Abstract
Aim
Bariatric surgery improves glycemic control, but not all patients achieve type 2 diabetes (T2D) remission. Thus, we aimed to identify metabolic determinants of T2D non-remission status following bariatric surgery at 12 and 24 months (m).
Methods
Forty adults (BMI: 36±3kg/m2, Age: 48±9y, HbA1c: 9.7±2%) undergoing bariatric surgery (i.e. RYGB or SG) were enrolled in STAMPEDE. T2D remission was defined as HbA1c <6.5% and fasting glucose <126 mg/dl without anti-diabetic medication. Indices of insulin secretion and sensitivity were calculated from plasma glucose, insulin and C-peptide during a 120 min MMTT. Body fat (DXA), incretins (GLP-1, GIP, ghrelin), and adipokines (adiponectin, leptin, TNF-α, hs-CRP) were also assessed.
Results
At 24m, 37 subjects had follow-up data (n = 18 RYGB and n = 19 SG). Bariatric surgery-induced 40% and 27% T2D remission rates at 12 and 24m, respectively. Total fat/abdominal fat loss, insulin secretion, insulin sensitivity, and β-cell function (C-peptide0–120/Glucose0–120 × Matsuda index) improved more in remitters at 12 and 24m than non-remitters. Incretin levels were unrelated to T2D remission, but, compared to non-remitters, hs-CRP decreased and adiponectin increased more in remitters. Only baseline adiponectin predicted lower HbA1c at 12 and 24m, and elevated adiponectin correlated with enhanced β-cell function, lower triglycerides and fat loss.
Conclusions
Smaller rises in adiponectin, a mediator of insulin action and adipose mass, depict T2D non-remission up to 2 years after bariatric surgery. Adjunctive strategies promoting greater fat loss and/or raising adiponectin may be key for higher T2D remission rates after bariatric surgery.
Keywords: gastric bypass, sleeve gastrectomy, glycemic control, diabetes, obesity, insulin secretion
INTRODUCTION
Bariatric surgery has proven efficacy in both long-term weight loss and glycemic control (1–5). In fact, bariatric surgery produces complete and persistent type 2 diabetes remission (6, 7), and is currently recognized by the International Diabetes Federation (8) and American Diabetes Association (9) as a viable treatment option for obese adults with type 2 diabetes. However, despite marked weight loss, approximately 30–70% of individuals do not achieve type 2 diabetes remission following bariatric surgery (10, 11).
Previous work reports that patients with longer pre-operative type 2 diabetes duration, poorer glycemic control, and higher medication use (i.e. insulin use or medication count) characterize individuals with a low propensity for type 2 diabetes remission (12, 13). However, these studies provide no insight into the metabolic determinants of type 2 diabetes non-remission status following bariatric surgery. Decompensated pancreatic β-cell insulin secretion for the prevailing degree of insulin resistance is the hallmark characteristic of type 2 diabetes disease severity (14, 15). The underlying etiology of this reduced insulin action is likely multifactorial, but excess body fat contributes to the dysregulation of adipokines, including high leptin and TNF-α as well as low adiponectin, that impair insulin signaling and secretion (16). Indeed, hypoadiponectemia is recognized is an independent biomarker for type 2 diabetes, and improvements in adipokines following bariatric surgery are related to favorable weight loss and insulin action (17). Recently, we demonstrated in the Surgical Treatment and Medication Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial that patients randomized to bariatric surgery achieved better glycemic control compared with intensive medical therapy (IMT). We had hypothesized that this enhanced reduction in blood glucose after surgery was related to improved GLP-1 and ghrelin responses that mediated greater fat loss and enhanced β-cell function (2, 18, 19). However, it is unclear if blunted improvements in these gastrointestinal hormones or adipokines determine type 2 diabetes non-remission. Therefore, we tested the hypothesis that non-remission status would be characterized by the presence of attenuated fat loss, smaller improvements in insulin sensitivity and β-cell function, blunted GLP-1 and ghrelin responses, and a less favorable adipokine profile (e.g. higher leptin and lower adiponectin) at 12 and 24 month following bariatric surgery. The observations gained from this work may contribute to the identification of metabolic profiles in patients with type 2 diabetes who have blunted glycemic responses to bariatric surgery. From a physiologic and clinical perspective, understanding if gut-mediated or adipose-derived inflammation plays a chief role in diabetes remission may optimize efforts to develop treatment strategies (e.g. incretin agonists or anti-obesity agents) that improve remission rates.
METHODS
Subjects and Design
The STAMPEDE study was a single center prospective study in which 150 patients were randomized to IMT, Roux-en-Y Gastric Bypass (RYGB) surgery + IMT or Sleeve Gastrectomy (SG) surgery + IMT, with stratification by use of insulin at screening (2). For purposes of this sub-study 40 participants who had undergone surgical intervention and underwent additional metabolic testing of glucose regulation were included in this analysis (18, 19). As part of the IMT all subjects received nutritional counseling by a certified diabetes educator, and ADA lifestyle guidelines combined with use of the latest FDA approved drug therapy, including incretin analogs or mimetics and insulin sensitizers. Prior to randomization, our nutrition, psychology, bariatricians, and surgery teams cleared subjects for bariatric surgery. Bariatric procedures were performed as previously reported (2). Type 2 diabetes remission at 12 and 24 months was defined as a HbA1c <6.5% and a fasting blood glucose <126 mg/dl with no diabetes medication therapy, while normal HbA1c is considered <6.0%. In the event a subject no longer met diabetes remission criteria at 24 months, they were analyzed as a non-remitter (n=5). Anti-diabetes medication sum was defined as the total number of diabetes agents prescribed. Participants were verbally briefed about the study and signed informed consent documents approved by the Cleveland Clinic Institutional Review Board.
