The aCSF composition was (in mm): 120 NaCl, 8 KCl, 1.26 CaCl2, 1.5 MgCl2, 21 NaHCO3, 0.58 NaH2PO4, 30 glucose; pH 7.4. Dissections and brainstem slicing were performed in this solution at 4°C. In a first step, the hindbrain was isolated from embryos following a rostral transverse section performed at the anterior limit of the rhombencephalon and a more caudal section made below the fourth cervical root. The isolated hindbrain was then embedded in an agar block with careful orientation and serially sectioned in the transverse plane using a vibratome (Leica, Wetzlar, Germany). A low-melting point agar was used at 4% and the embedding of the isolated preparations was done at a temperature at which the integrity of the tissue could be preserved while the agar was still malleable. Sections (200 μm thick) were made in the rostral-to-caudal direction until the facial motor nucleus (visible in direct light under a binocular microscope) was detected. Then the anterior limit of a 450 μm thick slice was set 250–300 μm caudal to the posterior limit of the facial nucleus. This slice encompasses the longitudinal column of the ventro-lateral medulla containing the highest density of rhythmically active interneurons expressing several anatomical markers of the preBötC network (Bouvier et al. 2010). From such slices we also obtained island preparations (Johnson et al. 2001), that were made under binocular control by cutting away the tissues in the slice that were outside the ventral respiratory group using a sharp razor blade. Slices and island preparations were then placed rostral side up in the recording chamber and continuously superfused with oxygenated aCSF maintained at 30°C. In addition, the preparations were held in place from above and below by slice support grids (made of a platinum ring with nylon threads) which maximized tissue oxygenation and avoided tissue movement during optical and electrophysiological recordings. A 30 min recovery period was systematically observed prior to the start of recording procedures.
Electrophysiology
Extracellular recordings of local population activity were made using an aCSF-filled glass micropipette positioned on the surface of the slice in the preBötC area and connected through a silver wire to a high-gain amplifier (7P511; Grass Instrument Co., Quincy, MA, USA). The signals were filtered (bandwidth 3 Hz to 3 kHz), integrated using an electronic filter (Neurolog system; Digitimer, UK) with a time constant of 100 ms, recorded and stored on a computer via a Digidata 1322 interface and analysed using the pCLAMP9 software (both from Molecular Devices, Sunnyvale, CA, USA). Because recordings were performed in vitro on reduced slice preparations, respiratory-related rhythmic activities were qualified as fictive eupnoeic-like and sigh-like activities.
Whole cell patch-clamp recordings were obtained under visual control using differential interference contrast and infrared video microscopy. Because the visual patch procedure allows detection of only the first two to three most superficial cell layers, our recordings were most likely to have been performed on inspiratory neurons of the ventral respiratory column, the majority of which belonging to the preBötC (Ruangkittisakul et al. 2011). Patch pipettes were fabricated with pulled borosilicate glass tubes (GC 150F; Clark Electromedical Instruments, Pangbourne, UK) filled with a solution containing 140 mm potassium gluconate acid, 1 mm CaCl2.6H2O, 10 mm EGTA, 2 mm MgCl2, 4 mm Na2ATP, 10 mm Hepes (pH 7.2) and having a tip resistance of 5–7 MΩ. The signals were acquired using an Axoclamp 2A amplifier (Molecular Devices), then stored and analysed via the same digitizing interface and pCLAMP9 software as for population recordings.
Calcium imaging
Slice preparations obtained as described above were incubated for 40 min in a solution of aCSF containing the cell-permeable acetoxymethyl (AM) ester form of the calcium indicator dye Calcium Green-1 (10 μm; Molecular Probes, Eugene, OK) maintained at room temperature under constant oxygenation. After dye loading, preparations were placed in the recording chamber and a 30 min delay was observed in order for excess dye to wash out and the preparation to stabilize in oxygenated aCSF at 30°C. Fluorescence images were captured through an E600-FN upright microscope (Nikon, Tokyo, Japan) equipped with a standard epifluorescent illumination system and a fluorescein filter coupled to a cooled CCD camera (Coolsnap HQ, Photometrics, Tucson, Az, US) operating with an exposure time of 100 ms in overlapping exposure and readout mode. Images were acquired over periods lasting 120–180 s and analysed using Metamorph software (Universal Imaging Corporation, West Chester, PA, USA) or the software developed and kindly provided by Dr Nicholas Mellen (Mellen & Tuong, 2009). For each image stack we measured the baseline fluorescence for each individual frame captured between two bursts of activity, averaged the values to obtain the mean baseline fluorescence (F) that was then used to normalize the variation of fluorescence (ΔF) in the region of interest, which was subsequently displayed as ΔF/F.
Pharmacological treatment
Strychnine and bumetanide (Sigma, St Louis, Mo, US) were dissolved in oxygenated aCSF and bath-applied for 15–30 min at their final concentrations of 1 μm and 20 μm, respectively. Because sigh activity occurs at a relatively low frequency, the assessment of drug effects were made over recording periods of 15–30 min. Data were analysed off-line using Clampfit software (Molecular Devices, Foster city, CA, US). Values are given as mean ± SEM. Statistical significance was assessed by Student's t test or a one-way ANOVA test when appropriate and mean values were considered to be significantly different at P < 0.05.
