Abstract
Objective
Characterize the status of RA in amnestic Mild Cognitive Impairment (MCI).
Method
We measured RA, AA, brain measures, Apolopoprotein-E status, and conversion to probable Alzheimer’s disease (AD) across 3 years in 15 individuals with MCI. We compared the severity of amnesia and brain atrophy in MCI to a group of patients with limited damage to the hippocampus (H) or more extensive damage to the medial temporal lobe (MTL).
Results
The MCI group exhibited modest AA together with severe RA covering nearly four decades before their diagnosis. Compared to H-MTL patients, the temporal extent of RA was disproportionate to the severity of AA. The MCI group exhibited more modest AA and MTL atrophy than H-MTL patients together with more severe RA and neocortical atrophy than H-MTL patients. The severity of AA corresponded to the integrity of MTL structures, whereas the severity of RA corresponded to the integrity of both MTL and neocortical structures. RA (but not AA nor measures of cognitive status) was related to Apolopoprotein-E status and subsequent diagnosis of probable AD. RA was predicted by heritable risk for AD in addition to the integrity of medial temporal lobe and neocortical structures.
Conclusions
Compared to H-MTL patients, the MCI group exhibited RA that was disproportionate to their AA and was more severe than would be expected if their atrophy were limited primarily to the MTL. Heritable risk for AD, as well as the integrity of brain regions within and beyond the MTL, are important for understanding RA in MCI.
Keywords: semantic memory, mild cognitive impairment, retrograde amnesia, anterograde amnesia
Introduction
Mild Cognitive Impairment (MCI) is considered a transitional stage between healthy aging and Alzheimer’s Disease (AD) (Petersen et al., 1999), and individuals with MCI are at an increased risk of developing AD (at a rate of 16% per year; Petersen et al., 2005). Amnestic MCI is associated with cognitive impairment limited to the domain of memory. These individuals exhibit anterograde memory impairment (difficulty learning new information) that is often intermediate in severity between normal controls and AD (Ally, Gold, & Budson, 2009; Petersen, et al., 1999). The severity of anterograde memory deficits in both MCI and AD are related to the integrity of structures in the medial temporal lobe (MTL), specifically the hippocampus and entorhinal cortex (Sexton et al., 2010; Walhovd et al., 2010).
MTL damage is also associated with retrograde amnesia, that is, difficulty remembering information acquired prior to the onset of amnesia (Bayley, Hopkins, & Squire, 2006; Smith, Frascino, Hopkins, & Squire, 2013). The status of retrograde amnesia in MCI has received relatively little attention, and the available studies have yielded mixed findings. Retrograde amnesia has been described as absent (Thomann et al., 2012--for non-autobiographical memory), mild and temporally limited (Irish, Lawlor, O’Mara, & Coen; Leyhe, Muller, Milian, Eschweiler, & Saur, 2009; Murphy, Troyer, Levine, & Moscovitch, 2008; Thomann, et al., 2012--for autobiographical memory), or severe and encompassing all time periods tested (Barbeau et al., 2012; Flicker, Ferris, Crook, & Bartus, 1987; Leyhe, Muller, Eschweiler, & Saur, 2010; Seidenberg et al., 2009). When overall performance on retrograde memory tests was examined (instead of the temporal extent of the impairment) impairment has been consistently observed in MCI (Bizzozero, Lucchelli, Saetti, & Spinnler, 2012; Gardini et al., 2013; Joubert et al., 2008).
The status of retrograde amnesia is clearer in AD. This group exhibits severe retrograde amnesia that frequently encompasses the entire lifespan prior to the onset of amnesia (see Meeter, Eijsackers, & Mulder, 2006 for a review of seven AD studies). Yet, atrophy in AD (though it begins in the MTL) eventually involves temporal, frontal, and parietal cortex (Braak & Braak, 1997; Dickerson et al., 2009; Thal, Rub, Orantes, & Braak, 2002; Thompson et al., 2003). Accordingly, the severity of retrograde amnesia in AD likely reflects atrophy not only of the MTL but also in the neocortex, most likely association cortices, where long-term memories are thought to be stored (Bayley, Gold, Hopkins, & Squire, 2005; Bright et al., 2006; Gilboa et al., 2005) or atrophy of the frontal lobe given its role in memory retrieval (Bayley, et al., 2005; Kopelman, 1991; Kopelman et al., 2003; Kopelman, Stanhope, & Kingsley, 1999; Kroll, Markowitsch, Knight, & von Cramon, 1997). Notably, like AD patients, individuals with MCI also exhibit variable neuroanatomical changes outside the MTL (Bakkour, Morris, & Dickerson, 2009; Fennema-Notestine et al., 2009; McDonald et al., 2009; McEvoy et al., 2009; Whitwell et al., 2007). The severity of retrograde amnesia in MCI may therefore depend on the extent of anatomical changes outside the MTL. Neuroanatomical information in conjunction with comprehensive neuropsychological testing should clarify the status of retrograde amnesia in MCI.
One approach to the assessment of retrograde amnesia in MCI is to consider their scores in comparison to other memory-impaired patient groups where neuroanatomical information is available. For example, memory-impaired patients with acute damage limited to the hippocampus (H) or with larger lesions of the MTL (hippocampus and parahippocampal gyrus) exhibited an orderly relationship between the extent of MTL damage, the severity of anterograde amnesia, and the severity of retrograde amnesia (Smith, et al., 2013). Specifically, when damage was limited to the hippocampus, anterograde amnesia was moderately severe and retrograde amnesia was limited to several years before the onset of amnesia. In contrast, larger lesions of the MTL produced more severe anterograde amnesia, and retrograde amnesia extended back several decades before the onset of amnesia. In patients with H or MTL damage, there was a strong relationship between the severity of anterograde amnesia and the extent of retrograde amnesia (r = 0.81, p < 0.05). Given these findings, one can ask whether the relationship between anterograde and retrograde amnesia in MCI is the same or different than in H and MTL patients. For example, if individuals with MCI have anterograde and retrograde memory scores that deviate substantially from the relationship observed in H and MTL patients, then one might expect atrophic changes to have occurred outside of or in addition to the MTL.
We assessed anterograde memory and retrograde memory (i.e., memory for public events) in 15 individuals with amnestic MCI and 21 healthy controls (Experiment 1). The relationship between anterograde and retrograde memory impairment was then evaluated in comparison to the scores on the same tests for 11 H and MTL patients (Experiment 2), as recently described (Smith, et al., 2013). The behavioral performance of the MCI group on these tests generated three predictions regarding the severity of atrophic changes in particular brain regions. These predictions were then tested using structural brain measures of the MTL and neocortex in 11 of the individuals with MCI and 11 controls (Experiment 3).
Experiment 1: Anterograde and Retrograde Memory in MCI
Method
Participants
The participants were 15 individuals diagnosed with amnestic Mild Cognitive Impairment (Table 1). The individuals were diagnosed and referred by the Alzheimer’s Disease Research Center (ADRC) at the University of California San Diego (UCSD) according to criteria proposed by Petersen and colleagues (Petersen et al., 2001). The criteria were memory complaints, impaired memory on psychometric testing, essentially normal activities of daily living, and otherwise normal general cognitive function. The individuals were diagnosed by consensus conference with neurologists, neuropsychologists, and staff that used all available clinical information, including psychometric test scores, standardized rating scales, and informant interviews. Individuals with MCI had no other significant neurologic illness, such as stroke. Individuals with amnestic MCI were referred to the study from the ADRC if they met three requirements: 1) indication of interest in participating in research, 2) an ability to speak and understand English, and 3) residence in the United States for most of his/her adult life. The latter two criteria were used because the measure of retrograde amnesia (see below) is in English and a portion of the test items query events that may not be well-known outside of the United States.
Table 1.
