Abstract
Melanin-concentrating hormone (MCH)-producing neurons are known to regulate a wide variety of physiological functions such as feeding, metabolism, anxiety and depression, and reward. Recent studies have revealed that MCH neurons receive projections from several wake-promoting brain regions and are integral to the regulation of rapid eye movement (REM) sleep. Here, we provide evidence in both rats and mice that MCH neurons express histamine-3 receptors (H3R), but not histamine-1 (H1R) or histamine-2 (H2R) receptors. Electrophysiological recordings in brain slices from a novel line of transgenic mice that specifically express the reporter ZsGreen in MCH neurons show that histamine strongly inhibits MCH neurons, an effect which is TTX insensitive, and blocked by the intracellular presence of GDP-β-S. A specific H3R agonist, α-methylhistamine, mimicks the inhibitory effects of histamine, and a specific neutral H3R antagonist, VUF 5681, blocks this effect. Tertiapin Q (TPQ), a G protein-dependent inwardly rectifying potassium (GIRK) channel inhibitor, abolishes histaminergic inhibition of MCH neurons. These results indicate that histamine directly inhibits MCH neurons through H3R by activating GIRK channels and suggest that that inhibition of the MCH system by wake-active histaminergic neurons may be responsible for silencing MCH neurons during wakefulness and thus may be directly involved in the regulation of sleep and arousal.
Introduction
Melanin-concentrating hormone (MCH) is a 19 amino acid cyclic neuropeptide first isolated in teleost fishes and amphibians. In mammals, MCH is expressed almost exclusively in the lateral hypothalamus (LH) and zona incerta (ZI) but projects broadly throughout the central nervous system (Bittencourt et al. 1992). In rodents, MCH activates one G protein-coupled receptor, MCHR1, which is expressed widely in the CNS (Saito et al. 2001).
The MCH system is known to modulate feeding and energy homeostasis, but is also involved in other functions such as metabolism, anxiety and depression, reward, and learning and memory (Monzon et al. 1999; Borowsky et al. 2002; Marsh et al. 2002; Adamantidis et al. 2005; Chaki et al. 2005; Chung et al. 2009; Garcia-Fuster et al. 2012). Of particular interest to the present study is its role in arousal and sleep. Central administration of MCH increases both rapid-eye movement (REM) (also known as paradoxical sleep) and non-REM (NREM or slow wave sleep (SWS)) sleep (Verret et al. 2003; Lagos et al. 2009; Torterolo et al. 2009). MCHR1 antagonists have been reported to cause sleep disruption (Chung et al. 2011a). MCH neurons are active during REM, but not during NREM sleep or wakefulness (Verret et al. 2003; Hassani et al. 2009), and, finally, it has recently been demonstrated that activation of MCH neurons promotes REM sleep (Jego et al. 2013). Most MCH neurons are GABAergic (Gao, 2009) and project to wake-promoting brain regions (Peyron et al. 2009; Del Cid-Pellitero & Jones, 2012), where they have been proposed to exert inhibitory effects. Taken together, these reports indicate that MCH promotes the maintenance of REM sleep (Peyron et al. 2009) through a mechanism that is not understood.
Despite the potential clinical relevance of the MCH system, much is unknown about how MCH neurons are regulated (Abbott et al. 2003) and which neurotransmitters regulate them (Gao, 2009). Histamine and MCH both regulate sleep, and several lines of indirect evidence suggest that histamine may regulate MCH neurons. Histaminergic neurons are active in a pattern reciprocal to that of MCH neurons as they are active during wakefulness, particularly during periods of heightened arousal, and are silent during sleep (Haas et al. 2008). They provide excitatory input to arousal-promoting brain regions active during wakefulness (Haas et al. 2008) and are critical mediators of arousal. Histamine-expressing neurons, located in the tuberomammillary nucleus (TMN) of the posterior hypothalamus (Haas et al. 2008), are also known to project to the LH and ZI cells of unknown identity (Eriksson et al. 2001) and one histamine receptor, the histamine-3 receptor (H3R), is expressed at high levels in the LH and ZI (Lintunen et al. 1998).
Because H3R is expressed in the same brain regions as MCH neurons and because histamine-expressing neurons project to the LH, we hypothesized that histaminergic transmission regulates the MCH system. We therefore investigated whether MCH neurons express histamine receptors using in situ hybridization, and then tested whether histamine affects the activity of MCH neurons by combining pharmacology and electrophysiological recording in brain slices from transgenic mice that selectively express the fluorescent reporter ZsGreen in MCH neurons.
Methods
Animals
Brains collected from adult male Sprague–Dawley rats (Charles River, Wilmington, MA, USA 200–400 g) or adult male C57/Bl6 mice (NCI, Rockville, MD, USA) were used for in situ hybridization experiments. Animals were anesthetized with CO2 and decapitated. Brains were then frozen in −20°C isopentane and stored at −80°C until ready to be sectioned. For electrophysiology experiments, hemizygous Tg(Pmch-cre)1Lowl/J (Jackson Laboratories, Bar Harbor, Maine, USA) mice that express Cre-recombinase (Cre) in MCH neurons, originally created in the laboratory of Dr Bradley Lowell (Kong et al. 2010), were crossed with homozygous Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J (Madisen et al. 2010; Jackson Laboratories) to create mice that express the fluorescent reporter ZsGreen in MCH-expressing neurons (referred to as ‘pMCH-cre/ZsGreen mice’). Mice used in electrophysiology experiments were 16–21 days old. Animals were group housed under controlled conditions (temperature 21°C ± 2°C; 12 h light–dark cycle, lights on at 06:00 h) with free access to water and food. All animal procedures were approved by the Institutional Animal Care and Use Committee, University of California, Irvine.
Probes and cRNA synthesis
The complete open reading frames (ORFs) of the rat MCH precursor (pMCH), mouse histamine receptor–1 (H1R), and mouse histamine receptor–2 (H2R) were cloned into the pBluescript vector. A 411 bp fragment of rat histamine receptor-3 (H3R) cloned into pBluescript with BamH1 and HindIII was generously provided by Dr Rainer Reinscheid (UC Irvine).
The MCH precursor probe was digested with Sma1 or HindIII and phenol–chloroform precipitated prior to synthesis. Antisense or sense RNA was generated using T7 and T3 polymerase, respectively, and labelled with digoxigenin (DIG) using a DIG RNA labelling kit (Roche Applied Science, Indianapolis, IN, USA). For double in situ hybridization, antisense probes for H1R, H2R and H3R were generated using EcoR1 for H1R and H2R, or BamHI for H3R and T7 RNA polymerase (Promega, Madison, WI, USA), while sense probes were generated with EcoR1 for H1R and H2R, or HindIII for H3R and T3 RNA polymerase (Promega). Transcription reactions were performed in the presence of 35S-UTP (Perkin Elmer, Waltham, MA). All probes were separated from unincorporated nucleotides using Sephadex G-50 columns (Roche Applied Science).