Immunostaining
Brainstem preparations were fixed for 3–4 h in 4% paraformaldehyde, cryoprotected in 30% sucrose–PBS overnight and then cryo-sectioned at 30 μm. For Islet1,2 (marker for motoneurons) and KCC2 double immunostaining, sections were incubated for 30 min in 1% bovine serum albumin (BSA) and 0.5% Triton X-100, followed by affinity-purified mouse anti-Islet1,2 (1/250, DSHB, Iowa city, Iowa, US) and rabbit anti-KCC2 (1/200, Millipore, Billerica, MA, US) overnight at room temperature. After rinsing in PBS, sections were exposed to secondary antibodies for 3 h: fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG, 1/1000 (Abcam, Paris, France); and Alexa Fluor 594-conjugated goat anti-rabbit IgG: 1/500 (Molecular Probes). After rinsing with PBS sections were coverslipped and mounted in PBS–glycerol (50/50). Control experiments in which the primary antibodies were replaced by normal serum exhibited no labelling.
Results
Emergence of sigh-like activity during embryonic development
The capability of the preBötC to generate different inspiratory-related activities during the prenatal period was assessed in preparations obtained at successive embryonic ages, ranging from E15.5 (the stage at which the respiratory oscillator becomes first active in the mouse; Thoby-Brisson et al. 2005) to E18.5 (the stage directly preceding birth in the mouse strain used in this study). Based on previous descriptions of post-natal sigh-like events in vitro (Lieske et al. 2000; Tryba et al. 2008) we first sought electrophysiological evidence for large-amplitude, biphasic inspiratory discharge (sigh-like bursts), in each case followed by a prolonged interval before the next eupnoeic burst and occurring at low frequency in intermittent coordination with smaller-amplitude eupnoeic bursts generated at higher frequency (Fig.1A). In all E15.5 slices examined (n = 32), preBötC population recordings revealed the exclusive presence of spontaneous rhythmic eupnoeic-like bursting activity (Fig.1A and B). At E16.5, the majority of preparations (n = 23 from 30) generated eupnoeic-like rhythmicity only, while the remaining preparations (n = 7/23, 23%) also generated low frequency bi-phasic sigh-like bursts (Fig.1A and B). At E17.5, however, 41% (n = 9/22) of the preparations spontaneously expressed the two types of burst (eupnoeic- and sigh-like), while at E18.5, fictive sigh bursts were detected in 88% (n = 23/26) of slices examined. Furthermore, as previously reported in the rat (Pagliardini et al. 2003), we found that the cycle frequency of eupnoeic-like burst activity increased progressively during this prenatal period. Eupnoeic discharges occurred at a mean frequency (bursts min−1) of 10.7 ± 0.2 (n = 32) at E15.5, 12.6 ± 0.2 (n = 30) at E16.5, 14.7 ± 0.3 (n = 22) at E17.5 and 16.3 ± 0.6 (n = 26) at E18.5 (P < 0.05, ANOVA test). In contrast, the frequency of sigh-like bursts when expressed in preparations, remained relatively constant over the same developmental period (P > 0.05, ANOVA test), occurring at mean rates (bursts min−1) of 1.3 ± 0.08 (n = 9) at E16.5, 1.5 ± 0.07 (n = 9) at E17.5 and 1.3 ± 0.06 (n = 23) at E18.5. It is noteworthy, finally, that at E18.5, we also recorded sigh- and eupnoeic-like activities in maximally reduced preBötC ‘island’ preparations (n = 6; Fig.1C; Johnson et al. 2001), thereby indicating that the neurons responsible for generating both rhythms are confined to this brainstem region. Together these results show that the developmental emergence of sigh-like activity occurs at E17.5–18.5, revealing that the generation of eupnoeic- and sigh-like activities have distinct times of onset, but are likely to share common neural substrates within, or in close proximity to, the preBötC.
Figure 1. Onset of sigh rhythmogenesis during mouse embryonic development.
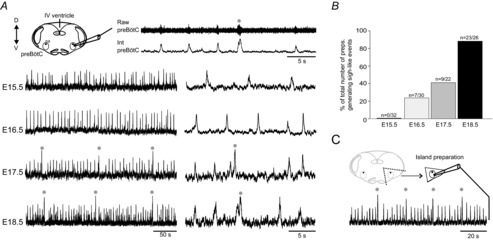
A, top left, schematic representation of the transverse brainstem slice preparation containing the isolated pre-Bötzinger complex (preBötC) from which respiratory network activity was recorded with an extracellular macro-electrode (top right, upper trace) and the raw signal integrated (bottom trace). Bottom, integrated recordings of preBötC activity at different embryonic stages (indicated at left) presented with two different time scales. Grey dots indicate sigh-related activity. B, histograms representing the proportion of preparations generating sigh events at the 4 different embryonic stages examined. C, schematic representation of the island preparation and associated integrated recording of preBötC activity obtained at E18.5. XII, hypoglossal nucleus; na, nucleus ambiguus; D, dorsal; V, ventral.