Anthropometrics
Height and weight were obtained in a standard hospital gown on a calibrated scale and wall-mounted stadiometer (Veeder-Root, Elizabethtown, NC) at baseline, 12, and 24 months. BMI was calculated as body mass (kg) divided by height (m)2. Total body fat and android body fat was assessed using dual-energy x-ray absorptiometry (iDXA, Lunar Prodigy, Madison, WI).
Glucose Regulation
After an approximate 12-hour overnight fast, a mixed meal tolerance test (MMTT; Boost™; 8 oz; 350 kcal; 55% CHO, 25% PRO, 20% Fat) was performed. Non-remitters were instructed to withhold anti-diabetic medications 24-hour prior to MMTT testing to minimize effects on glucose metabolism. Medication post-surgery was prescribed in attempt to manage HbA1c <6.0%. Blood samples were collected from an antecubital vein at 0, 30, 60, 90 and 120 min. Glucose, insulin, and C-peptide concentrations were used to calculate indices of glucose tolerance, insulin sensitivity (Matsuda index) and β-cell function (18). Since insulin sensitivity is mostly reflective of skeletal muscle glucose uptake, we multiplied fasting glucose and fasting free fatty acids (FFA) by fasting insulin, respectively, to estimate hepatic and adipose insulin resistance. Hepatic insulin resistance was divided by 1000 for data presentation. Pre-hepatic insulin secretion rate (ISR) was reconstructed by deconvolution from plasma C-peptide (20), and glucose-stimulated ISR incremental area under the curve (iAUC) was divided by glucose iAUC during the MMTT. iAUC was calculated by the trapezoidal model. Pancreatic β-cell function, or the oral disposition index, was calculated by multiplying glucose adjusted C-peptide secretion by the Matsuda index. Hepatic extraction of insulin was estimated by dividing C-peptide by insulin iAUC0–120 during the MMTT. Gastric inhibitory peptide (GIP), glucagon-like polypeptide-1 (GLP-1) and acylated ghrelin were determined at 0 and 60 minutes of the MMTT, and meal responses were defined as: Post-prandial – fasting (19).
Biochemical Analysis
Whole-blood glucose was measured immediately after collection using the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH). HbA1c was measured in whole blood by Turbidimetric Inhibition Immunoassay (Cleveland Clinic Laboratories, Cleveland, OH). The remaining blood was centrifuged at 4°C for 10 minutes and frozen at −70°C until subsequent analysis. To minimize inter-assay variability, blood measurements for each subject were analyzed on the same plate. Plasma triglycerides and cholesterol were analyzed using enzymatic methods with an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Plasma insulin and C-peptide was assayed by RIA (Linco Research, St. Charles, MO). GIP (total), GLP-1 (active), and acylated ghrelin were treated immediately with a DPP-4 and protease cocktail inhibitor (Sigma, St. Louis, MO) and were assayed using an ELISA (ALPCO diagnostics, Salem, NH and Kamiya Biomedical Company, Seattle, WA). FFAs were determined by standard colorimetry methods (Wako Chemical, Richmond, VA). Low-grade inflammation (hs-CRP) and adipokines (TNF-α, leptin and total adiponectin were also assayed by ELISA (R&D systems, Minneapolis, MN).
Statistical Analysis
An approximate 10% attrition rate occurred during the study leaving 18 subjects in the RYGB group and 19 in the SG group. Categorical factors were described using frequencies and percentages, while continuous measures were described using medians and quartiles. Group differences throughout the study were evaluated using Wilcoxon rank sum tests, while categorical group variables were assessed using Fisher exact tests. Associations were described using Spearman correlations, and significance was accepted as P≤0.05 using R software (Vienna, Austria).
RESULTS
Pre-operative Demographics
Non-remitters were older, had longer duration of type 2 diabetes, and took more anti-diabetic medications despite having similar BMIs and android body fat. Higher medication usage was predominantly from incretin mimetics (86.3% yes in non-remitters vs. 46.6% yes in remitters, P<0.05; Table 1). There was no difference in oral biguanide, insulin, secretagogues and thiazolidinedione use. At baseline, there were no differences in blood lipids or hypertension. However, fasting C-peptides were lower and plasma leptin, percent total body fat, and insulin sensitivity was higher in non-remitters compared with remitters (P<0.05; Table 2 and Figure 1).
Table 1.