In situ hybridization
Hybridization was performed as previously described with minor modifications (Clark et al. 2001). Prehybridized slides were removed from −20°C and warmed to room temperature. Next, slides were incubated at 60°C for 16–20 h with 0.5 ng ml−1 pMCH-DIG-labelled antisense probe mixed with 2 × 107 c.p.m. ml−1 of a 35S-UTP-labelled sense or antisense radiolabelled probe for one of the above-mentioned histamine receptors in hybridization buffer (50% formamide, 10% dextran sulfate, 0.3 m NaCl, 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin (BSA), 0.02% Ficoll, 10 mm dithiothreitol (DTT), 10 mm Tris, pH 8.0, 1 mm EDTA, pH 8.0, and 500 μg ml−1 tRNA). After hybridization, sections were treated with 20 μg ml−1 RNase A for 30 min at 37°C and washed in decreasing salinity (from 2 × to 0.1 × saline–sodium citrate buffer (SSC), 5–10 min each), followed by a 30 min incubation in 0.1 × SSC at 68°C and a brief rinse in room temperature 0.1 × SSC. Next, sections were rinsed in Genius Buffer 1 (GB1; 100 mm Tris-HCl, 150 mm NaCl, pH 7.5) briefly and then incubated in blocking buffer (GB1 + 5% non-fat dry milk and 0.25% Triton X-100) for 30–60 min at room temperature. Slides were then incubated at 37°C for 3 h in blocking buffer with 1 ml alkaline phosphatase-conjugated sheep anti-DIG antibody (Roche Diagnostics, Indianapolis, IN, USA) diluted 1:7500. Sections were then washed with GB1, and treated with 5-bromo-4-chloro-3-indolyl phosphate (0.09 mg ml−1) and nitroblue tetrazolium salt (NBT, 0.25 mg ml−1) for 20–60 min at room temperature while shielded from light. Sections were washed, dehydrated in ascending concentrations of ethanol, air dried, and exposed to β-Max film (Kodak, Rochester, NY, USA) for up to 7 days to determine signal intensity. Slides were then dipped in 0.3% Parlodion dissolved in isoamylacetate and dried overnight. Sections were then dipped in NTB emulsion (Kodak) and stored in the dark at 4°C for 4–8 weeks before development with D-19 (Kodak) followed by fixative. For images of in situ hybriziation experiments, brightness and contrast were adjusted using Adobe Photoshop and the image was sharpened to allow for easier visualization of silver grains.
Immunohistochemistry
After the animals were deeply anaesthetized with isoflurane, the chest was opened to expose the heart. Then pMCH-cre/ZsGreen mice were transcardially perfused with 0.9% saline followed by chilled 4% paraformaldehyde in 0.2 m Sorensen's phosphate buffer. The brains were removed rapidly from the cranium and postfixed overnight in 4% paraformaldehyde. Brains were then transferred to 20% sucrose in a 0.1 m phosphate buffer solution, pH 7.5 and stored at 4°C until sectioning (Sinchak & Micevych, 2001). Coronal brain sections (20 μm) through the lateral hypothalamus were cut on a cryostat and collected into wells containing phosphate-buffered saline (PBS, pH 7.5). MCH neurons were visualized using rabbit polyclonal anti-MCH antibody (antibody courtesy of W. Vale, Salk Institute, La Jolla, CA, USA). Sections were washed in PBS 3 times for 5 min and then placed into PBS containing 10% methanol and 3% hydrogen peroxide for 10 min to block endogenous peroxidases. Sections were washed in PBS 3 times for 5 min each and then placed in blocking buffer composed of PBS containing 1% normal goat serum (NGS), 1% BSA and 0.5% Triton X-100 for 60 min. The sections were then incubated for 2 days at 4°C in a solution containing MCH antibody diluted (1:150,000) in PBS blocking buffer. Sections were washed in PBS and then in Tris-buffered saline (TBS) twice each for 10 min. Slides were blocked for 30 min in 1% NGS, 0.17% Triton X-100 in TBS, and then incubated in goat anti-rabbit Alexa Fluor 647 for 2 h (Life Technologies). Sections were then washed 3 times for 10 min each in TNT buffer (0.1 m Tris-HCl, pH 7.5, 0.15 m NaCl, 0.05% Tween 20) to wash off unbound secondary antibody. The sections were washed in 0.1 m Tris, pH 7.5 and then mounted on Superfrost Plus slides (Fisher Scientific, Waltham, MA, USA). Slides were dried on a slide warmer (37°C) for 15 min and coverslipped using ProLong Gold Antifade Reagent (Life Technologies, Carlsbad, CA, USA) anti-fade mounting medium and allowed to dry in the dark. Slides were stored at −20°C until pictures were taken (Axiovert 200M equipped with an Axiocam, Zeiss, Oberkochen, Germany). No antibody amplification was necessary to visualize ZsGreen signal. MCH and ZsGreen colocalization was analysed in coronal sections through the rostrocaudal extent of the lateral hypothalamus. Control sections from Cre recombinase (Cre) negative littermates were also tested and showed positive immunoreactivity for MCH but no ZsGreen signal.
Electrophysiology
To prepare living brain slices, mice (postnatal day 16–21) were deeply anaesthetized with pentobarbital sodium (>100 mg kg−1, i.p.), rapidly decapitated and their brains removed. For electrophysiological experiments, 350 μm-thick coronal slices were cut with a vibratome (VT1200S, Leica Systems, Wetzlar, Germany) in sucrose-containing artificial cerebrospinal fluid (ACSF; in mm: 85 NaCl, 75 sucrose, 2.5 KCl, 25 glucose, 1.25 NaH2PO4, 4 MgCl2, 0.5 CaCl2 and 24 NaHCO3) oxygenated with 95% O2, 5% CO2. Slices were first incubated in sucrose-containing ACSF for 30 min to 1 h at 32°C.