Experiment 1. Characteristics of MCI and Control Groups
| Variables | Control Group | MCI Group | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Effect Size | ApoE Risk | ApoE No Risk | ||||
|
| ||||||
| Effect Size | ||||||
| Number of participants | 21 | 15 | -- | 7 | 8 | -- |
| Sex (male / female) | 12/9 | 10/5 | -- | 2/5 | 8/0 | -- |
| Age (years) | 76.3 ± 1.6 (65–89) | 78.7 ± 1.5 (66–87) | −0.3 | 78.8 ± 1.7 (71–86) | 78.5 ± 2.6 (66–87) | −0.1 |
| Education (years) | 15.3 ± 0.7 (12–21) | 15.3 ± 0.7 (12–20) | 0.0 | 15.4 ± 1.1 (12–19) | 15.1 ± 1.0 (12–20) | −0.3 |
| COGNITIVE STATUS | ||||||
| MMSE | 27.8 ± 0.5 (25–30) | -- | 27.7 ± 0.8 (25–30) | 27.9 ± 0.6 (26–30) | 0.1 | |
| CDR global score | 0.5, n=13; 1.0, n=2 | -- | 0.5, n=6; 1.0, n=1 | 0.5, n=7; 1.0, n=1 | -- | |
| CDR box Sum-of-Boxes | 2.5 ± 0.3 (0.5–5) | -- | 2.9 ± 0.5 (2–5) | 2.3 ± 0.5 (0.5–4) | 0.6 | |
| CDR Subscales | ||||||
| Memory | 0.80 ± 0.07 (0.5–1) | -- | 0.79 ± 0.10 (0.5–1) | 0.81 ± 0.09 (0.5–1) | 0.0 | |
| Orientation | 0.43 ± 0.14 (0–2) | -- | 0.57 ± 0.25 (0–2) | 0.31 ± 0.13 (0–1) | 0.5 | |
| Judgment/Problem Solving | 0.37 ± 0.08 (0–1) | -- | 0.50 ± 0.11 (0–1) | 0.25 ± 0.09 (0–0.5) | 0.9 | |
| Community Affairs | 0.47 ± 0.09 (0–1) | -- | 0.50 ± 0.15 (0–1) | 0.44 ± 0.11 (0–1) | 0.2 | |
| Home/Hobbies | 0.47 ± 0.09 (0–1) | -- | 0.50 ± 0.11 (0–1) | 0.44 ± 0.15 (0–1) | 0.3 | |
| Personal Care | 0.00 ± 0.00 (0–0) | -- | 0.00 ± 0.00 (0–0) | 0.00 ± 0.00 (0–0) | -- | |
| RETROGRADE MEMORY | ||||||
| Accuracy (percent correct) | 39.7 ± 2.8 (10.0 – 60.0) | 33.2 ± 4.1 (9.4–59.2) | 0.8* | 17.7 ± 3.6 (9.0–33.0) | 37.3 ± 5.3 (18.0–59.0) | 1.2A |
| Temporal Extent of Amnesia (yrs) | -- | 39.0 ± 5.4 (0–70) | -- | 50.0 ± 6.4 (25–70) | 29.4 ± 7.0 (0–50) | 0.9A |
| ANTEROGRADE MEMORY | ||||||
| Number of participants | 15 | 15 | -- | 7 | 8 | -- |
| DRS: Memory Subscale | 24.3 ± 0.2 (23–25) | 21.1 ± 0.8 (15–25) | 1.3* | 20.1 ± 1.2 (16–25) | 22.0 ± 1.2 (15–25) | 0.5 |
| WMS-R Logical Memory | 26.4 ± 1.9 (11–37) | 9.5 ± 1.6 (9–25) | 2.5* | 8.0 ± 2.7 (0–21) | 10.9 ± 2.0 (2–19) | 0.4 |
| Rey-Osterreith Complex Figure | 17.7 ± 1.1 (12–24) | 11.7 ± 1.4 (5–22) | 1.2* | 9.9 ± 1.2 (6–15) | 13.3 ± 2.4 (5–22) | 0.7 |
| Paired-Associate Learning | 20.6 ± 1.5 (11–29) | 10.9 ± 1.6 (0–21) | 1.6* | 11.3 ± 2.3 (2–21) | 10.5 ± 2.4 (0–18) | −0.1 |
Note. Data are the means ± SEMs (ranges). CDR ratings of 0.5 and 1 correspond to questionable and mild dementia, respectively. Cognitive status scores were not obtained for controls. Retrograde memory represents percent correct scores on the News Events Test for events that occurred prior to the onset of amnesia for individuals with MCI and for the same questions in healthy controls. Effect sizes for differences between groups are reported as Cohen’s d and reflect the difference between the means, in standard deviations.
CDR = Clinical Dementia Rating; MCI = Mild Cognitive Impairment; MMSE = Mini Mental State Examination; DRS = Dementia Rating Scale; WMS-R = Wechsler Memory Scale-Revised. Significant effects are highlighted in bold text and the levels of significance are as follows:
significant difference between the MCI and controls groups, p < 0.05;
significant difference between the Apolipoprotein E (ApoE) risk and no risk groups, p < 0.05 (see Experiment 1: Results).
Twenty-one healthy volunteers recruited from the San Diego community served as controls for the MCI group (Table 1). One to two controls were selected to match each member of the MCI group according to sex, age, and education. Controls had no significant neurologic illness or memory complaints. In addition, they exhibited no cognitive impairments (e.g., they performed within the normal range on the Information and Vocabulary subtests of the Wechsler Adult Intelligence Scale (WAIS-III) and within the normal range on a test of delayed prose recall [a test similar to the Wechsler Memory Scale-Revised (WMS-R) Logical Memory Subtest]). Fifteen of these individuals were available for the anterograde memory tests (described below).
Measuring Cognitive Status and Heritable Risk for AD for the MCI Group
Three standard indices of cognitive status were obtained for the MCI group: Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975; maximum score = 30; a score below 28 is considered impaired); Clinical Dementia Rating (Morris, 1993; CDR scores of 0, 0.5, 1, 2, and 3 correspond to normal, questionable dementia, mild dementia, moderate dementia, and severe dementia, respectively); Clinical Dementia Rating Sum of Boxes and the scores for each of its 6 subscales: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care (the score for each area ranges from 0 to 3; a lower score indicates less impairment). Note that two individuals with MCI obtained Clinical Dementia Rating global scores of 1.0. One individual transitioned from a score of 0.5 to 1.0 within the timeframe of the study, and the other individual had normal general cognitive function outside of the realm of memory. Apolipoprotein E (ApoE) status was also obtained for the MCI group.
Measuring Retrograde Memory for News Events
The test was constructed from a pool of 314 questions covering notable news events that occurred in a specific year between 1931 and 2005. Testing occurred between 2007 and 2010. The News Events Test was administered in a free recall format (e.g., What caused a suspension bridge to collapse over the Narrows at Tacoma, Washington? [1940]; Who killed John Lennon? [1980]; Who is Elizabeth Smart? [2003]; the year the event occurred was not provided). For each individual in the MCI group the questions ranged from the time of onset of amnesia to the time when the individual was 15 years old (mean = 242.1 ± 14.2 questions per individual). Earlier time periods were not queried, because adults are known to have limited knowledge of news events that occurred during childhood or early adolescence (Squire, 1974). The onset of amnesia was taken to be the year when the individual was diagnosed with MCI. Given that decline to a level of function consistent with MCI typically occurs within about 5 years (Acosta-Baena et al., 2011; Marquis et al., 2002), we presume that participants were healthy five years and longer prior to their diagnosis.
The percentage of questions answered correctly was calculated for each 5-year time interval–from the five years preceding the onset of amnesia to the time when each individual in the MCI group was 15 years old. For each individual in the MCI group, one or two controls were aligned in the same manner. The severity of retrograde amnesia was measured in two ways. First, for all participants, total accuracy was obtained by taking the mean of the mean percent correct scores across all time periods queried. Second, for the MCI group and for the H and MTL group the temporal extent of amnesia (in years) was calculated as follows. Data from all 21 controls were aligned to each individual relative to the year of onset of that individual’s amnesia. The temporal extent of retrograde amnesia was defined as the number of 5-year time intervals where the individual’s score was below the corresponding score of the controls (p < 0.05, one-sample t-test). A mean of 20.0 ± 5.0 questions were available for each 5-year time interval. For each individual with MCI, all the measures described above (cognitive status, anterograde memory, and retrograde memory) were obtained within 5.0 ± 0.8 months of each other.
Measuring Anterograde Memory
Anterograde Memory for News Events
Some of the news events questions covered events that occurred after the onset of amnesia, i.e., these questions assessed anterograde memory. Nine of the 15 individuals with MCI had questions that covered anterograde memory. A mean of 79.3 ± 17.8 questions was available for these nine individuals (and for their controls). Anterograde amnesia for news events was calculated for each individual with MCI as the mean percent correct score for these news events questions. Because these scores were not available for all 15 individuals in the MCI group, the scores were used primarily to confirm the relationship between anterograde and retrograde amnesia for the MCI group that was observed when anterograde memory was assessed with more conventional memory tests (see below). Specifically, anterograde amnesia for news events was not used for computing the correlations with brain measures. Instead, correlations with brain measures were carried out using the memory tests described below, which were available for all individuals in the MCI group.
Conventional Tests of Anterograde Memory
Delayed Recall of a Complex Figure
Participants copied a complex diagram (Rey-Osterrieth figure; Osterrieth, 1944) and then attempted to reproduce it from memory after a 10–15 min delay. The copy and the reproduction of the figure were each scored on a 36-point scale (Taylor, 1998).
Paired-Associate Learning
Participants completed three study-test trials with a list of 10 unrelated word pairs (Squire & Shimamura, 1986). To begin, the word pairs were displayed one at a time while the experimenter read each pair aloud. Immediately after all 10 pairs had been presented, participants were shown the first word from each pair and asked to recall the second word. This procedure was repeated two more times with the same pairs but each time in a different order. The score was the total number of pairs recalled (maximum = 30).
Dementia Rating Scale
Participants were administered the Memory Subscale from the Dementia Rating Scale (Mattis, 1976; maximum score = 25 points).
Wechsler Memory Scale-Revised (WMS-R) Logical Memory Subtest
Recall was tested for two short prose passages, each consisting of 25 segments. The first passage was read aloud to the participant, followed by an immediate recall test. The second passage was then read aloud, also followed by an immediate recall test. Recall of both passages was then tested 30 min later. The score was the sum of segments recalled from both passages at the 30-min test.
Testing for the anterograde memory tests followed testing on the retrograde memory test. Six controls were unavailable for further testing after completing the retrograde memory test. Based on the performance of the 15 remaining controls, z-scores were calculated for each individual with MCI for each of the four conventional anterograde memory tests. The four z-scores were then averaged to create a measure of anterograde memory impairment for each individual with MCI.
Analysis Plan
Comparisons between groups were carried out using between-subject t-tests. T-tests with pooled variance are reported unless the SD of one of the groups was more than 2 SD of the other group. In that case, t-tests with separate variance are reported. Arithmetic means and standard errors of the means are reported. The control group was matched to the MCI group according to sex, age, and education. Moreover, for the MCI group, neither age nor education was related to the severity of retrograde or anterograde amnesia. Specifically, age and education were unrelated to mean accuracy on the news events test (r = 0.22, p > 0.43; r = 0.05, p > 0.85, respectively), to the temporal extent of retrograde amnesia (r = 0.31, p > 0.20; r = 0.41, p > 0.10, respectively), or to mean z-scores from the four anterograde memory tests (r = −0.39, p > 0.15; r = 0.02, p > 0.90, respectively). Accordingly, age and education were not included as covariates in the between-group tests. To determine if there was significant impairment in the MCI group for the anterograde memory z-scores, these scores were compared to zero using a one-sample t-test. There was no correction for multiple comparisons.