Comparison of pre-operative demographic factors between remitters and non-remitters at 12 and 24 months.
| 12 month | 24 month | |||||||
|---|---|---|---|---|---|---|---|---|
| Demographics | Total (n) | Non-Remitter | Total (n) | Remitter | Total (n) | Non-Remitter | Total (n) | Remitter |
| Age (y)a | 22 | 50.6[42.7, 58.9] | 15 | 44.4[36.9, 51.2]* | 27 | 49.9[42.9, 58.1] | 10 | 39.3[36.52, 50.17]* |
| Female (n)b | 16 | 72.7 | 8 | 53.3 | 19 | 70.3 | 5 | 50 |
| White (n)b | 15 | 68.1 | 10 | 66.67 | 19 | 70.3 | 6 | 60 |
| Diabetes Duration (y)a | 22 | 9.5[4.7, 11.7] | 15 | 4[3, 8.5]* | 27 | 9 [4, 11] | 10 | 3.5[3, 7.5]* |
| Insulin use (%)b | 18 | 54.5 | 6 | 40 | 14 | 51.8 | 4 | 40 |
| T2D Medication Sum (n)a | 22 | 5[4,5] | 15 | 4[2.5, 4]* | 27 | 5 [4, 5] | 10 | 3.5[2.25, 4]* |
| Randomized SG (%)b | 15 | 68.1 | 4 | 26.6^ | 16 | 59.2 | 3 | 30 |
| Randomized RYGB (%)b | 7 | 31.8 | 11 | 73.3 | 11 | 40.7 | 7 | 70 |
Data are reported as
Median [25%, 75%] or
Percentage when appropriate. Type 2 diabetes remission defined as HbA1c <6.5%, fasting glucose < 126 mg/dl and no anti-diabetic medication.
Compared to non-remitter at baseline, Δ 12 month or Δ 24 month
P<0.05 using Wilcoxon ran sum test. Compared to non-remitter
P<0.05 using Fisher's Exact Test for Count Data. Medication sum defined as the total diabetes medication prescribed.
SG = sleeve gastrectomy. RYGB = Roux-en-Y Gastric Bypass. T2D = type 2 diabetes.
Table 2.
Comparisons between non-remitter and remitter following bariatric surgery on blood glucose regulation at 12 and 24 months.
| Non-Remitter | Remitter | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Baseline | Δ 12 month | Baseline | Δ 24 month | Baseline | Δ 12 month | Baseline | Δ 24 month |
| Body Composition | ||||||||
| BMI (kg/m2) | 35.5 [34.3, 3] | −8.3 [−9.7, −6.6] | 35.4[34.5,38.0] | −7.2 [−9.4, −6.2] | 37.1 [35.0, 37.7] | −9.8 [−11.2, −8.9]* | 37.5[35.4,38.8] | −10.1[−12.5, −8.1]* |
| Weight (kg) | 98.5 [86, 109.7] | −22.5 [−26, −18] | 98.9 [89.6, 109.5] | −18.5 [−28, −16.6] | 105.8 [96.7, 114] | −29.9[−34, −24.5]* | 108.9[97, 119.4] | −26.9[−38.4, −21.9]* |
| Body Fat (%) | 46.2 [42, 48.5] | −10 [−11.6, −7.6] | 44.6 [40.7, 47.6] | −7.8 [−11.0, −4.6] | 40.4 [38.7, 45.5]* | −14.4[−17, −12.6]* | 42.6[39.7, 46.1] | −10.4[−15.9, −7.8] |
| Android Fat (%) | 58 [55.4, 59.1] | −13.6 [−15, −11] | 57.1[53.5, 58.9] | −12.8 [−16.6, −8.3] | 56.9 [53.7, 60] | −18.6[−25, −16.7]* | 59.3[55.4, 60.3] | −13.3[−22.0, −11.4] |
| Glucose Regulation | ||||||||
| F-PG (mg/dl) | 184[129, 241] | −59 [−107, −29] | 196 [144.5, 232.5] | −100 [−123.5, −6] | 209 [170.5,224.5] | −127[−140, −81.5]* | 210.5 [150.5, 249.2] | −139[−170, −70] |
| F-PI (µU/ml) | 11.6[6,23.1] | −4.9[−11.3,0.6] | 14.5[7.0,25.4] | −5.7[−17.2, −1.3] | 24.6[18.5,50.0]* | −20[−39.3, −13.7]* | 22.5[17.6,51.8]* | −15.0[−44.1,10]* |
| F-C-pep (ng/ml) | 1.5[1.0,2.5] | −0.1[−0.9,0.4] | 1.9[1.1,2.6] | −0.4[−1.1,0.1] | 3.2[2.3,4.2]* | −1.4[−2.0, −0.8]* | 3.3[2.5,4.1]* | −1.8[−2.0, −1.2]* |