Our overall system of electrophysiological recordings and imaging has been described previously (Xu et al. 2010). Brain slices were transferred to the recording chamber with perfusing ACSF (in mm: 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, and 10 glucose) at 2 ml min−1 oxygenated with 95% O2, 5% CO2. To perform whole-cell recording, individual MCH neurons were visualized and selected at high magnification (×60 objective) via ZsGreen expression, and were patched under differential interference contrast optics with glass electrodes of 4–6 MΩ resistance that were filled with an internal solution containing (in mm) 126 potassium gluconate, 4 KCl, 10 Hepes, 4 ATP-Mg, 0.3 GTP-Na, and 10 phosphocreatine (pH 7.2, 300–305 mosmol l–1). The internal solution also contained 0.1% biocytin for cell labelling and post hoc morphological identification. Only neurons usually with initial resting potentials more negative than −55 mV and with good access resistance (<30 MΩ) were used. Once stable whole-cell recordings were achieved, basic electrophysiological properties were examined through a series of hyperpolarizing and depolarizing current injections with a duration of 500 ms or 1000 ms. Data were acquired with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), data acquisition boards (National Instruments, Austin, TX, USA), and a custom-modified version of Ephus software (Ephus, available at https://openwiki.janelia.org/). Data were digitized at 10 kHz, and stored on a computer. All physiological traces were processed using custom-made software in MATLAB (MathWorks, Natick, MA, USA). We focused on analysing neuronal resting membrane potentials, rheobases (minimum electric current amplitudes that result in action potentials), and spike rates at different steps of depolarizing current pulses in control ACSF and in the presence of pharmacological compounds. Spike shape analysis (Table1) was conducted as previously described (Xu et al. 2006). Histamine, the specific H3R agonist α-methylhistamine, the selective H3R neutral antagonist VUF 5681, 2–pyridylethylamine dihydrochloride (H1R agonist) and amthamine dihydrobromide (H2R agonist), the GIRK channel inhibitor Tertiapin Q (TPQ), and TTX were purchased from Tocris Bioscience (Bristol, UK). Guanosine 5′-[β-thio]diphosphate (GDP-β-S) trilithium salt was purchased from Sigma-Aldrich (St Louis, MO, USA). To examine the effect of blocking G protein signalling in MCH+ cells on histamine, GDP-β-S (1 mm) was added to the electrode internal solution and it was allowed to diffuse into MCH neurons for at least 10 min after the formation of whole-cell recordings. For bath application of a given drug, it was added to 20 ml of recirculated ACSF for a specified concentration. The bath application of drug for 20 min was estimated to produce full effects, while the washout of 20 min was considered to remove the added drug from the recording solution. Any neuron recordings in which the access resistance changed by >20% reflected by the testing current injections during the course of the experiment were not used for analysis.
Table 1.
Intrinsic electrophysiological properties of MCH neurons
| RMP (mV) | Rs (MΩ) | Rm (MΩ) | Cm (pF) | AP thresh (mV) | AP height (mV) | AP width at half-height (ms) | AHP (mV) | AHP time (ms) | AP rise time (ms) | AP fall time (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| −58.7 ± 2.4 | 33.5 ± 2.3 | 243.1 ± 53.4 | 32.4 ± 5.6 | −34.8 ± 1.9 | 65.6 ± 1.4 | 1.75 ± 0.2 | 14.2 ± 0.9 | 8.1 ± 2.8 | 1.2 ± 0.1 | 2.3 ± 0.3 |
Values are mean ± SEM. The quantification is based on selected MCH ZsGreen+ neurons (n = 9) recorded from lateral hypothalamus. Abbreviations: RMP, resting membrane potential; Rs, series resistance; Rm, membrane resistance; Cm, membrane capacitance; AP, action potential; AHP, afterhyperpolarization potential.
After all physiological assays had been completed, the brain slices were fixed in 4% paraformaldehyde in PBS overnight and transferred to 30% sucrose solution in PBS. The slices were stained against biocytin with 1:500 Cy3-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA, USA) to show the morphology of the recorded cells.
Statistical analysis
Data are presented as mean ± standard error of the mean. All statistical analysis was performed using GraphPad Prism 5.0 or MATLAB software. The data were checked for normality distribution and equal variance. If the criteria were met, a t test was performed to compare two groups; when the criteria were not met, a Mann–Whitney U test was used. For statistical comparisons across more than two groups, we used the Kruskal–Wallis test (non-parametric one-way ANOVA) and the Mann–Whitney U test for group comparisons. In all experiments, *corresponds to a P value of <0.05.
Results
MCH neurons express H3R but not H1R or H2R
The expression of histamine receptors by MCH neurons was analysed by double in situ hybridization. We first found that, in the rat brain, approximately 70% of all MCH neurons express H3R mRNA while very few express H1R and H2R (Fig.1A–C). The H3R colocalization is found in all subregions of the MCH neurons. We then extended these studies to the mouse, the species to be utilized for electrophysiology experiments, and found expression patterns nearly identical to those observed in rats. H1R and H2R displayed minimal colocalization with MCH, while H3R was expressed by most MCH neurons (Fig.1E–G). Sense probes for H3R demonstrated negligible signal in both rats and mice (Fig.1G and H), confirming probe specificity. While H1R and H2R were expressed at very low levels in the LH, they were expressed in several other brain regions (see online Supporting information Fig. S1) in a manner consistent with their published distribution patterns (Lintunen et al. 1998; Karlstedt et al. 2001). These results demonstrate that H3R, but not H1R or H2R, is expressed by MCH neurons in both mice and rats.
Figure 1. Double in situ hybridization of pMCH and histamine receptors in the lateral hypothalamus of the rat and mouse.
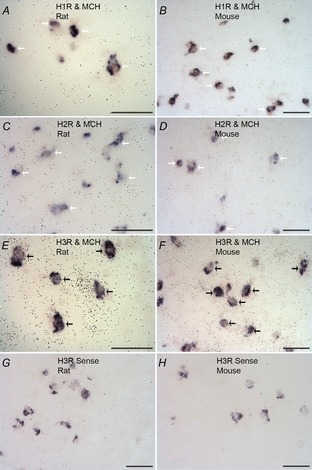
Sections hybridized with antisense 35S-labelled riboprobes (black grains) for histamine receptors and DIG-labelled MCH precursor (pMCH) (purple). A and B, H1R antisense probes in the rat and mouse, respectively. C and D, H2R antisense probes in the rat and mouse, respectively. E and F, H3R antisense probes in the rat and mouse, respectively. G and H, H3R sense probes in the rat and mouse, respectively. Black arrows show examples of colocalization; white arrows indicate cells that do not colocalize. Scale bars: 50 μm.