Cellular basis of embryonic sigh generation
In order to assess whether the separate eupnoeic- and sigh-like activities in the embryo arise from the same or different neuronal assemblages, we first examined the activity of preBötC inspiratory neurons in E18.5 slices using calcium imaging to monitor distributed cell populations within single preparations. Simultaneous optical recordings of multiple individual neurons in the preBötC network on one side were made concurrently with an electrophysiological recording of preBötC population activity on the contralateral side (Fig.2A). Cells exhibiting rhythmically organized fluorescence changes occurring in phase with overall network activity during either eupnoeic and/or sigh-like activities were detected (Fig.2A) and their location was mapped on the surface of the slice (Fig.2B). To cover the entire active region encompassing the preBötC, imaging data were acquired sequentially in two overlapping fields (see Fig.2B, lower panels). No cells outside the preBötC area were found to express either type of bursting, indicating that both inspiratory activities were indeed generated within the preBötC network and thus in accordance with observations made on island preparations (see above). From a total of 338 imaged neurons (from 9 preparations with a mean of 37 neurons per preparation), virtually all cells (336/338; i.e. 99.4%) displayed calcium signals during both eupnoeic and sigh-like burst discharge. Representative examples of the population maps of averaged burst discharges obtained in a single preparation during eupnoeic (n = 8 consecutive bursts) or sigh-like bursts (n = 6) are illustrated in lower left and middle Fig.2B, respectively. When superimposed these two maps show a complete overlap, thus appearing in yellow in the overlay image (right lower panel in Fig.2B). A similar fluorescence map correspondence was found in all nine preparations examined, further indicating that a highly significant proportion of cells were active during both eupnoeic and sigh burst genesis. Although these calcium imaging experiments indicated that both eupnoeic- and sigh-like activities appear to arise from a common neuronal population (with the exception of just two cells that exhibited detectable fluorescence changes during sigh bursts only), it was possible that an inability to reliably discriminate sigh from eupnoeic bursts due to detection limitations resulted in an underestimation of any ‘sigh-only’ cells in our analysis.
Figure 2. Spatial distribution of cells active during eupnoeic and sigh rhythms.
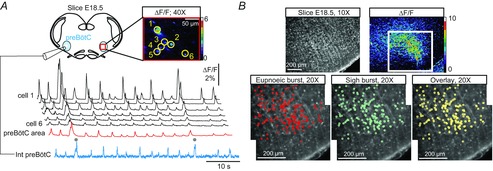
A, top left, schematic representation of the slice preparation from which an electrophysiological recording of preBötC activity on one side was made simultaneously with calcium imaging performed on the contralateral side. Top right, fluorescence image of 6 Ca2+-dye loaded cells displaying concomitant calcium transients (ΔF/F). Bottom, paired recordings of population electrical activity (blue trace) and calcium transients in the same individual neurons (traces 1–6) and in the cell population (spatially averaged signal over the area delimited by the red rectangle in top right) of the monitored preBötC region (red trace). The cells displayed fluorescence changes synchronized to rhythmic electrical activity recorded on the contralateral side during both eupnoea and sigh bursting (indicated by grey dots). B, fluorescence images of an E18.5 slice. Upper left, direct fluorescence; upper right, ΔF/F. The white square delimits the area observed at higher (×20) magnification in the bottom panels showing the distribution of rhythmically active cells during eupnoeic (lower left, red circles) and sigh events (lower middle, cyan circles). When superimposed, the two distribution maps overlap almost entirely indicating that the same cells are active during both eupnoea and sigh (appearing in yellow in the lower right overlay panel).
In a second step, therefore, we investigated the discharge patterns and synaptic inputs associated with eupnoeic- and sigh-like activities at E18.5 using patch-clamp recordings of individual inspiratory neurons. Out of 61 recorded neurons, 57 cells generated bursts of action potentials (APs) during both eupnoeic- and sigh-related events (Fig.3Aa), typically with more intense discharge occurring during the latter. On average, neurons at resting potential fired 13.5 ± 0.5 APs during sigh bursts versus 5.0 ± 0.1 APs during eupnoeic bursts (values obtained from 15 neurons that produced a combined total of 134 and 127 eupnoeic and sigh bursts, respectively). In voltage-clamp mode, we then measured the amplitude of the inward synaptic currents underlying the two types of discharge (Fig.3Ab). Commensurate with their elevated firing rates during sigh-related activity, the amplitude of the overall envelope of synaptic excitation in inspiratory neurons was significantly larger during sigh bursts (mean 80 ± 5 pA; n = 49) compared to eupnoeic bursts (mean 20 ± 2 pA; n = 122).
Figure 3. Membrane potential trajectories and firing properties of inspiratory neurons during eupnoeic and sigh bursting.
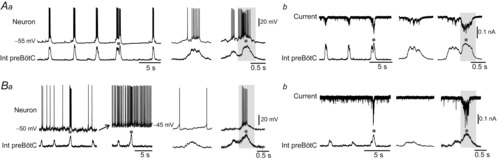
Aa, simultaneous patch-clamp recording from a rhythmic inspiratory neuron (top trace) and integrated population activity (bottom trace) recorded extracellularly from the preBötC network of an E18.5 slice preparation. The cell fired in time with both single-phase eupnoeic and bi-phasic sigh-like bursts (grey dot), as is further evident in the extended time scale presentations at right. Ab, same recorded neuron as in Aa, but in voltage-clamp mode with the cell held at −50 mV. The cell's more intense discharge during sigh bursts was associated with an underlying inward synaptic current that was also larger in amplitude than during eupnoeic bursts (also see faster time-base recording at right). Ba, current-clamp recordings from a different preBötC neuron that expressed bursting activity uniquely during the larger-amplitude sigh events, even when experimentally depolarized with injected current (left panels). Right panels, the cell fired impulse bursts during sigh-like events but not during eupnoeic-like bursts. Bb, same recorded neuron as in Ba, but in voltage-clamp mode with the cell held at −50 mV. Note the absence of any significant synaptic drive to this neuron during eupnoeic activity in contrast to the strong inward synaptic current associated with a sigh burst.