Results
Anterograde and Retrograde Amnesia in MCI
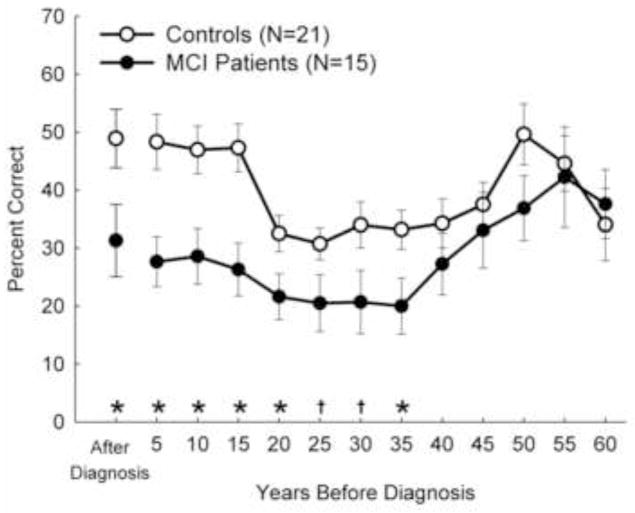
Retrograde memory was severely impaired, covering news events that occurred up to 35 years before the onset of amnesia (Figure 1). Specifically, accuracy on the news events test for those seven 5-year time periods was significantly poorer in the MCI group relative to controls (t values [df = 34] = 2.2–3.4, p values < 0.05–0.005, except time period 25 (t[34] = 1.9, p < 0.07) and time period 30 (t[34] = 2.0, p < 0.06). (Note that the estimate of 35 years is approximate because, as indicated above, there is a 5-year uncertainty about the time of onset of amnesia in individuals with MCI.) Despite the extent of retrograde memory impairment, the impairment was temporally limited. Scores for the MCI group were no different from controls for news events that occurred between 40 and 60 years before the onset of amnesia and were virtually identical for the two most remote time intervals (55 and 60 years before onset). Specifically, the t-tests comparing the MCI and control groups for the 5 most remote time periods were: t(34) = 1.0, p > 0.30; t(33) = 0.6, p > 0.5; t(33) = 1.6, p > 0.10; t(31) = 0.3, p > 0.80; t(24) = −0.4, p > 0.60, respectively. The MCI group also exhibited impaired anterograde memory for news events (Figure 1; t[21] = 2.2, p < 0.05). Likewise, for the four conventional tests of anterograde memory, impairment was observed for both verbal and pictorial material (Table 1). Across these four memory tests, the mean z-score for the MCI group was −2.5 ± 0.4 (and was significantly below zero: t[14] = 5.8, p < 0.001).
Figure 1.
Experiment 1. Anterograde and Retrograde (Semantic) Memory in MCI. Recall performance on a test of 314 news events that occurred from 1931 to 2005. The scores for each member of the MCI group, and one or two controls for each member, have been aligned relative to the year when that individual was diagnosed with amnestic mild cognitive impairment (MCI). The data point at 5 represents 1–5 years before diagnosis, the data point at 10 represents 6–10 years before diagnosis, and so on. The data point After Diagnosis represents the years after diagnosis (these scores are for 9 of the 15 individuals with MCI). Differences in performance between MCI and controls are indicated by *p < 0.05 and †p < 0.07. Error bars indicate SEM.
Relating Anterograde and Retrograde Memory to Heritable Risk for AD and Conversion to Probable AD
Performance on the news events test was related to both ApoE status and to subsequent diagnosis of probable AD. First, the MCI group was divided into two groups depending on whether they had any heritable risk for developing AD (ApoE allele 4/4 and ApoE allele 3/4; n = 7) or no heritable risk of developing AD (ApoE allele 3/3 and ApoE allele 2/3; n = 8). Individuals with a heritable risk for AD performed more poorly on the news events test (i.e., average accuracy score taken across all 5-year periods prior to diagnosis) and exhibited a temporal extent of retrograde amnesia that was substantially longer compared to those with no heritable risk (Table 1). By contrast, none of the measures of cognitive status or anterograde memory differed between these two groups (see Table 1). Moreover, there were also no differences between these two groups when anterograde memory was based on the z-score derived from the four conventional measures (t[13] = 1.1, p > 0.2) nor when it was based on the news events test (t[7] = 1.0, p > 0.3).
Next, individuals with MCI were followed for 3 years after completing the news events test. Three individuals in the MCI group were diagnosed with probable AD within 3 years after completing the news events test, and 9 continued to have a diagnosis of amnestic MCI (the remaining 3 participants were subsequently diagnosed with multiple-domain MCI or withdrew from follow-up visits). Compared to those who remained stable, the individuals who would be subsequently diagnosed with probable AD performed more poorly on the news events test (probable AD, 15.3 ± 2.7% correct; stable MCI, 30.6 ± 5.1 % correct; t[10] = 2.7, p < 0.05) and exhibited a temporal extent of retrograde amnesia that was substantially longer (probable AD, 56.7 ± 4.5 years; stable MCI, 37.2 ± 7.3 years; t[9.7] = 2.3, p < 0.05). None of the other measures in Table 1, including the severity of anterograde amnesia, were related to a subsequent diagnosis of probable AD (MMSE: t[10] = 0.2, p > 0.80; CDR Global Score: t[9.4] = 0.4, p > 0.6; CDR subscales: t values [df = 10] = −0.3 to 0.9, p values > 0.35 to 1.0; anterograde memory z-score: t[10] = −0.4, p > 0.70). In summary, in the MCI group, retrograde amnesia provided the most sensitive measure for detecting heritable risk of AD and conversion to probable AD.
Experiment 2: Relating Anterograde and Retrograde Memory in MCI and MTL Amnesia
In Experiment 1 the MCI group exhibited very severe retrograde amnesia together with significant, but modest anterograde amnesia. This finding was surprising because memory-impaired patients with retrograde amnesia as severe as the MCI group, also normally exhibit very severe anterograde amnesia (Smith, et al., 2013). Next, we directly compared the severity of anterograde amnesia and retrograde amnesia in the MCI group to a group of memory-impaired patients with amnesia resulting from acute damage limited to the hippocampus (H) or larger lesions limited to the medial temporal lobe (MTL).
Method
Participants
The MCI group (N=15) and control group (N=21) from Experiment 1 also participated in Experiment 2.
In addition, 11 H and MTL patients served as a comparison group for the MCI group. The H and MTL patients (1 female) averaged 56.5 ± 3.4 yrs of age and 13.0 ± 0.6 yrs of education. Additional information about this group appears elsewhere (Smith, et al., 2013) and in Supplemental Table S1. As a group, these patients were younger and less educated than the MCI group (t[24] = 6.5, p < 0.001; t[24] = 2.2, p < 0.05, respectively). To calculate the severity of anterograde and retrograde amnesia in the H and MTL group, their scores were compared to a group of 42 healthy controls (as reported previously, Smith, et al., 2013).
Measuring the Severity of Anterograde and Retrograde Amnesia
The scores from Experiment 1 for the severity of anterograde and retrograde amnesia for the MCI group were used for Experiment 2. Specifically, the severity of retrograde amnesia was the temporal extent of retrograde amnesia (in years) and the severity of anterograde amnesia was the mean z-score based on the four conventional anterograde memory tests. The same measures were calculated for the H and MTL group. For the severity of retrograde amnesia, eight to sixteen controls for each patient were identified from the 42 available controls, based on age, education, and when they took the News Events test relative to the patient (within about 1 year). Some controls were matched to more than one patient. The severity of anterograde amnesia for the H and MTL group was calculated relative to 11 the 42 controls who had scores for the four conventional anterograde memory tests.
Relating the Severity of Anterograde Amnesia and Retrograde Amnesia
To measure the relationship between the severity of anterograde and retrograde amnesia, a correlation was computed between the anterograde amnesia z-scores scores and the temporal extent of retrograde amnesia (in years). Separate correlations were computed for the MCI group and the H and MTL group. Because there is an advantage to using the same kind of test to assess both kinds of impairment (Kopelman, 2000; Mayes, Daum, Markowisch, & Sauter, 1997), we also assessed the relationship between anterograde and retrograde amnesia when both scores were taken from the news events test for the 9 individuals with MCI and for the 10 H and MTL patients for whom both scores were available.
Measuring the Amount of Disproportionate Retrograde Amnesia
To identify the amount of retrograde amnesia that was disproportionate to the severity of anterograde amnesia, the temporal extent of retrograde amnesia exhibited by each individual with MCI was subtracted from the temporal extent of retrograde amnesia predicted by the regression line computed from the correlation analysis for the H and MTL patients. Thus, when the anterograde amnesia score for an individual with MCI was less than a z-score of −3.4, the regression line predicted no retrograde amnesia. Accordingly, in these cases, the amount of disproportionate retrograde amnesia was equivalent to the extent of retrograde amnesia exhibited by that individual. Ten of the 15 individuals with MCI fit this circumstance. For each of the other five individuals with MCI, the amount of disproportionate retrograde amnesia was equivalent to the difference between the temporal extent of retrograde amnesia exhibited by the individual and the temporal extent of retrograde amnesia predicted (from the regression line) by an H or MTL patient with the same amount of anterograde amnesia. To identify whether the MCI group exhibited retrograde amnesia that was disproportionate to the severity of their anterograde amnesia, the mean score was compared to zero using a single-sample t-test.
Analysis Plan
The severity of anterograde and retrograde amnesia was compared between the MCI group and the H and MTL group using a repeated-measure, two-way ANOVA [Type of Amnesia X Group]. Follow-up, between-subject t-tests comparing the two groups were carried out for the severity of anterograde and retrograde amnesia. The relationship between the severity of anterograde and retrograde amnesia was calculated separately for the H and MTL group and the MCI group using Pearson’s r. Arithmetic means and standard errors of the means are reported.