Generation of a reporter mouse line for MCH neurons
We next sought to determine whether H3R functionally modulates the activity of the MCH system using in vitro electrophysiology. In order to facilitate targeting of MCH neurons, Tg(Pmch-cre)1Lowl/J mice (Kong et al. 2010; Jackson Laboratories), that express Cre recombinase specifically in MCH neurons, were crossed with Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J mice (Fig.2A; Madisen et al. 2010; Jackson Laboratories) that express the fluorescent reporter ZsGreen following Cre recombination. Bright green fluorescence was observed in the lateral hypothalamus and zona incerta of the resulting offspring, which we will refer to as the ‘pMCH-cre/ZsGreen’ mice, in a pattern consistent with that of MCH expression (Fig.2A). Quantification of ZsGreen expression specificity to MCH neurons in the LH and ZI using an anti-MCH antibody (Bittencourt et al. 1992) revealed that 96.5 ± 0.5% of ZsGreen-positive cells are MCH immunopositive and 95.2 ± 0.9% of MCH-immunopositive cells express ZsGreen (Fig.2B–E, n = 1793 cells pooled from 4 mice). However, in female mice, a small population of ZsGreen-expressing neurons was found in the medial preoptic area (MPO) that did not exhibit MCH immunoreactivity. It has been reported that MCH is expressed in the MPO of female rats during lactation (Rondini et al. 2010), thus the ZsGreen signal observed in the MPO may be the result of transient MCH expression in these neurons at some point during embryonic or neonatal development of female mice, which could result in Cre expression in these neurons throughout the animals’ lifetime. For the slice electrophysiology experiments described below, only ZsGreen-positive neurons (Fig.3A) in the LH and ZI were tested. Intrinsic electrophysiological properties of ZsGreen-expressing neurons were extensively characterized (Table1), and exhibited low firing rates at normal resting membrane potentials and large spike afterhyperpolarization, consistent with those previously reported for MCH neurons (van den Pol et al. 2004; Parsons & Hirasawa, 2011). ZsGreen-expressing cells were medium-sized multipolar neurons with several primary dendrites that often displayed secondary branching (Fig.2F), consistent with previous descriptions of MCH neurons (Bittencourt et al. 1992; van den Pol et al. 2004).
Figure 2. Generation of the pMCH-cre/ZsGreen mouse and characterization of ZsGreen-expressing MCH+ cells in the lateral hypothalamus.
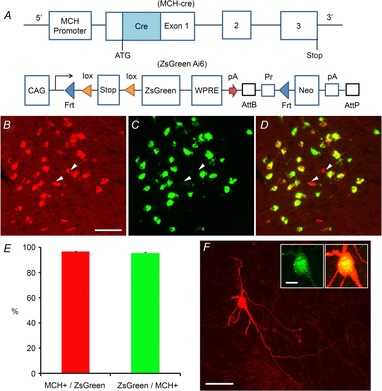
A, schematic diagram showing the transgenic regions of the MCH-cre and ZsGreen (Ai6) reporter mouse lines. The pMCH-cre mouse (Kong et al. 2010) is crossed with the Ai6 mouse (Madisen et al. 2010) to generate the pMCH-cre/ZsGreen mouse. In the ZsGreen reporter mouse, the Rosa26 locus is modified by targeted insertion of a construct containing the strong and ubiquitously expressed CAG promoter, followed by a loxP-flanked (‘floxed’) stop cassette-controlled fluorescent marker gene (ZsGreen). The construct uses the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and a pair of PhiC31 recognition sites (AttB/AttP) through which the Neo marker can be deleted from the reporter line. The FRT sites allow a Flp recombinase-mediated replacement strategy (‘Flp-in’) to swap other genes into the same locus at high efficiency. B–D, ZsGreen-expressing cells are MCH+ neurons in the lateral hypothalamus of the pMCH-cre/ZsGreen mouse. B, C and D are example images of ZsGreen expression in the lateral hypothalamus, immunostaining of MCH in the same section, and the merge of B and C, respectively. The arrowheads point to examples of a few MCH-immunopositive cells which do not express ZsGreen. Scale bar: 50 μm. E, summary of immunochemical data quantification obtained from 4 pMCH-cre/ZsGreen mice. F, typical morphology of single ZsGreen+/MCH+ neurons, revealed by post hoc biocytin staining. The insets show that this example cell expresses MCH. Scales: 50 and 10 μm.
Figure 3. The dose–response effect of different histamine concentrations on MCH+ cells.
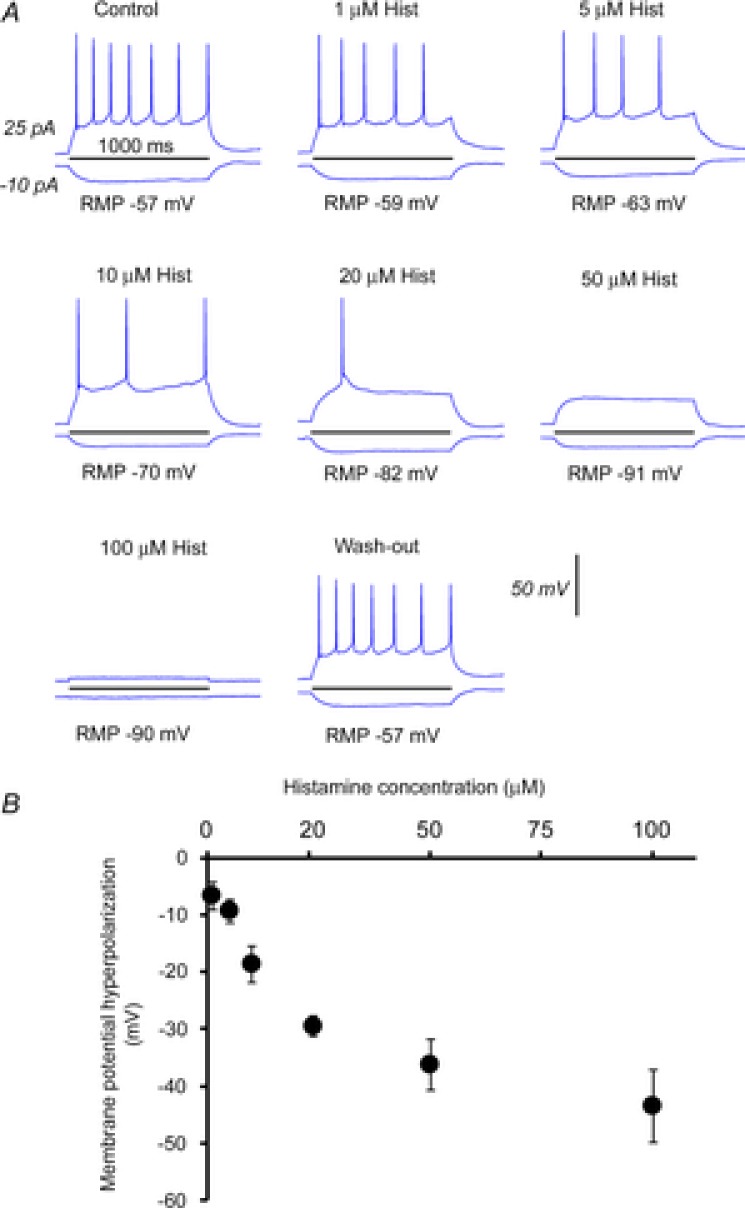
A, example current injection responses from a MCH+ cell in control, in the presence of different histamine concentrations of 1, 5, 10, 20, 50 and 100, and at washout. B, average cell membrane potential hyperpolarization (in mV) of MCH cells versus different histamine concentrations. Each data point (mean ± SEM) was calculated from test results of 4–6 cells.