As suggested by our calcium imaging experiments, a small fraction of patch-recorded neurons (4 of 61 cells) was found to express burst activity exclusively during sigh events (Fig.3Ba). The average discharge rate of these sigh-only cells was 9.5 ± 0.7 APs per burst, but otherwise their firing remained completely unrelated to eupnoeic-like activity, even when membrane depolarization by DC current injection was applied to reveal putative subthreshold eupnoea-timed synaptic modulation (Fig.3Ba). Moreover, in voltage-clamp mode, no detectable synaptic currents associated with eupnoeic activity were found in these cells, unlike during sigh bursts when a significant excitatory drive (mean 30 ± 3 pA) occurred (Fig.3Bb). Thus, single-cell electrophysiological recordings indicated that all neurons that fire during eupnoeic bursts are also active during sigh bursts and that the latter's larger amplitude is due to a stronger synaptic drive and resultant increased discharge throughout the inspiratory neuron ensemble. Taken together, therefore, these data strongly indicate that eupnoeic- and sigh-like activities are supported by the same preBötC neuronal population, with a possible contribution of a further very limited subset of cells that are active uniquely during sighs and which may be responsible for triggering sigh activity throughout the entire network.
Involvement of glycinergic inhibition in sigh burst formation
In newborn rodents, blockade of glycinergic synaptic inhibition has previously revealed that the two components of sigh burst activity, a larger-amplitude event that is normally coupled to, and arises from, an initial eupnoeic-like event can be generated independently (Lieske et al. 2000). We therefore wished to determine whether the similarly bi-phasic sigh burst pattern in the embryo is also composed of two separable components with distinct characteristics and subject to inhibitory synaptic control.
Bath-application of strychnine (Stry; 1 μm) to E18.5 slice preparations (n = 7) that were spontaneously generating both eupnoeic- and bi-phasic sigh-like discharge (Fig.4A), did not prevent the generation of the two burst types, which remained distinguishable in terms of their different maximal amplitudes and frequencies (Fig.4B). However, under strychnine the larger-amplitude sigh component no longer emerged from the peak of a foregoing eupnoeic-like component and no bi-phasic bursts could be detected (Fig.4B cf. A). Rather, under strychnine the time interval between the onset of a given large-amplitude burst and that of the immediately preceding eupnoeic-like discharge (T in Fig.4A and B) was significantly longer and more variable, with a mean T value of 3.15 ± 0.40 s (range 0.2–8.2 s; Fig.4B) compared to 0.38 ± 0.02 s (range 0.2–1.4 s) in control (Fig.4A; n = 77 events in Stry and 102 events in control (CTL) from 7 preparations; P = 1.3 × 10−8).
Figure 4. Role of glycinergic synapses in determining the biphasic waveform of sigh bursts.
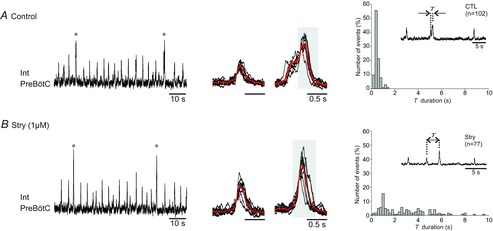
A, left, in vitro recording of spontaneous preBötC activity under control conditions in an E18.5 slice. Grey dots indicate sigh bursts. Middle, superimposed eupnoeic (left; n = 10) and sigh bursts (n = 7) with the corresponding averaged trace (in red). Note the two distinct phases of the sigh bursts. Right, histograms showing the distribution of the time intervals (T) separating the two components of sigh bursts measured from 102 events. Inset: T was defined as the interval between the large-amplitude component of a sigh burst and the immediately preceding eupnoeic-like component. B, same figure layout as in A after blockade of glycinergic synapses with 1 μm strychnine. The sigh bursts were now mono-phasic since they were decoupled from the smaller-amplitude eupnoeic-like bursts and consequently the T values were widely distributed.
The implication of glycinergic synapses in determining the relative timing of embryonic sigh bursts occurs during a developmental period when chloride-dependent synaptic transmission within preBötC circuitry undergoes substantial maturational changes, including a transition in postsynaptic action from excitation to inhibition (when ECl becomes negative relative to resting membrane potential; Ren & Greer, 2006). We therefore asked whether the typical bi-phasic sigh burst pattern might only be expressed when Cl−-mediated synaptic signalling had undergone this age-dependent switch, the timing of which can be inferred from changes in responsiveness to glycine receptor activation/inactivation (Ren & Greer, 2006). Our present results from the embryonic mouse, where we compared the inactivation of glycine receptors in developmental stages E16.5 and E18.5 (i.e. before and after the inception of sigh genesis, respectively), showed distinct effects on the frequency of spontaneous preBötC activity. Whereas strychnine application had no significant effect (P = 0.92) on rhythm rate in E16.5 slices (n = 12; mean value 12.4 ± 1.2 bursts min−1 in control vs. 12.5 ± 1.3 bursts min−1 in strychnine), at E18.5, the cycle frequency was significantly increased by 35 ± 8% (n = 15; mean value 19.3 ± 2.2 bursts min−1; P = 6 × 10−4) from a control mean value of 14.5 ± 1.6 bursts min−1. Thus the impact of glycine receptor blockade before and after sigh emergence corresponded either to a developmental change in inhibitory receptor function during this period or to an increased involvement of glycinergic neurons in respiratory rhythmogenesis.