Results
Relating Anterograde and Retrograde Memory in MCI and MTL Amnesia
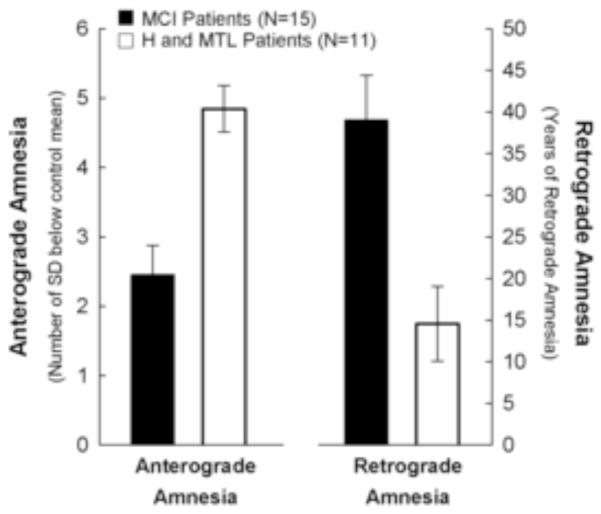
Figure 2 shows the severity of anterograde amnesia (based on the four conventional anterograde memory tests) and the temporal extent of retrograde amnesia for the MCI group1 (and H and MTL group for comparison). The severities of anterograde and retrograde amnesia were different for the MCI group and for the H and MTL group (Interaction: F[1,24] = 8.3, p < 0.01). Specifically, the MCI group exhibited less severe anterograde amnesia (t [24] = 4.2, p < 0.001) but more severe retrograde amnesia (t [24] = 3.3, p < 0.005).
Figure 2.
Experiment 2. Severity of anterograde amnesia and semantic retrograde amnesia for the MCI group and for a group of patients with bilateral lesions of hippocampus (H) or larger medial temporal lobe (MTL) lesions (H and MTL data from Smith, et al., 2013). H and MTL patients exhibited severe anterograde amnesia and limited retrograde amnesia. In contrast, the MCI group exhibited limited anterograde amnesia and severe retrograde amnesia. The dissociation between Group and Type of Amnesia was significant (p < 0.01). The anterograde amnesia score was derived from four tests of new learning ability (see Experiment 1: Methods and Table 1). The retrograde amnesia score represents the severity of retrograde amnesia (duration in years), calculated from the number of 5-year time periods where recall performance of an individual with memory impairment was significantly below control performance (see Experiment 1: Methods). For both anterograde scores and retrograde scores, higher scores indicate more severe impairment. Error bars indicate SEM.
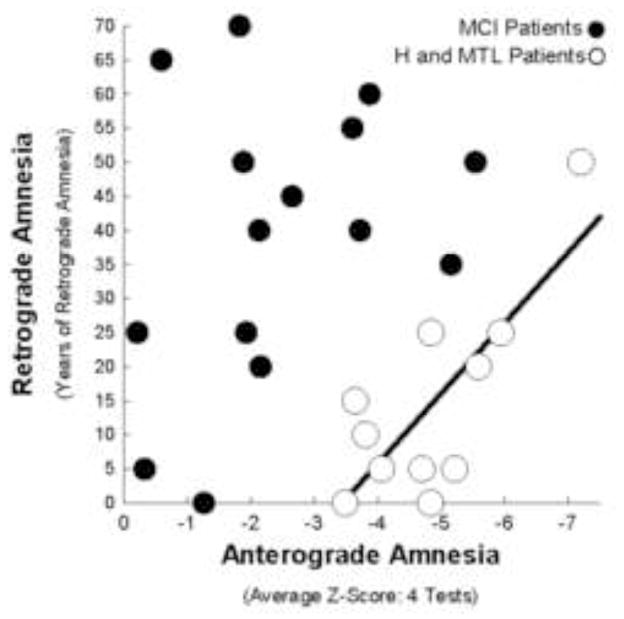
Figure 3 shows the relationship between the severity of anterograde amnesia and the temporal extent of retrograde amnesia for individuals with MCI and for individual H and MTL patients. While the severity of anterograde and retrograde amnesia was closely related in H and MTL patients (r = 0.77, p < 0.01), these measures were only weakly related in the MCI group (r = 0.34, p > 0.20). Moreover, the scores for all the individuals with MCI (except the individual who exhibited no detectable retrograde amnesia) lay outside the 95% confidence intervals for the regression line created from the scores of the H and MTL patients. This result indicates that, based on the relationship between anterograde and retrograde amnesia observed for H and MTL patients, the retrograde amnesia exhibited by the MCI group was disproportionate to anterograde amnesia. Specifically, the MCI group exhibited 30.4 ± 5.4 more years of retrograde amnesia on average than would be expected from the severity of their anterograde amnesia (t[10] = 5.2, p < 0.001).
Figure 3.
Experiment 2. Individual scores showing the relationship between the severity of anterograde amnesia and the severity of retrograde amnesia for the 15 individuals with MCI (black circles) and the 11 patients with bilateral lesions to hippocampus (H) or larger medial temporal lobe (MTL) lesions (white circles). The black regression line was based on the data from the H and MTL patients (Smith, et al., 2013). There was a close relationship between anterograde amnesia and retrograde amnesia for the H and MTL patients (r = 0.77, p < 0.01). For the MCI group the relationship was weak (r = 0.34, p > 0.20), and retrograde amnesia was variable. All individuals with MCI (except the single individual with no detectable retrograde amnesia) lay outside the 95% confidence intervals for the regression line. Anterograde amnesia and retrograde amnesia are defined as in Figure 2.
Relating Anterograde and Retrograde Memory with the Same Test
We also assessed the relationship between anterograde and retrograde amnesia for the MCI group and the H and MTL group when both scores were taken from the news events test. The finding of disproportionate retrograde amnesia in the MCI group was similar when anterograde memory was based on the news events test and when it was based on the four conventional measures of anterograde memory. That is, the MCI group still exhibited disproportionate retrograde amnesia relative to anterograde amnesia when both anterograde and retrograde amnesia were calculated from the news events test. Specifically, they exhibited 21.6 ± 3.1 more years of retrograde amnesia on average than would be expected from the severity of their anterograde amnesia (t[8] = 5.7, p < 0.001).
Experiment 3: Relating Predictions from Behavioral Findings to Brain Measures in the MCI Group
Three predictions about the brain emerged from the findings of Experiments 1 and 2 regarding the severity of anterograde and retrograde amnesia in MCI. First, the finding that the MCI group had less severe anterograde amnesia than H and MTL patients (Figure 2) suggests that they also have less severe atrophy of the MTL. Second, because anterograde and retrograde amnesia have an orderly relationship when atrophy is limited to the MTL (see Figure 3, H and MTL patients), one might suppose that individuals who deviate from that relationship have atrophy outside the MTL. Accordingly, the second prediction is that the MCI group has significant atrophy that extends beyond the MTL. The third prediction follows from the second. The extent to which the severity of retrograde amnesia in MCI is disproportionate to the severity of anterograde amnesia will be related to the integrity of cortical regions outside the MTL. These three predictions were tested for 11 of the 15 individuals in the MCI group for whom structural brain scans were available. Because structural scans were available for only these 11 individuals, analyses were limited to testing the three predictions that emerged from the behavioral findings and to the brain regions of interest (see below).
Brain measures were evaluated to test three predictions that emerged from the behavioral findings. Regional brain volumes and neocortical thickness were first measured for the MCI group and then compared to controls. Correlations were then carried out between brain measures in the MCI group and the severity of anterograde amnesia (composite z-score from 4 conventional tests) and the temporal extent of disproportionate retrograde amnesia (in years).
Method
Participants
Eleven individuals from the MCI group in Experiments 1 and 2 participated (2 females, 78.5 ± 2.0 years of age). The other four individuals with MCI were either unavailable or ineligible for magnetic resonance imaging (MRI). For comparison, structural MRI scans were obtained for 11 healthy control participants from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). In addition, percent reductions in the volumes of the hippocampus and parahippocampal gyrus are reported for nine of the 11 H and MTL patients reported in Experiment 2 for comparison. For two H patients (patients RB and GD), MRI scans were not available.
Obligatory Statement Regarding the ADNI Objective
The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many coinvestigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 subjects but ADNI has been followed by ADNI-GO and ADNI-2. To date these three protocols have recruited over 1500 adults, ages 55 to 90, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org.
Measuring Regional Brain Volumes and Neocortical Thickness
Image Acquisition and Processing
High-resolution, T1-weighted, structural MRI scans were obtained for participants in the MCI group. The scans were acquired on a General Electric (GE) 1.5T scanner at the UCSD Radiology Imaging Laboratory (4 individuals) or on a GE 3T scanner at the UCSD Center for Functional Magnetic Resonance Imaging (7 individuals). For all individuals with MCI an 8-channel, high-resolution head coil was used. In-plane resolution was 1 x 1 mm and thickness was 1 or 1.2 mm (Echo Times: 2.8–3.9 sec; Repetition Times: 6.5–8.8 sec; Flip Angles: 8 or 12 degrees). The average interval between the completion of behavioral testing and acquisition of the brain scan was 3.5 ± 1.4 months for the MCI group. For the control group, raw dicom files from high-resolution, T1-weighted, structural MCI scans were obtained from ADNI in August, 2010. The controls were matched to the 11 individuals with MCI on the basis of age (79.1 ± 1.6 years of age), sex (2 female), magnet strength (4 controls scanned at 1.5 T and 7 controls scanned at 3 T), as well as manufacturer and type of head coil (i.e., GE scanner with an 8-channel head coil). For 2 individuals with MCI, control scans were selected that matched the individual based on age, sex, and magnet strength but that had been acquired on a Siemens scanner.