Histamine directly inhibits MCH neurons by activating the H3 receptor
Because most MCH neurons were found to express H3R, we tested whether bath application of histamine to hypothalamic brain slices would alter the physiological properties of MCH neurons.
Histamine concentrations of 1, 5, 10, 20, 50 and 100 μm were bath applied to MCH neurons. Histamine was found to strongly hyperpolarize MCH neurons (Fig.3A and B, n = 4–6 cells per concentration tested) with an approximate half-maximal effect seen at 20 μm, a concentration that has been previously reported to induce robust H3R-mediated effects in other hypothalamic nuclei (Lundius et al. 2010). This concentration of histamine required 17.8 ± 0.9 min (Fig.4A, mean ± SEM, n = 8) to reach peak effect and strongly hyperpolarized the resting membrane potential (RMP) of MCH neurons (Fig.4D, −57.9 ± 2.6 mV vs. −77.1 ± 3.0 mV, n = 14; P < 0.01). This effect was TTX insensitive (Fig.4D, 68.4 ± 6.4 mV, n = 5; P < 0.05). Histamine also decreased input resistance (Fig.4E, 365.9 ± 35.7 MΩ vs. 260.7 ± 41 MΩ, n = 14; P < 0.05), and increased the rheobase (Fig.4F, 30.3 ± 9 pA vs. 63.9 ± 12 pA, n = 14; P < 0.01) of MCH neurons. We note that the action potential frequency of MCH+ cells increases with depolarizing current injections but then decreases as the injection current becomes stronger (Fig.4B and C). Histamine application shifted the cell response curve to the right, indicating reduced membrane excitability (Fig.4C).
Figure 4. Histamine inhibits MCH neurons.
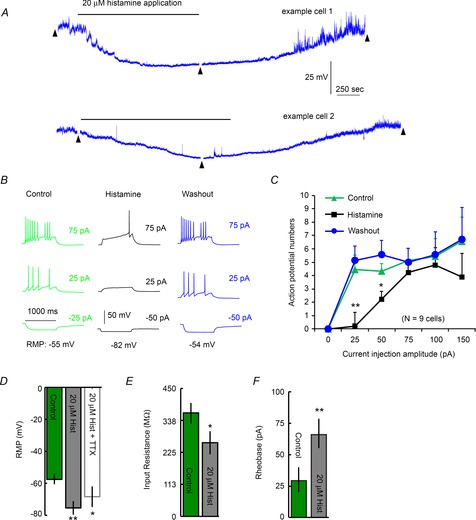
A, representative trace of membrane potentials from two example MCH+ cells showing the time course of the effect of bath application of 20 μm histamine (indicated by the horizontal bar over the trace). This recording trace is continuously presented except with short intervals (less than 2 min, indicated by black arrowheads) during which the current–voltage relationship (shown in B) was examined. Note that the MCH+ cells tend to show relatively large spontaneous fluctuations of membrane potentials. B, example current injection responses of the recorded cell displayed in A at control, histamine application and washout. C, plot of current injection amplitudes versus evoked action potential numbers in MCH cells (n = 6 cells) during control, in the presence of 20 μm histamine, and after washout. D, resting membrane potential (RMP) is hyperpolarized by bath application of 20 μm histamine. This effect is TTX insensitive. E, histamine reduces input resistance. F, increased current amplitudes are required to elicit neuronal firing in the presence of histamine. Control and histamine, n = 14 cells; histamine + TTX, n = 5 cells. *Statistical significance of P < 0.05; **statistical significance of P < 0.01.
Consistent with our in situ hybridization data showing that MCH neurons do not express H1R or H2R, a H1 agonist, 2-pyridylethylamine (100 μm) (Tabarean, 2013) and a selective H2 agonist, amthamine (5 μm) (Threlfell et al. 2008) had no effects on resting membrane potentials, input resistance, or rheobases of the recorded MCH+ cells (see online Supporting information Fig. S2, n = 7). Their average changes in membrane potential, input resistance and rheobase were −0.25 ± 0.3 mV, 5.6 ± 14.3 MΩ and 0 ± 0 pA, respectively, in the presence of both the H1 and H2 agonists. We next used a specific H3R agonist, α-methylhistamine, to determine whether the inhibitory effects of histamine on MCH neurons are mediated by H3R. Bath application of 10 μm α-methylhistamine significantly hyperpolarized MCH neurons (Fig.5A and B, −51.6 ± 1.5 mV vs. −66.2 ± 4.9 mV, n = 5; P < 0.05), increased the rheobase (Fig.5C, 42 ± 15.9 pA vs. 100 ± 15.8 pA, n = 5; P < 0.05), and decreased input resistance (Fig.5D, 393.6 ± 30.3 MΩ vs. 234.4 ± 37.6 MΩ, n = 5; P < 0.05), indicating that selective activation of H3R is sufficient to recapitulate the effects of histamine on MCH neurons. We also found that the effects of histamine are blocked by the highly selective H3R neutral antagonist VUF 5681, as no significant changes in membrane potential (Fig.5E and F, VUF application, −59.7 ± 2.2 mV vs. VUF + 20 μm histamine, −58 ± 2.2 mV, n = 9 cells), rheobase (Fig.5G, 36.1 ± 6 pA vs. 46.4 ± 5.8 pA), or input resistance (Fig.5H, 564 ± 90.8 MΩ vs. 611 ± 133 MΩ) were observed after histamine administration in the presence of VUF 5681. The application of VUF 5681 alone did not affect the activity or membrane potential of MCH neurons (data not shown). Taken together, this indicates that histamine inhibits MCH neurons via H3 receptors.
Figure 5. H3R regulates MCH neurons.
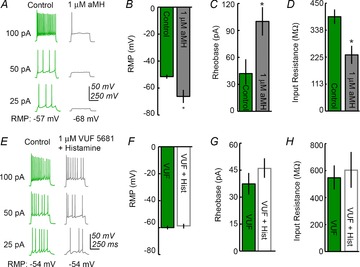
A, an example showing that MCH neurons are inhibited by bath application of the H3R agonist α–methylhistamine (aMH). B and C: α-methylhistamine hyperpolarizes and increases the rheobase of MCH neurons (n = 5). D, input resistance is reduced by α–methylhistamine. E, an example showing that the addition of the selective H3R neutral antagonist VUF 5681 abolishes the effect of histamine. F, G and H, VUF 5681 blocks histamine-induced changes in membrane potential, rheobase and membrane input resistance, respectively, of MCH neurons (n = 9). *Statistical significance of P < 0.05.