| F-FFA (mEq/L) | 0.6 [0.4, 0.8] | −0.0 [−0.2, 0.0] | 0.6 [0.4, 0.7] | 0.09 [−0.08, 0.2] | 0.62 [0.5, 0.74] | −0.1 [−0.2, −0.0] | 0.5 [0.5, 0.7] | −0.03 [−0.1, 0.0] |
| PG iAUC (mg/dl-2h) | 119[92.8, 141.9] | 19.1[−52.8, 77.3] | 115.3[82.5,134.2] | 24.5[−31.0, 66.6] | 87.6 [74.8, 117]* | −1.2 [−16.6, 31.2] | 88.8 [66.3, 113.6] | 6.6 [−28.9, 28.4] |
| PI iAUC (µU/ml-2h) | 16.8 [10.4, 32.2] | 38.9 [14.7, 56.8] | 19 [7.7, 34.3] | 39.6 [13.9, 71.0] | 34.1 [8.5, 46.2] | 88.7[58.1, 111]* | 34.6 [13.5, 37.4] | 71.4 [57.4, 90.4] |
| C-pep iAUC (ng/ml-2h) | 1.45 [0.67, 2.11] | 2.2 [1.6, 3.0] | 1.3 [0.6, 2.2] | 2.5 [1.3, 4.6] | 2.1 [0.7, 3.2] | 4.4 [3.9, 6.7]* | 2.1 [1.6, 3.1] | 3.6[2.5, 5.4] |
| Hepatic Extraction | 12.8 [9.4, 18.2] | −0.16[−4.0, 3.7] | 12.9[9.5,18.1] | −1.1[−3.9, 4.6] | 15.6 [12.0, 19.5] | 0.16[−5.6, 5.8] | 15.3[11.6, 20.3] | 0.8 [−3.4, 5.4] |
| Hepatic-IR | 1.9 [0.9, 3.4] | −1.0 [−2.3, −0.2] | 2.3 [1.0, 4.7] | −1.2 [−3.2, −0.2] | 5.3 [3.6, 9.1]* | −5.1 [−8.8, −3.3]* | 5.8[3.4, 8.6]* | −5.1[−8.2, −2.9]* |
| Adipose-IR | 4.4 [2.8, 12.0] | −3.9 [−10.6, −2.4] | 6.9 [2.8, 12.7] | −4.1[−12.1, −2.4] | 11.4[9.2, 16.4]* | −12.0[−13.7, −9.0]* | 11.72 [10.3, 13.0]* | −12.2[−13.5, −11.0]* |
| Matsuda Index | 3.6 [2.0, 6.3] | 1.33 [0.0, 2.8] | 2.8 [1.5, 6.3] | 1.8[0.1, 2.9] | 1.3[0.8, 1.8]* | 5.3 [3.5, 7.0]* | 1.3 [1.1, 1.8]* | 4.8[3.4, 5.7]* |
| ISR iAUC0–120 | 0.8 [−0.3, 1.5] | 1.7[1.3, 2.4] | 0.8[−0.4, 1.5] | 2.2[0.8, 3.8] | 0.9 [−0.4, 1.4] | 3.9 [2.6, 7]* | 1.0 [0.3, 1.4] | 3.1[1.9, 5.6] |
| ISR/Glucose iAUC0–120 | 0.01 [0, 0] | 0.01 [0, 0.0] | 0.01 [0, 0.0] | 0.02[0, 0.03] | 0.01 [0, 0.02] | 0.05 [0.03, 0.0]* | 0.01 [0.01, 0.01] | 0.04 [0.02, 0.07] |
| Gut Hormones | ||||||||
| F-GIP (pg/ml) | 3.6 [2.6, 8.7] | −1.5[−3.5, −0.2] | 3.5 [2.3, 8.7] | −0.2 [−2.9, 1.6] | 2.7 [1.35, 7.4] | −0.2 [−1.9, 0] | 3.0 [1.2, 8.2] | 0 [−3.8, 0.6] |
| GIP stim (pg/ml) | 33.7 [16.5, 53.0] | −4.4 [−20, 19.4] | 34.5 [19.2, 53.8] | −14.5[−26.2, 23.0] | 41.2 [26.8, 70.7] | −17.7 [−55, −4.0] | 39.9 [27.3, 70.7] | −16.2 [−38.1, −9.2] |
| F-GLP-1 (pg/ml) | 1.1 [0.6, 2.0] | 0 [−0.3, 0.6] | 1.1 [0.6, 1.7] | 0 [−0.3, 0.7] | 0.8 [0.6, 1.5] | 0 [−0.1, 0.2] | 0.7 [0.6, 2.0] | 0 [0, 1.0] |
| GLP-1 stim (pg/ml) | 2.0 [1.05,3.4] | 9.9[5.1, 18.1] | 2 [1.0, 3.3] | 8.1 [4.6, 16.1] | 2.6 [1, 3.1] | 9.2 [5, 11.15] | 2.6 [1.0, 3.1] | 3.7 [−0.5, 5.3]* |
| F-ghrelin (fmol/ml) | 6.2[2.8, 11.2] | −2.7 [−6.3, −0.5] | 6.7 [2.8, 11.0] | −4.2 [−6.8, −1.6] | 5.7 [3.1, 8.7] | −1.0 [−3.9, 0.9] | 4.8 [2.4, 6.3] | −0.6 [−3.7, 1.3] |
| Ghrelin supp (fmol/ml) | −1.5 [−3.2, 1.2] | −0.9[−2.8, 1.6] | −1.6 [−3.9, 1.2] | −1.01 [−3.5, 2.5] | −1.7 [−4.4, −0.1] | 0.8 [−3.7, 3.8] | −1.4 [−3.6, −0.4] | −0.31 [−1.1, 2.3] |
There were 22 (59%) and 27 (73%) non-remitters at 12 and 24 months, respectively. Data are reported as median [25%, 75%]. Δ Refers to the change between Post-Pre.
Compared to non-remitter at baseline, Δ 12 month or Δ 24 month
P<0.05 using Wilcoxon ran sum test.