In a further test of the relationship between the emergence of the bi-phasic sigh burst pattern and the maturation of Cl−-mediated synaptic signalling, we examined the preBötC network operation of transgenic animals knocked-down for the chloride co-transporter KCC2 (responsible for neuronal Cl− extrusion), thereby exhibiting an abnormal chloride gradient maturation (Woo et al. 2002; Stil et al. 2011). Initial control immunostaining for KCC2 confirmed that the co-transporter was widely expressed in the preBötC of wild-type embryos at E18.5 (Fig.5A), but was virtually absent in the same region from KCC2 knock-down embryos (Fig.5B). Moreover, whereas bursts with two distinct amplitudes were evident in recordings from both wild-type (n = 6) and mutant (n = 6) littermate preparations, in the latter, only monophasic (Fig.5B) rather than bi-phasic (Fig.5A) sigh-like burst events were observed. Correspondingly, in the mutant the delay between the large-amplitude bursts and each immediately preceding smaller, eupnoeic-like burst was now highly variable (with a mean T value of 1.92 ± 0.26 s, range 0.4–4.8 s; n = 65 bursts; Fig.5B, right) in a manner similar to the T value dispersion observed in wild-type preparations under glycinergic synaptic blockade (cf. Fig.4B, right).
Figure 5. KCC2 knock-down embryos generate sigh bursts uncoupled from a preceding eupnoeic-like burst.
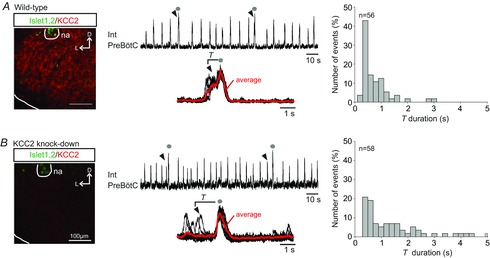
A, left, expression of KCC2 (red) in the preBötC region (located ventral to the motoneurons of the nucleus ambiguus (na) that express Islet1,2 (green) immunostaining) of a preparation from a wild-type embryo at E18.5. D, dorsal; L, lateral. Middle, integrated trace of preBötC activity displaying eupnoeic and sigh bursts (indicated with grey dots). Below are typical bi-phasic sigh bursts superimposed (n = 7) with averaged trace (in red). Arrowheads indicate the eupnoeic burst immediately preceding the larger-amplitude sigh burst. Right, distribution of the time intervals (T) separating the two burst components of 56 sigh events. B, same layout as in A for a KCC2 knock-down embryo. The absence of KCC2 expression in the preBötC area was verified by immunostaining (left). In these mutants, large-amplitude sigh bursts (grey dots) were now monophasic with a wider distribution of T values measured from 58 events.
Altogether these data show that chloride-mediated signalling at glycinergic synapses plays an important role in governing the relative timing of inspiratory bursts within the embryonic preBötC network and underline the importance of their maturation to the inception of proper sigh burst production.
Appropriate chloride-mediated signalling maturation is required for bi-phasic sigh burst generation
In a small number of E16.5 (n = 9) and E18.5 (n = 6) preparations, independent eupnoeic-like and large-amplitude monophasic bursts were generated spontaneously but with no apparent temporal relationship (Fig.6A). In these cases, therefore, the time intervals between large- and preceding small-amplitude bursts showed a large variability and high mean value (2.9 ± 0.3 s; Fig.6A; cf. Fig.4A). In reasoning that an incomplete chloride gradient maturation that remained incompatible with inhibitory synaptic signalling might be responsible for the temporal decoupling of the two burst types, we performed a rescue experiment in which bumetanide (20 μm), a blocker of the NKCC1 chloride co-transporter that mediates chloride entry into cells (Payne et al. 2003), was bath-applied to these slices. In preparations from E16.5 embryos, the presence of bumetanide had no evident effect on the distribution of the intervals between large and eupnoeic-like bursts, with the mean T (3.2 ± 0.2 s) remaining similar to the control value (Fig.6B cf. A). Thus, impairing NKCC1 function at this developmental stage was unable to induce bi-phasic burst expression via an enhancement of temporal coupling. In contrast, in E18.5 preparations that were spontaneously generating dissociated bursts (Fig.6C), the same bumetanide treatment led to bi-phasic burst production in all (6/6) cases, characterized by diminished and less variable T values to 1.1 ± 0.1 s under the antagonist (Fig.6D) from 5.7 ± 0.6 s in control (Fig.6C). In these experiments, therefore, blocking the NKCC1 co-transporter at E18.5 significantly augmented the coordination between large and small-amplitude burst pairs, a finding consistent with the hypothesis that mature chloride-mediated inhibitory signalling is required for the preBötC network to generate normal bi-phasic sigh bursting.
Figure 6. Blockade of the cation–chloride co-transporter NKCC1 increases the phase-coupling between the two sigh burst components at E18.5 but not at E16.5.
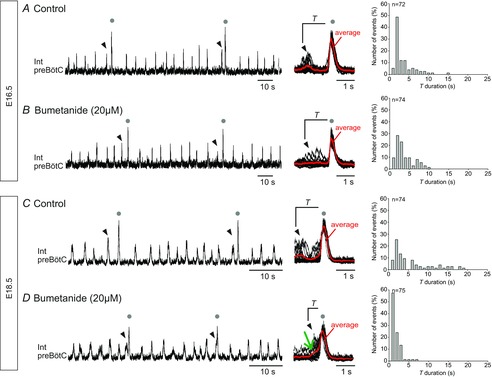
A, left, recording of preBötC activity in an E16.5 slice preparation that spontaneously generated independent small- (eupnoeic) and large-amplitude (sigh, grey dots) bursts. The arrowheads indicate the eupnoeic burst immediately preceding each large-amplitude sigh burst. Middle, superimposed large-amplitude bursts (n = 14) and averaged trace in red showing their mono-phasic waveform. Right, histograms showing the wide distribution of associated T values (from 72 bursts). B, left, in the presence of the NKCC1 blocker bumetanide (20 μm), the same preparation continued to generate large-amplitude sigh bursts that occurred independently of eupnoeic bursts. Middle, superimposed bursts (n = 15) and averaged trace (red trace, as in A) showing the still mono-phasic waveform of large-amplitude bursts (n = 74). C and D, same figure layout and description as in A and B, respectively, for an E18.5 slice preparation. At this developmental stage, bumetanide's presence facilitated the expression of bi-phasic large-amplitude bursts (green arrow indicates the initial eupnoeic-like component in the averaged red trace at middle, n = 20 bursts) that resembled typical biphasic sigh activity. The tighter phase-coupling between the large- and preceding small-amplitude components resulted in a narrower range of T value distributions (right).