Histamine activates G protein-dependent inwardly rectifying potassium (GIRK) channels in MCH neurons
We next determined the molecular mechanism underlying how histamine inhibits MCH neurons. Because the inhibitory effect of histamine on MCH neurons is TTX insensitive, we investigated whether a postsynaptic G protein-coupled receptor (GPCR) is responsible for inhibiting MCH neurons. GDP-β-S (1 mm), an inhibitor of G protein signalling, was loaded into the pipette recording solution and allowed to diffuse into MCH neurons prior to recording. We found that histamine has no effect on RMP or input resistance in the presence of intracellular GDP-β-S (Fig.6A–C), indicating that the effect of histamine is mediated through a postsynaptic GPCR.
Figure 6. GDP-β-S intracellularly blocks the effects of histamine on MCH+ cells.
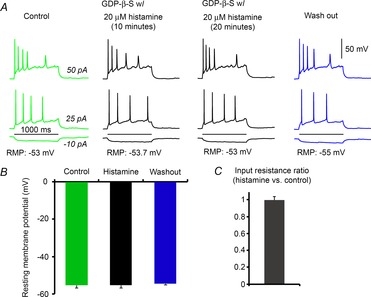
A, representative cell responses to current injections showing that histamine has no significant effects on electrophysiological properties of MCH+ cells in the intracellular presence of GDP-β-S. Cell responses to current injections are shown. The durations (10 min and 20 min) of histamine application are indicated over the example traces. B and C, histamine application does not affect average resting membrane potential or input resistance of MCH+ cells in the presence of GDP-β-S.
Orphanin FQ/nociceptin is known to inhibit MCH neurons by activating GIRK channels (Parsons & Hirasawa, 2011), and H3R activates GIRK channels in vitro (Sahlholm et al. 2007). In light of this, and because histamine hyperpolarizes membrane potentials and reduces the input resistance of MCH neurons, we hypothesized that H3R may inhibit MCH neurons by activating GIRK channels. To investigate this possibility, we tested whether histamine inhibits MCH neurons in the presence of the GIRK channel inhibitor Tertiapin Q (TPQ, 0.5 μm). We found that TPQ abolishes histaminergic inhibition of MCH neurons, as histamine application caused no changes in RMP (Fig.7A and B; −66.2 ± 2 mV vs. −66.5 ± 2.2 mV; n = 5 cells), rheobase (Fig.7C; 50 ± 15.8 pA vs. 56.3 ± 14 pA), and input resistance (Fig.7D; 327 ± 26 MΩ vs. 362 ± 27.3 MΩ) in the presence of TPQ. This indicates that H3R-mediated inhibition of MCH neurons is dependent on GIRK channels. To our knowledge, this is the first time that postsynaptic H3R has been demonstrated to activate GIRK channels in mammalian cells.
Figure 7. Histamine activates GIRK channels in MCH neurons.
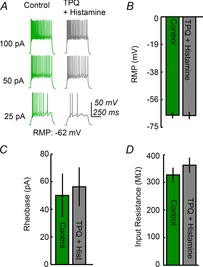
A, an example showing that the GIRK channel inhibitor Tertiapin Q (TPQ) blocks histamine-induced hyperpolarization of MCH neurons. B and C, histamine has no effect on resting membrane potential or firing threshold of MCH neurons (n = 5) in the presence of TPQ. D, TPQ blocks the alterations in input resistance elicited by histamine.
Discussion
In the CNS, histamine is produced exclusively by neurons in the tuberomammillary nucleus (TMN) of the posterior hypothalamus that project throughout the brain to regulate arousal and wakefulness (Thakkar, 2011). Two excitatory receptors, H1R and H2R, and one inhibitory receptor, H3R, mediate the effects of histamine in the brain (Lin et al. 2011). H1R and H2R are typically expressed postsynaptically while H3R classically functions as an inhibitory autoreceptor for histaminergic neurons that provides negative feedback on histamine release and expression (Lin et al. 2011), but also commonly functions as a presynaptic heteroreceptor on other neuron types that inhibits neuronal activity and transmitter release (Schlicker et al. 1999; Doreulee et al. 2001). Only a few instances of H3R functioning postsynaptically are known (Zhou et al. 2006; Lundius et al. 2010).
Our study reveals that most MCH neurons express H3R and that histamine regulates MCH neurons via postsynaptic H3R. This finding is in agreement with a previous report demonstrating histaminergic projections to the LH (Eriksson et al. 2001). Although not often observed, H3R has been shown to function postsynaptically in the preoptic area (Lundius et al. 2010) and basal ganglia (Zhou et al. 2006). Thus our identification of another instance of H3R postsynaptic activity suggests that this phenomenon may be more common than previously believed. We also found that a GIRK channel inhibitor blocks the effects of histamine, indicating that histamine inhibits MCH neurons by activating GIRK channels. Although we cannot rule out the potential contribution of other currents, we can conclude that most of histamine's inhibitory effect on MCH neurons results from activating GIRK channels. Because activation of H3R was found to exert an entirely inhibitory effect, this effect may be mediated through the Gi/o signalling pathway, but further studies are needed to confirm this. Human H3R has been shown to activate GIRK channels in oocytes (Sahlholm et al. 2007), but to the best of our knowledge, this is the first case in which H3R has been shown to activate GIRK channels in the brain. As orphanin FQ/nociceptin has also been demonstrated to activate GIRK channels in MCH neurons (Parsons & Hirasawa, 2011), other transmitters may also regulate the MCH system through this mechanism.
We propose that histaminergic neurons interact with the MCH system as part of a circuit that regulates arousal and wakefulness (Fig.8). As has been previously described, the MCH system is thought to dampen arousal and promote REM sleep (Peyron et al. 2009). Central injection of MCH promotes both REM and, to a lesser extent, NREM sleep (Verret et al. 2003), and activation or blockade of MCHR1 in the dorsal raphe and the basal forebrain (Lagos et al. 2009, 2012) alters REM sleep patterns (Lagos et al. 2009, 2011). MCH neurons are active during REM sleep but not during wakefulness or NREM (Hassani et al. 2009), indicating that they play a role in the maintenance of REM sleep. MCH neurons are typically inhibitory and project widely to brain regions that are inactive during REM sleep, suggesting that MCH neurons contribute to keeping these neurons silent during this phase. A recent study (Jego et al. 2013) has confirmed this hypothesis by demonstrating that optogenetic activation of MCH neurons induces REM sleep and MCH neurons directly inhibit histaminergic neurons through GABAA receptors.