BMI = body mass index. F-PG = fasting plasma glucose. F-PI = fasting plasma insulin. F-C-pep = fasting plasma C-peptide. F-FFA = fasting free fatty acid. Stim = stimulation. Supp = suppression. iAUC = incremental area under the curve. Hepatic insulin resistance was divided by 1000 for data presentation. ISR = insulin secretion rate derived from deconvolution of plasma C-peptide. GIP = gastric inhibitory polypeptide. GLP-1 = glucagon-like polypeptide-1. Multiply ghrelin fmol/ml by 3.37 to get pg/ml. Gut hormone stimulation/suppression = Post-prandial (60 min) – Fasting (0 min). Gut hormone stimulation/suppression = Post-prandial (60 min) – Fasting (0 min).
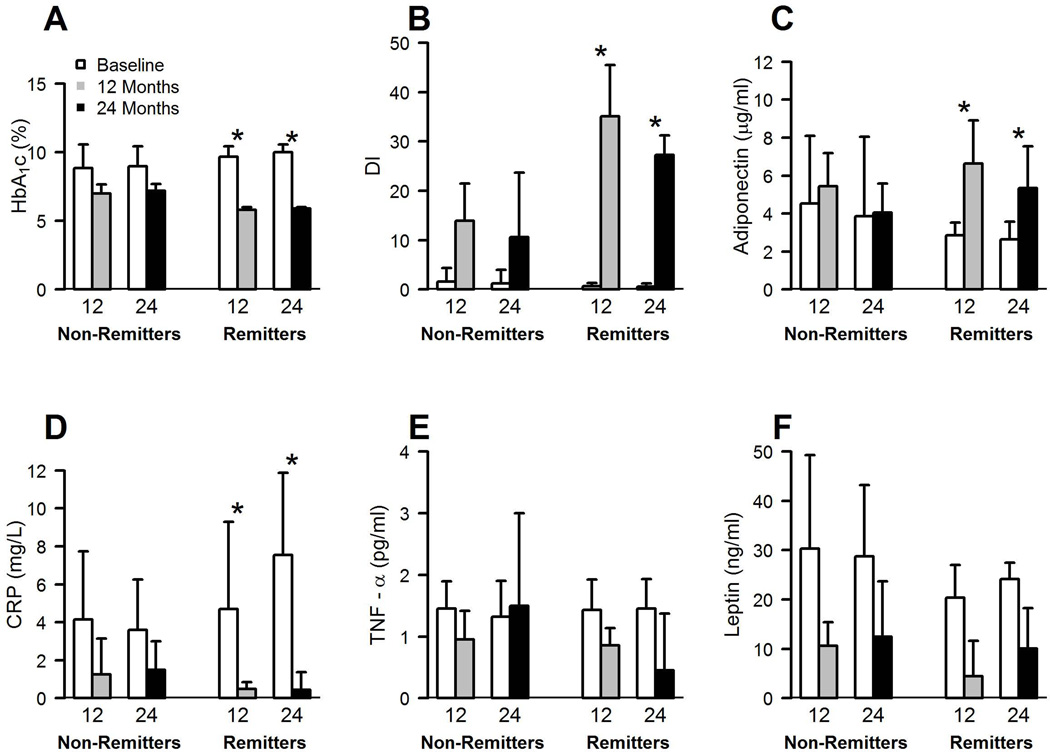
Figure 1.
Comparisons between non-remitter and remitter after bariatric surgery on glucose regulation and adipokines at 12 and 24 months. DI = disposition index or pancreatic β-cell function. TNF-α = tumor necrosis factor-α. CRP = high sensitivity C-reactive protein.
Blood Glucose Regulation
Of the 37 subjects included in this analysis, there were 22 (59%) and 27 (73%) non-remitters at 12 and 24 months, respectively. Bariatric surgery reduced fasting glucose and HbA1c after 12 and 24 months in both remitters and non-remitters. However, as expected, non-remitters had attenuated reductions in fasting glucose and HbA1c at 12 and 24 months compared with remitters (P<0.05; Table 2 and Figure 1). Non-remitters required anti-diabetic medication following surgery at 12 (median[25%,75%]; 1[0,1.7] vs. 0[0,0], P<0.01) and 24 months (1[0,2] vs. 0[0,0], P<0.01) compared with remitters. The main medications prescribed at 12 and 24 months to non-remitters were biguanides (~55–62 vs. 0%, P<0.01) and incretin mimetics (~22–29 vs. 0%, trend: P≤0.07). Improvements in insulin sensitivity and pancreatic β-cell function were blunted in non-remitters compared with remitters at 12 and 24 months (P<0.01; Table 2 and Figure 1). Plasma FFAs and hepatic insulin extraction was not different between remitters and non-remitters following bariatric surgery. Fasting and meal stimulated GLP-1, GIP and acylated ghrelin were also not statistically different at 12 months between remitters and non-remitters. However, the GLP-1 meal response was statistically higher in non-remitters compared with remitters at 24 months (P<0.05; Table 2).
Body Composition
At 12 months, compared with remitters, non-remitters were characterized by smaller reductions in body weight (P<0.05), percent total body fat (P<0.01; Table 2), and android body fat (P<0.01). These blunted improvements in body weight (P<0.05) and total body fat (P<0.09) persisted up to 24 months in non-remitters compared with remitters (Table 3).
Adipokines and Blood Lipids
Circulating TNF-α and leptin were not statistically different between remitters and non-remitters before or after bariatric surgery, although hs-CRP was reduced less in non-remitters at 12 and 24 months (P<0.05; Figure 1). In addition, plasma adiponectin was elevated to a greater extent in remitters than non-remitters at 12 and 24 months, respectively (P<0.01; Figure 1), and these results were independent of changes in body weight, diabetes duration and age. Plasma triglycerides or total cholesterol were not statistically different between groups after bariatric surgery, although HDL levels were significantly higher in remitters than non-remitters at 12 months (P<0.05; Supplemental Table 1).