Measures of cortical thickness, cortical volume, and hippocampal volume were calculated for the MCI and control groups using FreeSurfer software (version 5.1) (Dale, Fischl, & Sereno, 1999; Fischl et al., 2002; Fischl, Sereno, & Dale, 1999; Fischl et al., 2004). Briefly, the automated software divides the brain into cortical grey matter, white matter, cerebrospinal fluid (CSF), and deep, grey matter structures. Next, each voxel within the cerebral cortex is assigned a neuroanatomical label according to a probabilistic atlas (Desikan et al., 2006). For volume segmentation and cortical surface reconstruction, the cortical surface is divided into 34 distinct areas in each hemisphere. Cortical thickness of each area is calculated as the distance from the grey/white boundary to the grey/CSF boundary, averaging across multiple measurements within each area. Cortical thickness does not depend on head size, so this measure was not adjusted for intracranial volume. However, cortical volumes and hippocampal volume were adjusted relative to intracranial volume as calculated by Freesurfer (estimated total intracranial volume; Buckner et al., 2004). Manual intervention was carried out to correct errors associated with distinctions between brain and pia/skull boundaries and between grey matter and white matter boundaries. Implementation of these corrections was carried out blind to group membership.
For the 9 H and MTL patients, volumes for the hippocampus and parahippocampal gyrus were estimated based on high-resolution, T1-weighted magnetic resonance images. The scores were compared to 19 age-matched, healthy males for KE, EP, GP, RS, GW, and JRW, and 11 age-matched, healthy females for patient LJ (Gold & Squire, 2005). These brain measures have been reported previously (Bayley, et al., 2006; Press, Amaral, & Squire, 1989; Smith, et al., 2013). Briefly, the hippocampus and parahippocampal gyrus (temporopolar cortex, entorhinal cortex, perirhinal cortex, and parahippocampal cortex) were drawn on the structural MRI images according to histological landmarks readily visible on MRI (Franko, Insausti, Artacho-Perula, Insausti, & Chavoix, 2014; Insausti et al., 1998). Values for the left and right hemispheres were combined. The volume of each region was then divided by the total brain volume. For patients LM and WH, the percent reduction scores were based on measurements of the average area of the hippocampus and parahippocampal gyrus obtained from three consecutive 5-mm images from T1-weighted, structural MRI scans (starting from the pes hippocampus and proceeding caudally). The areas for the left and right hemispheres were then combined. The area of each region was then divided by the total area of the temporal lobe bilaterally (from the fundi of the collateral sulcus to the inferior limiting sulci of the insular cortex). The scores for these two patients were compared to 4 controls, as previously reported by Press, Amaral, and Squire (1989).
The percent volume reduction measure was calculated for all individuals in the MCI and the H and MTL groups relative to their respective controls. To determine if each group exhibited smaller volumes of the hippocampus and parahippocampal gyrus, the percent volume reductions were compared to zero using a one-sample, t-test.
A Priori Areas of Interest
Analyses (see below) were carried out for seven brain areas of interest. These were areas previously related to the severity of retrograde amnesia (Barr, Goldberg, Wasserstein, & Novelly, 1990; Bayley, et al., 2005; Bayley, et al., 2006; Bright, et al., 2006; Eustache et al., 2004; Kopelman, 1991; Kopelman, et al., 2003; O’Connor, Butters, Miliotis, Eslinger, & Cermak, 1992; Reed & Squire, 1998), areas where atrophy has previously been reported in MCI (Bakkour, et al., 2009; Fennema-Notestine, et al., 2009; McDonald, et al., 2009; McEvoy, et al., 2009; Whitwell, et al., 2007), or areas where atrophy has been associated with decline from MCI to probable AD (Bakkour, et al., 2009; McEvoy, et al., 2009; Whitwell, et al., 2007). The seven areas were as follows: hippocampus, parahippocampal gyrus, lateral temporal cortex, inferior parietal lobule, precuneus, posterior cingulate gyrus, and the prefrontal cortex.
Analysis Plan
The objective was to identify brain areas where measures (volumes and cortical thickness) were different between the MCI group and controls or where brain measures were associated with behavioral measures (anterograde or retrograde amnesia). Statistical tests were carried out for each of the seven a priori areas of interest. When necessary, the areas computed by FreeSurfer were combined together to obtain the measures for an area of interest (parahippocampal gyrus = temporal pole + entorhinal cortex + parahippocampal cortex; lateral temporal cortex = fusiform gyrus + inferior + middle + superior temporal gyri; posterior cingulate gyrus = posterior cingulate cortex + isthmus cingulate; prefrontal cortex = caudal and rostral anterior cingulate gyri + caudal and rostral middle frontal gyri + lateral and medial orbitofrontal gyri + paracentral lobule + pars opercularis + pars orbitalis + pars triangularis + superior frontal gyrus + frontal pole). For volume measures, the values were summed together prior to correcting for intracranial volume. For thickness measures, the values were combined using a weighted average based on surface area. These methods were carried out separately for each hemisphere. Measures of regional brain volumes and neocortical thickness for the MCI group and controls were compared using between-subject t-tests. Detection of between-group differences may be affected by differences in scanner strength (Han et al., 2006). Accordingly, tests comparing brain measures between the MCI group and controls included a covariate for scanner strength (1.5 T or 3T). By contrast, detection of within-subject, brain-behavior relationships is robust to differences in field strength (Dickerson et al., 2008). Accordingly, correlational analyses investigating the relationship between brain measures and behavioral measures within the MCI group did not include scanner strength as a covariate. Within the MCI group, the relationships between brain measures (brain volumes, neocortical thicknesses) and behavioral measures (anterograde amnesia, retrograde amnesia) were assessed with Pearson’s r. Because age and education were uncorrelated with the severity of anterograde and retrograde amnesia (See Experiment 1: Methods), these measures were not included as covariates in the brain-behavior correlations. Probabilities (uncorrected for multiple comparisons) are reported for each a priori area of interest. If a statistical test was significant for the left or right hemispheres for each region of interest, a bilateral test was also carried out. For completeness, significant results obtained in areas other than the a priori areas of interest are reported as well. Arithmetic means and standard errors of the means are reported.
Finally, a best subsets regression analysis was carried out to determine which variables best predicted the amount of disproportionate retrograde amnesia in the MCI group. For this analysis, the predictor variables were the 5 brain regions associated with the amount of disproportionate retrograde amnesia (see Table 2 and Results: Prediction 3, below) and ApoE status (see Table 1). Follow-up, linear regression analyses were carried out on the regression models with the fewest predictors and that explained the most variance (according to adjusted R squared).
Table 2.
Experiment 3. Brain Measures for the MCI and Control Groups
| Region of Interest | Control Group (N=11) | MCI Group (N=11) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mean (SEM) | Mean (SEM) | Effect Size (MCI vs. Control) | Correlation with Retrograde Amnesia | Correlation with Anterograde Amnesia | ||||||
|
| ||||||||||
| Cohen’s d | Pearson r | Pearson r | ||||||||
|
|
|
|||||||||
| VOL | THK | VOL | THK | VOL | THK | VOL | THK | VOL | THK | |
| MEDIAL TEMPORAL LOBE | ||||||||||
| L Hippocampus | .229 (.010) | -- | .182 (.009) | -- | 1.5** | -- | .59† | -- | .76* | -- |
| R Hippocampus | .239 (.011) | -- | .199 (.011) | -- | 1.1** | -- | .50 | -- | .56† | -- |
| Bil. Hippocampus | .468 (.021) | -- | .381 (.018) | -- | 1.3** | -- | .61* | -- | .74** | -- |
| L Parahippocampal Gyrus | .377 (.013) | 2.95 (.08) | .327 (.019) | 2.68 (.10) | 0.9* | 0.9† | .33A | −.03 | .48A | .16 |
| R Parahippocampal Gyrus | .364 (.012) | 3.01 (.09) | .292 (.016) | 2.65 (.12) | 1.5** | 1.1* | .43 | .23 | .17 | −.14 |
| Bil. Parahippocampal Gyrus | .742 (.023) | 2.98 (.07) | .620 (.032) | 2.67 (.10) | 1.3** | 1.1* | .41 | .12 | .38 | .00 |
| NEOCORTEX | ||||||||||
| L Lateral Temporal Cortex | 2.525 (.077) | 2.46 (.03) | 2.436 (.087) | 2.35 (.06) | 0.3 | 0.7 | .47 | −.17 | .08 | .13 |
| R Lateral Temporal Cortex | 2.510 (.060) | 2.52 (.02) | 2.456 (.086) | 2.39 (.06) | 0.2 | 0.9* | .56†B | −.03 | .08 | −.07 |
| Bil. Lateral Temporal Cortex | 5.035 (.136) | 2.49 (.02) | 4.892 (.167) | 2.37 (.05) | 0.3 | 0.9† | .53† | −.07 | .06 | .03 |
| L Inferior Parietal Lobule | .712 (.027) | 2.25 (.05) | .758 (.049) | 2.29 (.05) | −0.4 | −0.2 | .23 | −.02 | .35 | −.03 |
| R Inferior Parietal Lobule | .889 (.036) | 2.24 (.04) | .889 (.052) | 2.28 (.06) | −0.0 | −0.2 | .46 | −.17 | .36 | .15 |
| L Precuneus | .535 (.022) | 2.17 (.05) | .521 (.005) | 2.16 (.05) | 0.2 | 0.0 | .24 | −.12 | .17 | .01 |
| R Precuneus | .521 (.018) | 2.11 (.04) | .537 (.023) | 2.11 (.06) | −0.2 | 0.0 | .04 | −.05 | −.17 | .06 |
| L Posterior Cingulate Gyrus | .328 (.011) | 2.32 (.04) | .327 (.012) | 2.31 (.07) | 0.0 | 0.1 | .46C | .35 | −.05 | .36 |
| R Posterior Cingulate Gyrus | .317 (.013) | 2.35 (.05) | .325 (.014) | 2.26 (.04) | −0.2 | 0.6 | −.10 | −.22 | −.38 | .00 |
| L Prefrontal Cortex | 4.151 (.109) | 2.36 (.03) | 4.373 (.143) | 2.33 (.06) | −0.5* | 0.2 | .19 | −.30 | .16 | −.32 |
| R Prefrontal Cortex | 4.117 (.096) | 2.35 (.03) | 4.332 (.114) | 2.31 (.05) | −0.6* | 0.3 | .17 | −.27 | .22 | −.37 |
| Bil. Prefrontal Cortex | 8.268 (.203) | 2.35 (.03) | 8.704 (.276) | 2.32 (.05) | −0.5 | 0.2D | .19 | −.29 | .19 | −.35 |
Note. Means and (SEM) for volume (VOL) and thickness (THK, mm) for the Mild Cognitive Impairment (MCI) group and the control group for 7 regions of interest (see Experiment 3: Methods). Volume measures for each region denote the percentage of total intracranial volume. Brain-behavior correlations were carried out to relate volume and thickness measures to anterograde amnesia (derived from 4 tests of new learning ability) and retrograde amnesia (derived from a test of notable news events) in the MCI group. The measure of retrograde amnesia was the extent to which the severity of retrograde amnesia was disproportionate to the severity of anterograde amnesia (see Experiment 2: Methods). Effect sizes for differences between the MCI and control groups are reported as Cohen’s d and reflect the difference between the means, in standard deviations. Effect sizes for the brain-behavior correlations are reported as Pearson r. Significant differences in the mean volume or mean thickness for the MCI group relative to controls or for brain-behavior correlations within the MCI group are: Significant effects are highlighted in bold text and the levels of significance are indicated by the symbols:
p < 0.01,
p < 0.05, and
p < 0.10.