Figure 8. Schematic diagram of how histamine may regulate the MCH system during sleep and wakefulness.
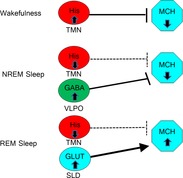
During wakefulness, histaminergic neurons in the tuberomammillary nucleus (TMN) silence MCH neurons. During NREM sleep, histaminergic neurons are inactive and do not affect MCH neurons, but NREM-active GABAergic neurons in the ventrolateral preoptic area (VLPO) keep MCH neurons silent. During REM sleep, wake-active and NREM-active neurons are silent and MCH neurons are activated by REM-active glutamatergic neurons in the sublaterodorsal nucleus (SLD).
Histaminergic inhibition of the MCH system is likely to be part of the circuitry responsible for silencing sleep-active neurons during wakefulness (Fig.8), which is in agreement with reports that other arousal-promoting brain regions also inhibit the MCH system during wakefulness (Bayer et al. 1999; van den Pol et al. 2004; Jones, 2005; Yoshida et al. 2006; Peyron et al. 2009). Histaminergic neurons in the TMN are highly active during wakefulness (Haas et al. 2008) and promote wakefulness and arousal. Because MCH promotes sleep and may inhibit arousal-promoting brain regions, inhibition of the MCH system would presumably promote arousal. The large magnitude of hyperpolarization induced by histamine suggests that this circuit may be a key player in keeping MCH neurons inactive during wakefulness. This supports the model described by van den Pol et al. in which multiple populations of wake-active neurons inhibit the MCH system (van den Pol et al. 2004). Conversely, because histaminergic neurons are silent during REM sleep (Takahashi et al. 2006), the lack of histaminergic inhibition of MCH neurons during this period may contribute to their greatly increased activity during REM sleep.
Histamine and MCH are involved in behaviours and functions such as food intake, anxiety and reward, and may regulate behaviours relevant to schizophrenia. Systemic administration of H3R antagonist decreases food intake and may have antipsychotic and anxiolytic effects (Haas et al. 2008). Conversely, MCH increases food intake (Rossi et al. 1997), is anxiogenic (Smith et al. 2006), modulates drug reward (Chung et al. 2009; Morganstern et al. 2010), and may promote behaviours associated with psychiatric disorders (Chung et al. 2011b). However, it is unclear whether histaminergic regulation of MCH neurons is relevant to these functions because H3R antagonists would be expected to cause increased activation of MCH neurons, which should in turn cause an increase in food intake, anxiety and drug reward if this pathway mediates histaminergic influence on these functions. Because H3R antagonists exert the opposite effect as would be expected of increased activity of the MCH system, histamine and MCH may affect these functions independently of each other, but further study is required to investigate this hypothesis.
In conclusion, we have identified a novel circuit in which histamine directly inhibits MCH neurons through H3R by activating GIRK channels. This strongly suggests that histamine and MCH act together in the regulation of arousal and sleep. Indeed, a recent study (Jego et al. 2013) utilized optogenetic stimulation of MCH terminals to confirm that MCH neurons inhibit the histamine system in the TMN by activating GABAA receptors, thus demonstrating a reciprocal interaction between the two systems. Together, these data indicate that histamine regulates the MCH system, representing a new circuit that may be involved in regulating sleep and other physiological functions.
Funding
This work was supported by National Institutes of Health grants DA024746 to O.C. and NS078434 to X.X. Additional funding was provided by a NARSAD Established Investigator Award and a grant from the Tourette's Syndrome Foundation to O.C., and a NARSAD Young Investigator Award to X.X. O.C. is holder of the Eric L. and Lisa D. Nelson Chair of Pharmacology.
Acknowledgments
We would like to thank Dr Rainer Reinscheid for providing the H3R probe, and Drs Yan Zhang and Naoto Hoshi for critical discussion and support. We would also like to thank Andrew San Antonio, Krupa Gohil, Justin Matsuura, Andy Vu and Tracy Lu for excellent technical assistance.
Glossary
- DIG
digoxigenin
- GIRK channel
G protein-dependent inwardly rectifying potassium channel
- H1R
histamine-1 receptor
- H2R
histamine-2 receptor
- H3R
histamine-3 receptor
- LH
lateral hypothalamus
- MCH
melanin-concentrating hormone
- MCHR1
melanin-concentrating hormone receptor-1
- NREM sleep
non-rapid eye movement sleep
- REM sleep
rapid eye movement sleep
- RMP
resting membrane potential
- TMN
tuberomammillary nucleus
- TPQ
tertiapin Q
- ZI
zona incerta
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
G.S.P., N.D.O., O.C. and X.X. conceived of and designed the study. G.S.P., N.D.O., T.I., N.M.S. and X.X. analysed the data. G.S.P., N.D.O., T.I., N.M.S. and L.W. performed experiments. Z.W. created new tools and reagents for the study. G.S.P., N.D.O., O.C. and X.X. wrote the manuscript. All authors were involved in interpreting data and critically editing the manuscript, and all approved the final version of the manuscript.
Key points
Melanin-concentrating hormone (MCH) neurons express histamine-3 receptor (H3R) mRNA but not histamine-1 (H1R) or histamine-2 (H2R) receptor mRNA.
Histamine inhibits MCH neurons by activating postsynaptic H3R. This effect is mediated through G protein-dependent inwardly rectifying potassium (GIRK) channels.
Histamine may function to silence MCH neurons during wakefulness.
Supporting Information
The following supporting information is the online version of this article.
H1R and H2R are expressed in the expected brain regions.
Bath application of H1R and H2R agonists does not affect resting membrane potentials or intrinsic spiking patterns of MCH+ neurons.