Correlation Analysis
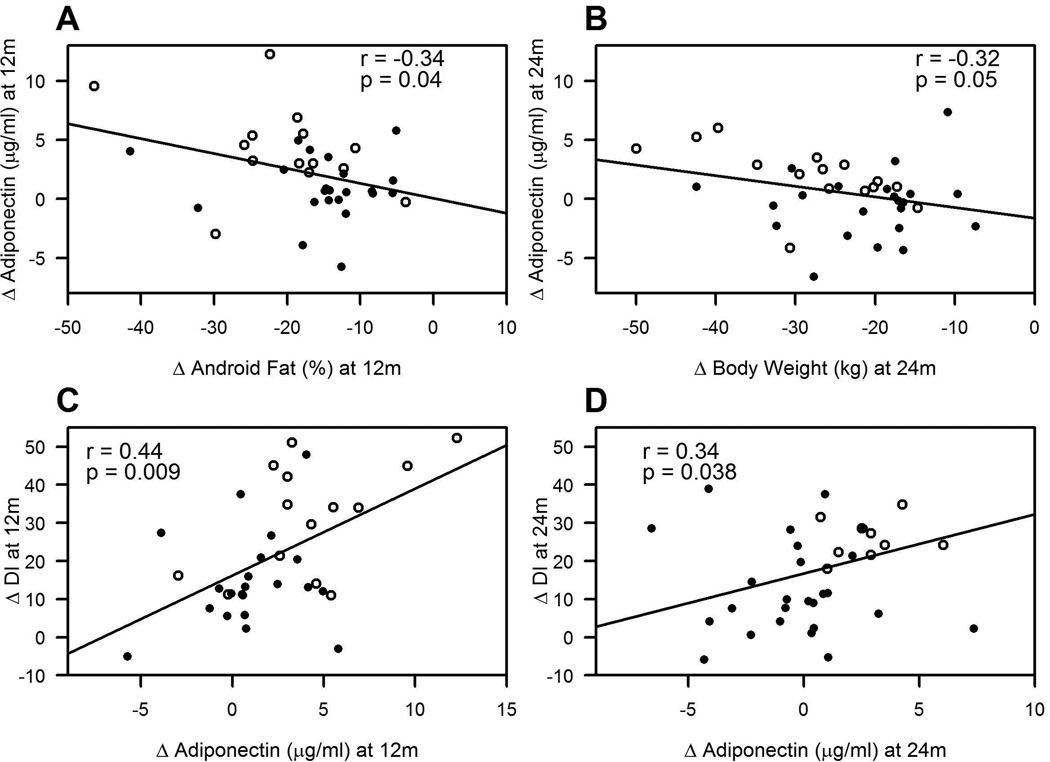
At 12 months, normal HbA1c correlated with low baseline adiponectin (r=0.34, P<0.04), low MMTT glucose iAUC (r=0.39; P<0.04), and less need for medication (r=0.35, P<0.05), but not meal-induced changes in GLP-1 (r=0.05, P=0.75), GIP (r=−0.08, P =0.62) or ghrelin (r=0.23, P=0.18). At 24 months, normal HbA1c also correlated with low baseline adiponectin (r=0.33, P<0.05), and less medication usage (r = 0.40, P<0.02), but not following meal-induced changes in GLP-1 (r=−0.02, P=0.92), GIP (r=−0.15, P =0.38) or ghrelin (r=0.28, P=0.09). Increased adiponectin (i.e. Post minus Pre) at 12 months was associated with reduced android body fat (r=−0.35, P<0.05; Figure 2a), less hepatic insulin resistance (r=−0.29, P=0.08), enhanced glucose-stimulated insulin secretion (GSIS; r=0.33, P<0.05), and β-cell function (r=0.44, P<0.009 Figure 2c). Elevated plasma adiponectin at 24 months was linked with reduced body weight (r=−0.32, P=0.05 Figure 2b), lower hepatic insulin resistance (r=−0.40, P<0.02), decreased adipose insulin resistance (r=−0.51, P<0.01), reduced triglycerides (r=−0.35, P<0.04), and enhanced glucose-stimulated insulin secretion (r=0.40, P<0.02) and β-cell function (r=0.34, P<0.038 Figure 2d). It is worth noting, however, that at 12 and 24 months, changes in BMI and total body fat did not correlate with changes in adiponectin (data not shown). While no other adipokine was associated with HbA1c, at 12 months, decreased hs-CRP correlated with decreased fasting glucose (r=−0.57, P<0.001) and increased GSIS (r=−0.45, P<0.01). At 24 months, reduced hs-CRP also correlated with lower fasting glucose (r=0.44, P<0.01) and higher GSIS (r=−0.38, P<0.04) and β-cell function (r=−0.35, P<0.04).
Figure 2.
Correlation between the change (Δ) in adiponectin and the in Δ body fat/weight and Disposition Index (DI, or pancreatic β-cell function) at 12 (a, c) and 24 months (b, d). Closed circles = non-remitters. Open circles = remitters.