Parahippocampal Cortex was marginally significant when examined separately;
The correlation was stronger (r = 0.59, p < 0.06) when the fusiform gyrus was omitted;
Posterior Cingulate Cortex was significant when examined separately;
Ventrolateral Prefrontal Cortex was significant when examined separately.
Results
Prediction 1: The MCI group will exhibit less severe MTL atrophy than the H and MTL group
As indicated by structural MRI scans, the MCI group had a smaller volume of the hippocampus bilaterally (18.7 ± 3.9% reduction in volume) and parahippocampal gyrus bilaterally (16.5 ± 4.3% reduction in volume) than controls (Table 2). In addition, parahippocampal gyrus bilaterally was 10.4 ± 3.2% thinner in the MCI group relative to controls (Table 2, Figure 4). The H and MTL group exhibited significant volume reduction in the hippocampus (55.9 ± 8.5% reduction; t[8] = 6.4, p < 0.001). For the parahippocampal gyrus, the two MTL patients exhibited substantial volume reduction in the parahippocampal gyrus (94.0 ± 0.0% reduction in volume), whereas the seven H patients exhibited almost none (1.7 ± 4.1% reduction in volume). As a group, the H and MTL patients did not exhibit significant volume reduction in the parahippocampal gyrus (22.2 ± 13.9% reduction; t[8] = 1.6, p > 0.10). Accordingly, volume reduction in the hippocampus was more severe in the H and MTL group than in the MCI group (t[11.3] = 3.9, p < 0.005), whereas volume reduction in the parahippocampal gyrus was similar for the two groups (t[9.5] = 0.4, p > 0.70). Thus, consistent with Prediction 1, volume loss in the MTL was less severe in the MCI group than in the H and MTL group.
Figure 4.
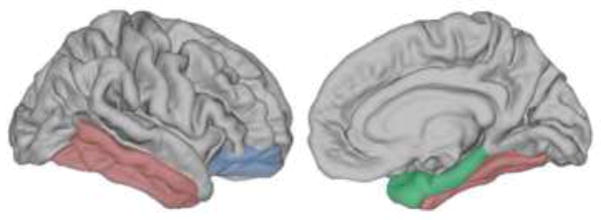
Brain regions where neocortex was thinner in the MCI group (N=11) compared to controls (N=11). (Lateral view on the left, medial view on the right). The MCI group exhibited thinner cortex (and smaller volumes) in the structures of the medial temporal lobe (MTL)--parahippocampal gyrus 10% thinner and 16% reduced in volume [green] and hippocampus 19% reduced in volume [not pictured]. Consistent with Prediction 1 (see Experiment 3), the reduction in volume of MTL structures was less severe in the MCI group than in the H and MTL group. Consistent with Prediction 2 (see Experiment 3), the MCI group also exhibited atrophy of areas outside of the MTL. Specifically, neocortex was 6% and 7% thinner than controls bilaterally in lateral temporal cortex (red) and bilaterally in ventrolateral prefrontal cortex (blue), respectively. The lateral temporal cortex includes fusiform, inferior temporal, and middle temporal gyri. Ventrolateral prefrontal cortex includes lateral orbitofrontal and parsorbitalis cortices. Parahippocampal gyrus includes temporopolar, entorhinal, and parahippocampal cortices. All comparisons between the MCI group and controls were significant at p < 0.05. Abbreviations are as in Figure 2.
Interestingly, the severity of anterograde amnesia in the MCI group, as measured by the four conventional anterograde memory tests, was related to the volume of the hippocampus bilaterally (r = 0.74, p < 0.01). Table 2). Anterograde amnesia was also weakly related to the volume of left parahippocampal cortex (r = 0.57, p < 0.07) and significantly related to the volume of the left caudal anterior cingulate was also associated with anterograde amnesia (r = 0.64, p < 0.05). For all these areas, more severe anterograde amnesia was associated with smaller volumes. Measures of cortical thickness were not correlated with the severity of anterograde amnesia.
Prediction 2: The MCI group will exhibit significant brain atrophy that extends beyond the MTL
Consistent with Prediction 2, the MCI group exhibited significantly thinner neocortex outside of the MTL (Table 2, Figure 4). Specifically, cortex was thinner in the MCI group than in controls in the right lateral temporal cortex (5.2 ± 2.2% thinner) and was thinner bilaterally when the superior temporal gyrus was excluded (6.1 ± 2.1% thinner; t[20] = 2.7, p < 0.05). In addition, thinner cortex was observed in the MCI group relative to controls in two adjacent areas in the ventrolateral prefrontal cortex (bilateral pars orbitalis, 8.2 ± 2.7% thinner, t[20] = 3.5, p < 0.01; bilateral lateral orbital frontal gyrus, 6.1 ± 2.9% thinner, t[20] = 3.1, p < 0.01). Measures of neocortical volume outside the MTL did not differ between the MCI group and controls (Table 2), except for left and right prefrontal cortex which were a little larger in the MCI group than in controls (5.3 ± 3.4% and 5.2 ± 3.3% larger, respectively; note though that the volume of prefrontal cortex bilaterally was not different between the MCI and control groups; t[20] = 1.6, p > 0.10).
Prediction 3: The degree that retrograde amnesia is disproportionate to anterograde amnesia will be related to the integrity of neocortical brain regions
Consistent with Prediction 3, the amount of disproportionate retrograde amnesia in MCI was related to the volume of left posterior cingulate cortex (r = 0.64, p < 0.05) and paracentral lobule bilaterally (r = 0.60, p < 0.05), a region adjacent to the posterior cingulate cortex. The amount of disproportionate retrograde amnesia was also (marginally) related to the volume of bilateral lateral temporal cortex (r = 0.53, p < 0.10; Table 2). A stronger relationship was observed for the right lateral temporal cortex when the fusiform gyrus was excluded (r = 0.59, p < 0.06). For all these regions, smaller volumes were associated with more disproportionate retrograde amnesia (Figure 5). A relationship was also observed between the amount of disproportionate retrograde amnesia and the volume of the hippocampus bilaterally (r = 0.61, p < 0.05; Table 2), and a marginal relationship was observed for the volume of left parahippocampal cortex (r = 0.58, p < 0.07), though the volumes of these regions were also related to the severity of anterograde amnesia. It is important to note that the amount of disproportionate retrograde amnesia was not related to the volumes in the areas of prefrontal cortex that were thinner in MCI relative to controls (bilateral pars orbitalis, r = −0.42, p > 0.20; bilateral lateral orbital frontal gyrus, r = 0.16, p > 0.60) nor was it related to the volume of the prefrontal cortex as a whole (Table 2). Measures of neocortical thickness outside of the MTL were not correlated with the disproportionality of retrograde amnesia in the MCI group (Table2). Thus, regions in the MTL were related to the severity of both anterograde and retrograde amnesia, whereas neocortical regions outside the MTL were related only to the severity of retrograde amnesia.
Figure 5.
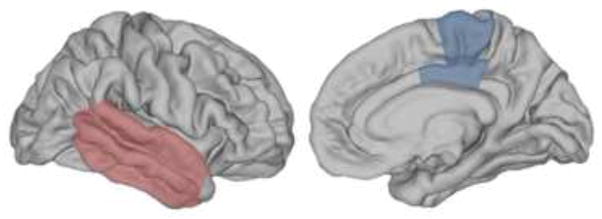
Consistent with Prediction 3 (see Experiment 3), smaller volumes of right lateral temporal cortex (p < 0.06, red) and left posterior cingulate cortex and paracentral lobule bilaterally (p values < 0.05, blue), were associated with the extent to which the severity of retrograde amnesia was disproportionate to the severity of anterograde amnesia in the MCI group (N=11; see Experiment 2, Measuring the Amount of Disproportionate Retrograde Amnesia). (Lateral view on the left, medial view on the right). The lateral temporal cortex includes inferior, middle, and superior temporal gyri. Anterograde amnesia and retrograde amnesia are defined as in Figure 2.