References
- Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ. Bloom SR. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144:3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- Adamantidis A, Thomas E, Foidart A, Tyhon A, Coumans B, Minet A, Tirelli E, Seutin V, Grisar T. Lakaye B. Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur J Neurosci. 2005;21:2837–2844. doi: 10.1111/j.1460-9568.2005.04100.x. [DOI] [PubMed] [Google Scholar]
- Bayer L, Risold PY, Griffond B. Fellmann D. Rat diencephalic neurons producing melanin-concentrating hormone are influenced by ascending cholinergic projections. Neuroscience. 1999;91:1087–1101. doi: 10.1016/s0306-4522(98)00678-2. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W. Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ. Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone–1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Hirota-Okuno S, Nishiguchi M, Shimazaki T, Iijima M, Grottick AJ, Kanuma K, Omodera K, Sekiguchi Y, Okuyama S, Tran TA, Semple G. Thomsen W. Anxiolytic- and antidepressant-like profile of ATC0065 and ATC0175: nonpeptidic and orally active melanin-concentrating hormone receptor 1 antagonists. J Pharmacol Exp Ther. 2005;313:831–839. doi: 10.1124/jpet.104.081711. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A. Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009;106:6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Parks GS, Lee C. Civelli O. Recent updates on the melanin-concentrating hormone (MCH) and its receptor system: lessons from MCH1R antagonists. J Mol Neurosci. 2011a;43:115–121. doi: 10.1007/s12031-010-9411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Verheij MM, Hesseling P, van Vugt RW, Buell M, Belluzzi JD, Geyer MA, Martens GJ. Civelli O. The melanin-concentrating hormone (MCH) system modulates behaviors associated with psychiatric disorders. PloS One. 2011b;6:e19286. doi: 10.1371/journal.pone.0019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SD, Nothacker HP, Wang Z, Saito Y, Leslie FM. Civelli O. The urotensin II receptor is expressed in the cholinergic mesopontine tegmentum of the rat. Brain Res. 2001;923:120–127. doi: 10.1016/s0006-8993(01)03208-5. [DOI] [PubMed] [Google Scholar]
- Del Cid-Pellitero E. Jones BE. Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience. 2012;223:269–276. doi: 10.1016/j.neuroscience.2012.07.072. [DOI] [PubMed] [Google Scholar]
- Doreulee N, Yanovsky Y, Flagmeyer I, Stevens DR, Haas HL. Brown RE. Histamine H3 receptors depress synaptic transmission in the corticostriatal pathway. Neuropharmacology. 2001;40:106–113. doi: 10.1016/s0028-3908(00)00101-5. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE. Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB. Electrophysiological effects of MCH on neurons in the hypothalamus. Peptides. 2009;30:2025–2030. doi: 10.1016/j.peptides.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Parks GS, Clinton SM, Watson SJ, Akil H. Civelli O. The melanin-concentrating hormone (MCH) system in an animal model of depression-like behaviour. Eur Neuropsychopharmacol. 2012;22:607–613. doi: 10.1016/j.euroneuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA. Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Lee MG. Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D. Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Karlstedt K, Senkas A, Ahman M. Panula P. Regional expression of the histamine H2 receptor in adult and developing rat brain. Neuroscience. 2001;102:201–208. doi: 10.1016/s0306-4522(00)00464-4. [DOI] [PubMed] [Google Scholar]
- Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Bruning JC. Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos P, Monti JM, Jantos H. Torterolo P. Microinjection of the melanin-concentrating hormone into the lateral basal forebrain increases REM sleep and reduces wakefulness in the rat. Life Sci. 2012;90:895–899. doi: 10.1016/j.lfs.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Lagos P, Torterolo P, Jantos H, Chase MH. Monti JM. Effects on sleep of melanin-concentrating hormone (MCH) microinjections into the dorsal raphe nucleus. Brain Res. 2009;1265:103–110. doi: 10.1016/j.brainres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Lagos P, Torterolo P, Jantos H. Monti JM. Immunoneutralization of melanin-concentrating hormone (MCH) in the dorsal raphe nucleus: effects on sleep and wakefulness. Brain Res. 2011;1369:112–118. doi: 10.1016/j.brainres.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sergeeva OA. Haas HL. Histamine H3 receptors and sleep-wake regulation. J Pharmacol Exp Ther. 2011;336:17–23. doi: 10.1124/jpet.110.170134. [DOI] [PubMed] [Google Scholar]
- Lintunen M, Sallmen T, Karlstedt K, Fukui H, Eriksson KS. Panula P. Postnatal expression of H1-receptor mRNA in the rat brain: correlation to l-histidine decarboxylase expression and local upregulation in limbic seizures. Eur J Neurosci. 1998;10:2287–2301. doi: 10.1046/j.1460-9568.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J. Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES. Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH. Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzon ME, de Souza MM, Izquierdo LA, Izquierdo I, Barros DM. de Barioglio SR. Melanin-concentrating hormone (MCH) modifies memory retention in rats. Peptides. 1999;20:1517–1519. doi: 10.1016/s0196-9781(99)00164-3. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG. Leibowitz SF. Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiol Behav. 2010;101:428–437. doi: 10.1016/j.physbeh.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP. Hirasawa M. GIRK channel-mediated inhibition of melanin-concentrating hormone neurons by nociceptin/orphanin FQ. J Neurophysiol. 2011;105:1179–1184. doi: 10.1152/jn.00791.2010. [DOI] [PubMed] [Google Scholar]
- Peyron C, Sapin E, Leger L, Luppi PH. Fort P. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides. 2009;30:2052–2059. doi: 10.1016/j.peptides.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Donato J, Jr, Rodrigues BdeC, Bittencourt JC. Elias CF. Chemical identity and connections of medial preoptic area neurons expressing melanin-concentrating hormone during lactation. J Chem Neuroanat. 2010;39:51–62. doi: 10.1016/j.jchemneu.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA. Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Sahlholm K, Nilsson J, Marcellino D, Fuxe K. Arhem P. The human histamine H3 receptor couples to GIRK channels in Xenopus oocytes. Eur J Pharmacol. 2007;567:206–210. doi: 10.1016/j.ejphar.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM. Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Werthwein S. Zentner J. Histamine H3 receptor-mediated inhibition of noradrenaline release in the human brain. Fundam Clin Pharmacol. 1999;13:120–122. doi: 10.1111/j.1472-8206.1999.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Sinchak K. Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behaviour. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL, Nomikos GG. Gehlert DR. Melanin-concentrating hormone–1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology. 2006;31:1135–1145. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- Tabarean IV. Functional pharmacology of H1 histamine receptors expressed in mouse preoptic/anterior hypothalamic neurons. Br J Pharmacol. 2013;170:415–425. doi: 10.1111/bph.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Lin JS. Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15:65–74. doi: 10.1016/j.smrv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Exley R, Cragg SJ. Greenfield SA. Constitutive histamine H2 receptor activity regulates serotonin release in the substantia nigra. J Neurochem. 2008;107:745–755. doi: 10.1111/j.1471-4159.2008.05646.x. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Sampogna S. Chase MH. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009;1268:76–87. doi: 10.1016/j.brainres.2009.02.055. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR. Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C. Luppi P-H. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Olivas ND, Levi R, Ikrar T. Nenadic Z. High precision and fast functional mapping of cortical circuitry through a novel combination of voltage sensitive dye imaging and laser scanning photostimulation. J Neurophysiol. 2010;103:2301–2312. doi: 10.1152/jn.00992.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD. Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A. Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS. Zhou FM. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol. 2006;96:1581–1591. doi: 10.1152/jn.00148.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H1R and H2R are expressed in the expected brain regions.
Bath application of H1R and H2R agonists does not affect resting membrane potentials or intrinsic spiking patterns of MCH+ neurons.