Discussion
Here, we report that the preBötC network in the prenatal mouse is capable of generating two distinct inspiratory-related motor rhythms: one corresponding to normal respiratory activity (eupnoea-like), generated from the time (E15.5) when the preBötC first becomes active (Thoby-Brisson et al. 2005), and a second rhythm that emerges at E18.5 and corresponds to the augmented inspiratory bursting that typifies sigh genesis. We also found that the later inception of sigh-related activity is dependent on the establishment of effective synaptic interactions involving inhibitory glycinergic neurotransmission.
Developmental aspects
To our knowledge the present study provides the first description of the developmental onset of sigh rhythmogenesis in mammals. One significant finding is that although the eupnoeic and sigh rhythms are tightly linked and co-expressed from birth and throughout life, they emerge and mature at different developmental times during embryogenesis. Our data also indicate that the emergence of sigh rhythm production requires the maturation of network synaptic properties and in particular of glycinergic synaptic transmission associated with a mature chloride gradient. Interestingly, the period of rodent embryonic development when the preBötC network normally acquires the ability to generate sighs corresponds closely to the time when chloride-mediated conductances switch from depolarizing (excitatory) to hyperpolarizing (inhibitory) synaptic actions (Ren & Greer, 2006). Thus, the capacity for generating different inspiratory-related rhythmic activities is likely to depend upon maturational changes in synaptic properties within the preBötC network itself, this process being most probably accompanied by a maturation of neuronal membrane properties required for the large-amplitude inspiratory burst generation (Lieske & Ramirez, 2006; Koch et al. 2013).
The mechanism(s) by which glycinergic synapses achieve the phase-coupling between the two components of sigh bursts remains unclear. The predominant role of synaptic inhibition in coordinating neuronal bursting in rhythmogenic motor networks is well established (Getting, 1989; Marder & Calabrese, 1996; Grillner, 2006). Moreover, both theoretical and experimental studies have demonstrated that inhibitory connections between neurons may achieve stable network operation through the promotion of synchronized rather than desynchronized bursting (Van Vreeswijk et al. 1994; Elson et al. 2002; Belykh et al. 2010). Such an inhibitory in-phase coupling process involving glycine synapses may underlie the closely overlapping coordination between the two components of sigh-related discharge, and in turn would explain their temporal dissociation in response to glycine receptor inactivation. A further possibility, at least in theory, could involve a hitherto unidentified neuronal assemblage within the preBötC region that decouples the small- and large-amplitude sigh burst components but which is normally inhibited by glycinergic inputs in control conditions. Although distinguishing between such possibilities awaits further experimentation, nevertheless our present results highlight the crucial role played by glycinergic neurons, potentially including pacemaker ones (Morgado-Valle et al. 2010) in sigh rhythmogenesis and is in accordance with the recently proposed importance of this cell type in controlling respiratory rhythm generation and inspiratory burst amplitude in newborn rodents (N. Koshiya, H. Koizumi, R. Zhang & J. Smith, unpublished communication).
Same or separate circuits for the genesis of distinct respiratory activities
In the newborn mouse preBötC, previous studies have strongly suggested that both eupnoea and sigh generation arise from within a single neuronal population: stereotaxic mapping of rhythmic cell distributions, intracellular recordings and calcium imaging revealed that almost all neurons active during eupnoeic rhythmicity were also active during sigh bursting (Lieske et al. 2000; Ruangkittisakul et al. 2008). However, despite constituting a very limited population (13/265 neurons; <5%), another cell type that was active exclusively during sigh bursts was also identified in the postnatal preBötC (Tryba et al. 2008). We also found an equivalent sigh-only-type neuron in the embryo, here again representing an extremely small population (4/61 patch-recorded neurons). Due to its rarity, the precise role of this very limited cell population in sigh production remains unclear, although it has been recently reported that the activation of only a few neurons in the preBötC can be sufficient to trigger a full population burst and thus drastically affect network activity (Kam et al. 2013). Therefore it remains plausible that the activity of a restricted sigh-only neuron subset within the preBötC serves as a major determinant of sigh production.
Moreover, since excitatory glutamatergic connections are widespread in the preBötC network (Funk et al. 1993; Rekling & Feldman, 1998; Wallen-Mackenzie et al. 2006), it is likely that the cell populations responsible for the two types of inspiratory activity interact during rhythmogenesis. Consequently, it is possible that sigh-only neurons receive rhythmic excitatory inputs from other inspiratory neurons during eupnoeic bursts, thereby effectively causing them to discharge in time with both rhythms. Similarly, inspiratory neurons exclusively active during eupnoeic bursts would be difficult to distinguish as they could in turn receive an excitatory drive from the sigh-only neuron population. Therefore, distinguishing the separate cell populations responsible for the two inspiratory-related activities is rendered difficult, although such an extensive overlap could at least partially explain how two different burst types are apparently generated within a single network. It is interesting in this context that pacemaker inspiratory neurons that are capable of expressing both endogenous eupnoeic and sigh-like bursting properties have been identified in the newborn mouse (Tryba et al. 2008), although whether such bimodal pacemakers already exist and contribute to sigh generation at the prenatal stage remains to be determined. In any case, our present data indicate that while eupnoea and sigh activities arise mostly from the same neuronal population, there also exists a subset of inspiratory neurons that are probably endowed with membrane and synaptic properties specialized for promoting sigh burst production.