Next, we asked which variables best predicted the amount of disproportionate retrograde amnesia in the MCI group. A best subsets regression analysis was carried out using the five brain regions that were significantly or marginally correlated with the amount of disproportionate retrograde amnesia (see Figure 5; bilateral hippocampus, left parahippocampal cortex, right lateral temporal cortex [minus the fusiform gyrus], left posterior cingulate cortex, and paracentral lobule bilaterally). In addition, ApoE status was included as a predictor because it was closely related to the severity of retrograde amnesia (see Table 1 and Experiment 1). The best subsets regression analysis indicated that adjusted R squared was highest for a model that included ApoE status and the hippocampus and paracentral lobule bilaterally. A follow-up, linear regression analysis revealed that these three variables could reliably predict the amount of disproportionate retrograde amnesia in the MCI group (Adjusted R squared = 0.56; F [3,7] = 5.3, p < 0.05).
Discussion
Anterograde memory and retrograde memory for news events were assessed in 15 individuals with MCI and in controls. For the MCI group, anterograde memory was modestly impaired (Table 1). In contrast to the modest anterograde memory impairment exhibited by the MCI group, retrograde amnesia for news events was severe and spanned nearly 40 years before the onset of amnesia (Figure 1; Table 1). The severity of retrograde amnesia (and not the severity of anterograde amnesia or other measures of cognitive status), was the most predictive of heritable risk of AD and conversion to probable AD in the MCI group (Table 1).
Interestingly, in comparison to a group of 11 patients with acute damage limited to hippocampus or larger lesions of the MTL, the MCI group exhibited less severe anterograde amnesia and more temporally extensive retrograde amnesia (Figure 2). The severity of retrograde amnesia exhibited by the MCI group was disproportionate to their anterograde amnesia (whether anterograde amnesia was assessed by the test of news events or was assessed by the four conventional anterograde memory tests) and was more severe than would have been expected if their atrophy was limited to the MTL (Figure 3).
These behavioral findings generated three predictions regarding the integrity of brain regions in the MCI group. First, relative to H and MTL patients, the more modest anterograde amnesia exhibited by the MCI group should also be associated with more modest MTL atrophy. This prediction was confirmed for the hippocampus bilaterally (Table 2). Second, individuals with MCI who deviate from the orderly relationship between anterograde and retrograde amnesia exhibited by H and MTL patients would be expected to have atrophy outside the MTL. This expectation was confirmed by an analysis of cortical thickness in the MCI group, which revealed that lateral temporal cortex bilaterally and ventrolateral prefrontal cortex bilaterally were thinner than in controls (Table 2, Figure 4). Third, the extent to which the severity of retrograde amnesia was disproportionate to the severity of anterograde amnesia should be related to the integrity of neocortical regions outside the MTL. The volumes of three areas in neocortex (Table 2, Figure 5) were related to the disproportionality of retrograde amnesia in the MCI group (right lateral temporal cortex, left posterior cingulate cortex, and paracentral lobule bilaterally). Overall, the pattern was that the integrity of the MTL was associated with both anterograde and retrograde amnesia, whereas the integrity of neocortical regions was uniquely associated with the disproportionality of retrograde amnesia relative to anterograde amnesia (note that because of the relatively small sample size [N=11] for the brain analyses, it is possible that the present findings do not identify all the relevant brain regions important for anterograde or retrograde amnesia). Finally, the volumes of both the paracentral lobule and hippocampus bilaterally (in conjunction with ApoE status) were successful at predicting the amount of disproportionate retrograde amnesia in the MCI group.
When identifying the year of onset of amnesia in individuals with MCI, some time must undoubtedly elapse between the onset of symptoms and the time when the diagnosis can be made. The decline from healthy cognitive status to a level of function consistent with a diagnosis of MCI typically occurs within about 5 years (Acosta-Baena, et al., 2011; Marquis, et al., 2002). Accordingly, performance on some parts of the news events test could reflect impaired anterograde memory, i.e., impaired memory for events that occurred after the onset of symptoms but before diagnosis. Thus, the estimate for the temporal extent of retrograde amnesia in MCI could be as much as five years too long. Note, though, that even allowing for a 5-year error in the estimate, the MCI group still exhibited severe retrograde amnesia that was disproportionate to the severity of their anterograde amnesia (i.e., see Figure 3 and subtract five years from the retrograde amnesia score of each member of the MCI group).
We considered using a test of autobiographical memory, in addition to the news events test. Unfortunately, the tools available to measure autobiographical memory do not easily lend themselves to the analysis carried out here. Specifically, most of the tests (Kopelman, Wilson, & Baddeley, 1989; Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002) sample only a few time periods. Better tests could be constructed [see Bayley, Hopkins, and Squire (2003) for use of the method introduced by Crovitz and Schiffman (1974)], but even then it would be difficult to achieve the 5-year temporal resolution across the life span that is available from the news events test. In any case, the present findings relate to the relationship between anterograde amnesia and retrograde amnesia for news events. A different relationship might be observed between anterograde amnesia and retrograde amnesia for autobiographical information.
The volume loss in lateral temporal cortex of the MCI group may be particularly important for understanding their retrograde amnesia. In other individuals with damage to lateral temporal cortex (as the result of temporal lobectomy, encephalitis, or an abscess), damage to this region has been associated with severe retrograde amnesia that appeared disproportionate to the severity of anterograde amnesia (Barr, et al., 1990; Bright, et al., 2006; O’Connor, et al., 1992; Reed & Squire, 1998). Additionally, the integrity of lateral temporal cortex in AD and MCI has been previously associated with memory for semantic retrograde memory (Barbeau, et al., 2012; Gilboa, et al., 2005; Woodard et al., 2009). It is possible that variability in the integrity of lateral temporal cortex, the hippocampus and paracentral lobule bilaterally, and ApoE status (as revealed the linear regression analysis in Experiment 3) might account for differences in the severity of retrograde amnesia that have been reported in studies of MCI (Leyhe, et al., 2010; Leyhe, et al., 2009; Murphy, et al., 2008; Seidenberg, et al., 2009; Thomann, et al., 2012).
Note that frontal lobe damage and lateral temporal lobe damage have both been associated with retrograde amnesia (Bayley, et al., 2005; Kopelman, 1991; Kopelman, et al., 2003; Kopelman, et al., 1999; Kroll, et al., 1997). In the present study, we found no relationship in the MCI group between retrograde amnesia and the integrity of the prefrontal cortex. Though the MCI group exhibited thinner cortex in ventrolateral prefrontal cortex bilaterally, neither the thickness nor volume of this area was related to their disproportionate retrograde amnesia (in addition, the volumes of left and right prefrontal cortex were larger in the MCI group than in controls, Table 2). Thus, their disproportionate retrograde amnesia is likely related to the integrity of lateral temporal neocortex rather than to the integrity of frontal neocortex.
If the severity of retrograde amnesia in MCI is related to atrophic changes in neocortex, which stores (at least in part) the content of semantic memory, why then does the MCI group exhibit retrograde amnesia that is temporally limited? Should not all memories be compromised regardless whether they are recent or remote? One way to understand the sparing of remote memory in MCI is to suppose that memories become strengthened with repeated rehearsal and that the oldest memories have the most opportunity for rehearsal/replay and strengthening (Fuster, 2009; McClelland, McNaughton, & O’Reilly, 1995; Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006). Indeed, in healthy adults and in AD, how often memories have been retrieved was a better predictor of news event memory than how long ago the event had occurred (Muller et al., 2014). Likewise, memories that are especially personally significant (and that are also likely to be strong) tend to be less vulnerable to disruption in AD (Martinelli, Anssens, Sperduti, & Piolino, 2013). According to this idea, when neocortex is compromised, the stronger memories are more likely to be spared than weaker memories, and memories are strongest in the most remote time periods.
Measures of semantic retrograde memory, such as the News Events Test, may be particularly sensitive predictors of cognitive decline and conversion to AD. In the present study, the members of the MCI group who converted to probable AD in the three years following testing had approximately 20 more years of retrograde amnesia than the members who remained stable during that time frame. Consistent with this finding, performance of healthy adults on a test of famous names from the remote past (individuals who became famous 45–60 years prior to testing) predicted cognitive decline across 1.5 years (Seidenberg et al., 2013). Furthermore, brain activity in a network of brain regions (including lateral temporal cortex and posterior cingulate gyrus) by the same individuals when performing this task also predicted cognitive decline across the same time period (Woodard et al., 2010). It is notable that another study of MCI, which used a combined measure of semantic memory (famous faces, general facts, and public events), did not predict conversion to AD during a 3-year period (Barbeau, et al., 2012). It would be interesting to analyze the data in that study separately for public events. Performance on semantic memory tests for public events or famous people may be more predictive of cognitive decline and conversion to AD than performance on tests concerning general knowledge.
In summary, the MCI group exhibited only modest anterograde amnesia but extensive retrograde amnesia for news events, compared to patients with lesions limited the H and MTL. Compared to a group of H and MTL patients, given the modest severity of anterograde amnesia in the MCI group, the severity of their retrograde amnesia was greater than would have been expected if their atrophy were limited to the MTL. The modest severity of the anterograde amnesia corresponded to modest atrophy in the MTL, whereas the severe retrograde amnesia corresponded to atrophy in three neocortical regions. Volumes of structures in the MTL were related to both anterograde and retrograde amnesia, whereas volumes in specific regions of neocortex were related only to the degree that the temporal extent of retrograde amnesia was disproportionate to the severity of anterograde amnesia. The amount of disproportionate retrograde amnesia in the MCI group was best predicted by a model that incorporated heritable risk for AD in addition to the integrity of medial temporal lobe and neocortical structures.