DISCUSSION
The major finding from the present analysis is that greater increases in circulating adiponectin characterize type 2 diabetes remission status 2 years after bariatric surgery irrespective of changes in incretin/gut hormone responses (i.e. GLP-1, GIP or ghrelin). The diabetes remission rates are consistent with prior work from our group (11), and suggest that lower remission rates at 24 vs. 12 months may relate to weight regain. Adiposopathy, or “sick fat”, is a term used to describe pathogenic adipose tissue that contributes to insulin resistance and β-cell dysfunction (21). Adiponectin is an anti-inflammatory hormone demonstrated to reduce atherosclerosis and increase insulin sensitivity (22). Bariatric surgery raises circulating adiponectin levels in part by elevating gene expression of adiponectin from omental fat (23). Higher adiponectin is clinically relevant as it correlates with the degree of multi-organ insulin sensitivity (i.e. skeletal muscle, liver, and adipose) and reductions in both blood lipids and hs-CRP (22, 24). While lifestyle-induced weight loss decreases TNF-α in relation to improved insulin sensitivity (25), bariatric surgery seems to have no consistent effect on plasma TNF-α despite changes in hs-CRP or adiponectin (26). Our finding of blunted adiponectin responses and elevated hs-CRP in non-remitters following bariatric surgery is consistent with work by Hirsch and colleagues who characterized non-remitters as having ongoing subclinical inflammation (17). We recognize that total adiponectin in the blood comprises of distinct forms: globular, low molecular weight trimer, mid-molecular weight hexamer, and high-molecular weight complex, and that the latter is considered a key isoform responsible for insulin sensitizing effects. However, lower circulating total adiponectin is associated with higher risk of type 2 diabetes (27), suggesting that our measurement of adiponectin has biological relevance for diabetes remission. Thus, our data suggest that reduced adiposopathy is important for diabetes remission following bariatric surgery.
Improved and persistent glycemic control after bariatric surgery is in part related to marked weight loss and long-term maintenance of fat loss (13). At 12 and 24 months in the current study, non-remitters lost less weight and fat compared to remitters. Interestingly, android body fat was significantly reduced following bariatric surgery in the remitters compared to non-remitters, and lower android body fat and greater weight loss at 12 and 24 months was associated with enhanced adiponectin levels (Figure 1), although BMI and total body fat was unrelated to adiponectin. Collectively, these associations suggest that adiponectin may relate to adipose tissue mass, and are consistent with studies reporting an inverse correlation between adiponectin and visceral adiposity (28). We cannot determine from this study design the mechanism by which remitters lost more weight or increased adiponectin to a greater extent than non-remitters, but higher adiponectin is typically related to smaller adipocytes and elevated fat oxidation (28), which together may contribute to reductions in substrate availability for ectopic lipid accumulation (e.g. pancreas, liver or skeletal muscle) that impair insulin action. While fat biopsies were not collected in the current study to characterize cell size or adiponectin expression in relation to diabetes remission, our observations suggest that weigh/fat mass changes explain a small extent (r=−0.32 to −0.35) of the variance in adiponectin levels. Subsequently, the enhanced adiponectin rise in remitters is unlikely to be driven entirely by greater fat loss after bariatric surgery, and changes in an unknown gut-derived factor (e.g. bile acids or gut microbiota) or adipose insulin resistance may have influenced adiponectin secretion. Indeed, we detected a significant correlation between increased adiponectin and lower plasma triglyceride levels and adipose insulin resistance at 24 months, suggesting that elevated systemic adiponectin levels may serve as a biomarker for metabolic health independent of weigh loss.
Gastrointestinal hormone secretion is often evoked to explain the increased capacity of pancreatic β-cells to secrete insulin and lower plasma glucose following bariatric surgery (29, 30). GLP-1 is a leading candidate for explaining this enhanced insulin responsiveness, and has been reported to characterize individuals achieving diabetes remission (17). However, some suggest that gut hormones are unlikely to be the sole factor involved in the restoration of blood glucose (31). In the current study, changes in fasting or meal-induced GLP-1, GIP, or acylated ghrelin were not statistically related to lower HbA1c levels at 12 or 24 months, suggesting that changes in these gut hormones were not primary mediators of diabetes remission rates at 2 years post-surgery. Indeed, contrary to our expectations, GLP-1 levels were higher at 24 months in non-remitters compared with remitters. One possible explanation for this response is that higher GLP-1 levels occurred as compensatory mechanism to secrete more insulin during the MMTT in response to the pro-inflammatory induced insulin resistant state. Since non-remitters in our study had lower pancreatic β-cell function at 24 months, compared with remitters, our data suggest that non-remitters may have pancreatic β-cell resistance to the actions of GLP-1. We recognize that our current data do not negate the benefits of bariatric surgery to enhance GLP-1 stimulation in relation to improving insulin secretion and glucose control (18, 32, 40–42), as circulating GLP-1 may not equate to GLP-1 action on the pancreas. Moreover, our data do not exclude the potential of GLP-1 responsiveness prior to bariatric surgery as a predictive tool for characterizing diabetes remission (40–42), as our sample size is modest. However, our observations strengthen the view that independent of incretin responses, adiponectin plays a role in promoting diabetes remission by partly enhancing pancreatic β-cell function (Figure 2). It is known that adiponectin receptors are expressed on β-cells in humans and rats (33–35), although conflicting reports on the ability of adiponectin to stimulate in vitro insulin secretion do exist (34, 35), and elevated plasma adiponectin has been associated with β-cell function in some studies (36–37) by reducing lipid accumulation within the pancreas (38).