It is also significant that under certain natural and experimental conditions the two inspiratory rhythms can be expressed independently. Specifically, we observed (i) eupnoea in the absence of sighs prior to E17.5, (ii) eupnoea and sighs without any phase relationship under strychnine, in KCC2 mutants and in some immature preparations at E16.5 and E18.5. Note that in these latter conditions, because of the monophasic form of these large-amplitude bursts they could represent a further type of sigh-related activity that is probably expressed more frequently in embryos than newborn rodents. Therefore, the two rhythms are not mutually interdependent, but when co-expressed, their strict temporal coupling must result from network synaptic wiring and from the fact that there are neurons that participate in both activities. The functional advantage for an augmented inspiratory (sigh) effort being normally immediately preceded by a smaller eupnoeic event remains unclear, although one possibility is that the latter acts as a priming stimulus for the secondary component of the biphasic sigh pattern (Thach & Taeusch, 1976; but also see Wulbrand et al. 2008).
In conclusion, our data show that sigh-like rhythmic activity can be generated by the mouse preBötC at embryonic stages and that different types of inspiratory activities emerge sequentially during fetal development. Gaining insights into sigh-generating mechanisms as they become established prenatally has potential clinical relevance since premature babies often suffer from ARDS associated with an immaturity of the respiratory centres and lungs (Di Fiore et al. 2013). Thus, a better understanding of the cellular and network processes underlying sigh expression and its developmental emergence might help to improve therapeutical approaches to countering disorders such as ARDS.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale to M.T.-B., Société de Pneumologie de Langue Française (SPLF) to M.T.-B., Fond de Recherche sur la Santé Respiratoire to C.C. and the Agence Nationale de la Recherche (ANR12-BSV4-0011-01) to M.T.-B.
Acknowledgments
We thank Drs Laurent Vinay and Jean-Charles Viemari for providing the KCC2−/− pregnant mice.
Glossary
- aCSF
artificial cerebrospinal fluid
- AP
action potential
- ARDS
acute respiratory distress syndrome
- E
embryonic day
- preBötC
pre-Bötzinger complex
- SIDS
sudden infant death syndrome
- Stry
strychnine
Additional information
Competing interests
The authors declare no competing financial interests.
Author contributions
Experiments were carried out in INCIA (Bordeaux) and N&D (Gif sur Yvette). Conception and design of the experiments: M.T.-B. Collection, analysis and interpretation of data: C.C., S. A. and M.T.-B. G.F., J.S. and M.T.-B. wrote the paper. All the authors have read and approved the final submission.
Key points
The respiratory oscillator of the pre-Bötzinger complex (preBötC) can generate distinct inspiratory motor patterns underlying eupnoeic and sigh-related rhythmic activities.
The preBötC can generate ‘fictive’ eupnoea at embryonic stages, but its ability to also generate sigh-like activity remains unexplored at prenatal stages.
Here, using mouse brainstem slice preparations, we show that sigh-like activity emerges during embryonic development but later than eupnoeic rhythmogenesis. Inspiratory cells active during the latter are also active during fictive sighing, although a small subset of neurons was found to fire exclusively during sighs. Effective glycinergic inhibitory signalling is also required for sigh generation.
We conclude that the developmental emergence of a sigh-generating capability occurs after the onset of eupnoeic rhythmogenesis and requires an appropriate maturational state of chloride-mediated glycinergic synaptic transmission.
References
- Bell HJ, Ferguson C, Kehoe V. Haouzi P. Hypocapnia increases the prevalence of hypoxia-induced augmented breaths. Am J Physiol Regul Integr Comp Physiol. 2009;296:R334–R344. doi: 10.1152/ajpregu.90680.2008. [DOI] [PubMed] [Google Scholar]
- Belykh I, Jalil S. Shilnikov A. Burst-duration mechanism of in-phase bursting in inhibitory networks. Regul Chaotic Dyn. 2010;15:148–160. [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A. Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, von Euler C, Glogowska M. Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol Scand. 1981;111:349–360. doi: 10.1111/j.1748-1716.1981.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Davis GM. Moscato J. Changes in lung mechanics following sighs in premature newborns without lung disease. Pediatr Pulmonol. 1994;17:26–30. doi: 10.1002/ppul.1950170106. [DOI] [PubMed] [Google Scholar]
- Di Fiore JM, Martin RJ. Gauda EB. Apnea of prematurity – Perfect storm. Respir Physiol Neurobiol. 2013;189:213–222. doi: 10.1016/j.resp.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson RC, Selverston AI, Abarbanel HD. Rabinovich MI. Inhibitory synchronization of bursting in biological neurons: dependence on synaptic time constant. J Neurophysiol. 2002;88:1166–1176. doi: 10.1152/jn.2002.88.3.1166. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco P, Verheulpen D, Valente F, Kelmanson I, de Broca A, Scaillet S, Groswasser J. Kahn A. Autonomic responses to sighs in healthy infants and in victims of sudden infant death. Sleep Med. 2003;4:569–577. doi: 10.1016/s1389-9457(03)00107-2. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC. Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR. Feldman JL. Normal breathing requires preBotzinger complex neurokinin–1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Hummler H, Gerhardt T, Gonzalez A, Claure N, Everett R. Bancalari E. Increased incidence of sighs (augmented inspiratory efforts) during synchronized intermittent mandatory ventilation (SIMV) in preterm neonates. Pediatr Pulmonol. 