Supplementary Material
Acknowledgments
I thank Larry R. Squire, Ashley Knutson, Susan McDuff, Mark Starr, Erin Light, Jennifer Frascino, Anna van der Horst, Flora Suh, James Brewer, Marc Bondi, Adam Fleisher, Adam Dede, and the Alzheimer’s Disease Research Center at UCSD for assistance. The work was funded by an NSF grant SMA-1041755 to the Temporal Dynamics of Learning Center, an NSF Science of Learning Center, NIMH Grant 5 RO1 MH24600, and NIA Grant P50 AG005131. Data collection and sharing for this project was partially funded by ADNI (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
Footnotes
Note that when the estimate for the temporal extent of retrograde amnesia for the MCI group was based on all 21 controls (39.0 ± 5.3 years, see Figure 2) it was almost identical to when it was based on only the 15 controls who completed both the four anterograde tests and as well as the retrograde test (38.7 ± 5.1 years).
References
- Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, Saldarriaga A, Lopera F. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet neurology. 2011;10(3):213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. Brain Cogn. 2009;69(3):504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau EJ, Didic M, Joubert S, Guedj E, Koric L, Felician O, Ranjeva JP, Cozzone P, Ceccaldi M. Extent and neural basis of semantic memory impairment in mild cognitive impairment. Journal of Alzheimer’s disease : JAD. 2012;28(4):823–837. doi: 10.3233/JAD-2011-110989. [DOI] [PubMed] [Google Scholar]
- Barr WB, Goldberg E, Wasserstein J, Novelly RA. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990;28:243–256. doi: 10.1016/0028-3932(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005;46(5):799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38(1):135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26(51):13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzozero I, Lucchelli F, Saetti MC, Spinnler H. Autobiographical memory in amnestic Mild Cognitive Impairment. Neurological sciences. 2012;33(5):1145–1153. doi: 10.1007/s10072-011-0928-2. [DOI] [PubMed] [Google Scholar]
- Braak E, Braak H. Alzheimer’s disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol. 1997;93(4):323–325. doi: 10.1007/s004010050622. [DOI] [PubMed] [Google Scholar]
- Bright P, Buckman JR, Fradera A, Yoshimasu H, Colchester ACF, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learning & Memory. 2006;13:545–557. doi: 10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder A. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn B, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39(1):10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron JC, Desgranges B. ’In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain. 2004;127(Pt 7):1549–1560. doi: 10.1093/brain/awh166. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Hagler DJ, Jr, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AM. Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Hum Brain Mapp. 2009;30(10):3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany RJ, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically Parcelling the Human Cerebral Cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Crook T, Bartus RT. Implications of memory and language dysfunction in the naming deficit of senile dementia. Brain Lang. 1987;31(2):187–200. doi: 10.1016/0093-934x(87)90069-1. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State - Practical Method for Grading Cognitive State of Patients for Clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franko E, Insausti AM, Artacho-Perula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. 2014;35(1):248–256. doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. Journal of Cognitive Neuroscience. 2009;21(11):2047–2072. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cuetos F, Fasano F, Pellegrini FF, Marchi M, Venneri A, Caffarra P. Brain structural substrates of semantic memory decline in mild cognitive impairment. Current Alzheimer research. 2013;10(4):373–389. doi: 10.2174/1567205011310040004. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Ramirez J, Kohler S, Westmacott R, Black SE, Moscovitch M. Retrieval of autobiographical memory in Alzheimer’s disease: relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15(4):535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15(1):79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Irish M, Lawlor BA, O’Mara SM, Coen RF. Exploring the recollective experience during autobiographical memory retrieval in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 16(3):546–555. doi: 10.1017/S1355617710000172. [DOI] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau EJ, Didic M, Poncet M, Ceccaldi M. Patterns of semantic memory impairment in Mild Cognitive Impairment. Behav Neurol. 2008;19(1–2):35–40. doi: 10.1155/2008/859657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD. Frontal dysfunction and memory deficits in the alcoholic Korsakoff syndrome and Alzheimer-type dementia. Brain : a journal of neurology. 1991;114(Pt 1A):117–137. [PubMed] [Google Scholar]
- Kopelman MD. Focal retrograde amnesia and the attribution of causality: An exceptionally critical review. Cognitive Neuropsychology. 2000;17(7):585–621. doi: 10.1080/026432900750002172. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Lasserson D, Kingsley DR, Bello F, Rush C, Stanhope N, Stevens TG, Goodman G, Buckman JR, Heilpern G, Kendall BE, Colchester AC. Retrograde amnesia and the volume of critical brain structures. Hippocampus. 2003;13(8):879–891. doi: 10.1002/hipo.10140. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N, Kingsley D. Retrograde amnesia in patients with diencephalic, temporal lobe or frontal lesions. Neuropsychologia. 1999;37:939–958. doi: 10.1016/s0028-3932(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical and Experimental Neuropsychology. 1989;5:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kroll NE, Markowitsch HJ, Knight RT, von Cramon DY. Retrieval of old memories: the temporofrontal hypothesis. Brain. 1997;120(Pt 8):1377–1399. doi: 10.1093/brain/120.8.1377. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Leyhe T, Muller S, Eschweiler GW, Saur R. Deterioration of the memory for historic events in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia. 2010;49:4093–4101. doi: 10.1016/j.neuropsychologia.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Leyhe T, Muller S, Milian M, Eschweiler GW, Saur R. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia. 2009;47(12):2464–2469. doi: 10.1016/j.neuropsychologia.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, Camicioli R. Independent predictors of cognitive decline in healthy elderly persons. Archives of Neurology. 2002;59(4):601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Anssens A, Sperduti M, Piolino P. The influence of normal aging and Alzheimer’s disease in autobiographical memory highly related to the self. Neuropsychology. 2013;27(1):69–78. doi: 10.1037/a0030453. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. In: Bellack R, Keraso B, editors. Geriatric PsychiatryX. New York: Grune and Stratton; 1976. pp. 77–121. [Google Scholar]
- Mayes AR, Daum I, Markowisch HJ, Sauter B. The relationship between retrograde and anterograde amnesia in patients with typical global amnesia. Cortex. 1997;33(2):197–217. doi: 10.1016/s0010-9452(08)70001-7. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;3:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr, Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73(6):457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Holland D, Karow DS, Pung CJ, Brewer JB, Dale AM. Alzheimer Disease: Quantitative Structural Neuroimaging for Detection and Prediction of Clinical and Structural Changes in Mild Cognitive Impairment. Radiology. 2009;251(1):195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter M, Eijsackers EV, Mulder JL. Retrograde amnesia for autobiographical memories and public events in mild and moderate Alzheimer’s disease. J Clin Exp Neuropsychol. 2006;28(6):914–927. doi: 10.1080/13803390591001043. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Muller S, Mychajliw C, Hautzinger M, Fallgatter AJ, Saur R, Leyhe T. Memory for Past Public Events Depends on Retrieval Frequency but not Memory Age in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2014;38(2):379–390. doi: 10.3233/JAD-130923. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, Troyer AK, Levine B, Moscovitch M. Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia. 2008;46(13):3116–3123. doi: 10.1016/j.neuropsychologia.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M, Butters N, Miliotis P, Eslinger P, Cermak LS. The dissociation of anterograde and retrograde amnesia in a patient with herpes encephalitis. Journal of Clinical and Experimental Neuropsychology. 1992;14:159–178. doi: 10.1080/01688639208402821. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complexe [The test of copying a complex figure] Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Press GA, Amaral DG, Squire LR. Hippocampal abnormalities in amnesic patients revealed by high-resolution magnetic resonance imaging. Nature. 1989;341:54–57. doi: 10.1038/341054a0. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. Journal of Neuroscience. 1998;(18):3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Zhang Q, Gander A, Antuono P, Rao SM. Semantic knowledge for famous names in mild cognitive impairment. Journal of the International Neuropsychological Society : JINS. 2009;15(1):9–18. doi: 10.1017/S1355617708090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Kay CD, Woodard JL, Nielson KA, Smith JC, Kandah C, Guidotti Breting LM, Novitski J, Lancaster M, Matthews M, Hantke N, Butts A, Rao SM. Recognition of famous names predicts cognitive decline in healthy elders. Neuropsychology. 2013;27(3):333–342. doi: 10.1037/a0032226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Lonie JA, Bastin ME, Terriere E, O’Carroll RE, Ebmeier KP. MRI correlates of episodic memory in Alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Res. 2010;184(1):57–62. doi: 10.1016/j.pscychresns.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Smith CN, Frascino JC, Hopkins RO, Squire LR. The nature of anterograde and retrograde memory impairment after damage to the medial temporal lobe. Neuropsychologia. 2013;51(13):2709–2714. doi: 10.1016/j.neuropsychologia.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Remote memory as affected by aging. Neuropsychologia. 1974;12:429–435. doi: 10.1016/0028-3932(74)90073-6. [DOI] [PubMed] [Google Scholar]
- Squire LR, Shimamura AP. Characterizing amnesic patients for neurobehavioral study. Behavioral Neuroscience. 1986;100:866–877. doi: 10.1037//0735-7044.100.6.866. [DOI] [PubMed] [Google Scholar]
- Taylor LB. Scoring criteria for the Rey-Osterrieth Complex Figure Test. In: Spreen O, Strauss E, editors. A compendium of neuropsychological tests. Administration, norms, and commentary. New York: Oxford University Press; 1998. pp. 350–351. [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Thomann PA, Seidl U, Brinkmann J, Hirjak D, Traeger T, Wolf RC, Essig M, Schroder J. Hippocampal morphology and autobiographic memory in mild cognitive impairment and Alzheimer’s disease. Current Alzheimer research. 2012;9(4):507–515. doi: 10.2174/156720512800492558. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS, Salmon DP, Fennema-Notestine C. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging. 2010;31(7):1107–1121. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130(Pt 7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132(Pt 8):2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Guidotti L, Zhang Q, Butts A, Hantke N, Lancaster M, Rao SM. Prediction of cognitive decline in healthy older adults using fMRI. Journal of Alzheimer’s disease : JAD. 2010;21(3):871–885. doi: 10.3233/JAD-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.