1997;24:195–203. doi: 10.1002/(sici)1099-0496(199709)24:3<195::aid-ppul5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N. Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: The pre-Bötzinger complex ‘island’. J Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Kam K, Worrell JW, Ventalon C, Emiliani V. Feldman JL. Emergence of population bursts from simultaneous activation of small subsets of preBötzinger complex inspiratory neurons. J Neurosci. 2013;33:3332–3338. doi: 10.1523/JNEUROSCI.4574-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, Zanella S, Elsen GE, Smith L, Doi A, Garcia AJ, 3rd, Wei AD, Xun R, Kirsch S, Gomez CM, Hevner RF. Ramirez JM. Stable respiratory activity requires both P/Q-type and N-type voltage-gated calcium channels. J Neurosci. 2013;33:3633–3645. doi: 10.1523/JNEUROSCI.6390-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske SP. Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J Neurophysiol. 2006;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P. Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lijowska AS, Reed NW, Chiodini BA. Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J Appl Physiol. 1997;83:219–228. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA. Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara F, Lijowska AS. Thach BT. Spontaneous arousal activity in infants during NREM and REM sleep. J Physiol. 2002;538:263–269. doi: 10.1113/jphysiol.2001.012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Mellen NM. Tuong CM. Semi-automated region of interest generation for the analysis of optically recorded neuronal activity. Neuroimage. 2009;47:1331–1340. doi: 10.1016/j.neuroimage.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM. Feldman JL. Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas TE, Power JH. Barr HA. The pulmonary consequences of a deep breath. Respir Physiol. 1982;49:315–324. doi: 10.1016/0034-5687(82)90119-0. [DOI] [PubMed] [Google Scholar]
- Orem J. Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol. 1993;74:761–769. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Ren J. Greer JJ. Ontogeny of the pre-Bötzinger complex in perinatal rats. J Neurosci. 2003;23:9575–9584. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC. St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Patroniti N, Foti G, Cortinovis B, Maggioni E, Bigatello LM, Cereda M. Pesenti A. Sigh improves gas exchange and lung volume in patients with acute respiratory distress syndrome undergoing pressure support ventilation. Anesthesiology. 2002;96:788–794. doi: 10.1097/00000542-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J. Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK. Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Poets CF, Rau GA, Neuber K, Gappa M. Seidenberg J. Determinants of lung volume in spontaneously breathing preterm infants. Am J Respir Crit Care Med. 1997;155:649–653. doi: 10.1164/ajrccm.155.2.9032208. [DOI] [PubMed] [Google Scholar]
- Qureshi M, Khalil M, Kwiatkowski K. Alvaro RE. Morphology of sighs and their role in the control of breathing in preterm infants, term infants and adults. Neonatology. 2009;96:43–49. doi: 10.1159/000201738. [DOI] [PubMed] [Google Scholar]
- Rekling JC. Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Ren J. Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci. 2006;26:3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD. Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Panaitescu B. Ballanyi K. K+ and Ca2+ dependence of inspiratory-related rhythm in novel “calibrated” mouse brainstem slices. Respir Physiol Neurobiol. 2011;175:37–48. doi: 10.1016/j.resp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW. Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PSG, Grillner S, Selverston A, editors; Stuart DG, editor. Neurons, Networks and Motor Behaviors. Cambridge, MA, USA: MIT Press; 1997. [Google Scholar]
- Stil A, Jean-Xavier C, Liabeuf S, Brocard C, Delpire E, Vinay L. Viemari JC. Contribution of the potassium-chloride co-transporter KCC2 to the modulation of lumbar spinal networks in mice. Eur J Neurosci. 2011;33:1212–1222. doi: 10.1111/j.1460-9568.2010.07592.x. [DOI] [PubMed] [Google Scholar]
- Sürmeli-Onay O, Korkmaz A, Yiğit S. Yurdakök M. Surfactant therapy in late preterm infants: respiratory distress syndrome and beyond. Turk J Pediatr. 2012;54:239–246. [PubMed] [Google Scholar]
- Takeda M. Matsumoto S. Discharge patterns of dorsal and ventral respiratory group neurons during spontaneous augmented breaths observed in pentobarbital anesthetized rats. Brain Res. 1997;749:95–100. doi: 10.1016/s0006-8993(96)01168-7. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM. Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT. Lijowska A. Arousals in infants. Sleep. 1996;19:S271–273. doi: 10.1093/sleep/19.suppl_10.s271. [DOI] [PubMed] [Google Scholar]
- Thach BT. Taeusch HW., Jr Sighing in newborn human infants: role of inflation-augmenting reflex. J Appl Physiol. 1976;41:502–507. doi: 10.1152/jappl.1976.41.4.502. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J. Fortin G. Emergence of the pre-Bötzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M. Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vreeswijk C, Abbott LF. Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, Fujiyama F, Fortin G. Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NS, Lu J, England R, McClellan R, Dufour S, Mount DB, Deutch AY, Lovinger DM. Delpire E. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K–Cl cotransporter gene. Hippocampus. 2002;12:258–268. doi: 10.1002/hipo.10014. [DOI] [PubMed] [Google Scholar]
- Wulbrand H, McNamara F. Thach BT. The role of arousal related brainstem reflexes in causing recovery from upper airway occlusion in infants. Sleep. 2008;31:833–840. doi: 10.1093/sleep/31.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]