This study has limitations and strengths. We acknowledge that the associations observed herein do not equate to causality and further work is needed to determine if targeting adipose tissue produces more durable and/or enhanced diabetes remission rates following bariatric surgery. It is also possible that the observed metabolic profiles between remitters and non-remitters may be underestimated because surgical interventions (RYGB and SG) were combined for this study (39). However, recent work demonstrates that RYGB and SG have comparable effects on diabetes remission up to 3 years (2, 40), and we observed no statistical difference between RYGB and SG on HbA1c, BMI, and adiponectin responses within the non-remitter group at 12 or 24 months post-surgery (data not shown; all P>0.37). Thus, our findings suggest that improved adiponectin concentrations and fat loss may be important for diabetes remission regardless of surgery type. Moreover, medication withdraw 24-hours prior to metabolic studies at baseline may have improved outcomes of interest to some extent, and in turn underestimate the true effects of bariatric surgery on glucose regulation at 12 and 24 months. Females typically present with higher adiponectin levels than males, and we are underpowered to determine if a sexual dimorphism in adiponectin responses occur post-bariatric surgery. It is also worth considering that our non-remitters had reduced insulin secretion capacity as indicated by low baseline fasting C-peptide levels. This observation may have contributed to overall lower insulin concentrations in non-remitters that overestimated baseline insulin sensitivity compared with remitters. However, subjects served as their own controls thereby allowing us to determine the relative improvements following bariatric surgery, and despite marked improvements in meal-stimulated insulin secretion, insulin sensitivity was robustly improved in remitters following surgery. A strength of the current study is that using the MMTT increases the generalization of our findings and provides physiologic insight to the interactions of HbA1c, adipokines, and pancreatic β-cell function. In addition, obese subjects with type 2 diabetes were randomized to bariatric surgery and long-term follow up was conducted under vigorously controlled conditions. Therefore, our findings suggest that non-remitters have unique characteristics that warrant personalized medical therapy to induce disease resolution.
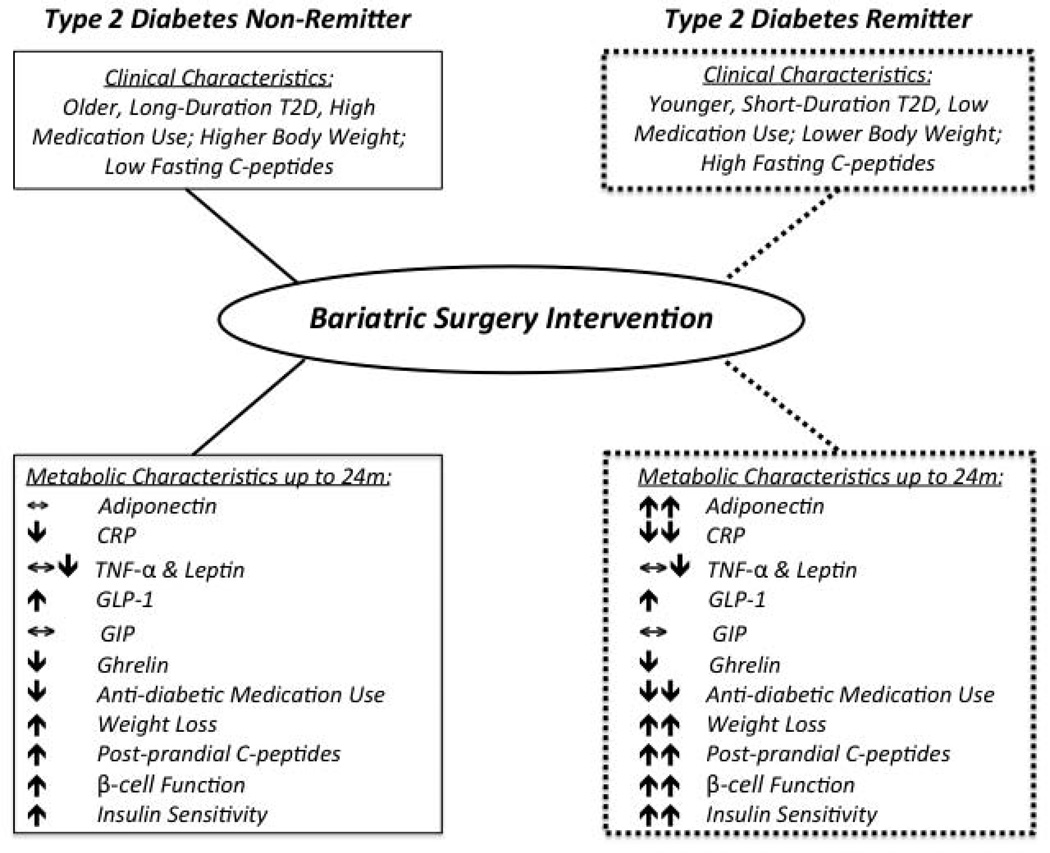
In conclusion, attenuated increases in circulating adiponectin and inadequate fat loss following bariatric surgery characterize non-remission of type 2 diabetes in obese adults. Elevated adiponectin was linked to improved glycemic control and paralleled by higher pancreatic β-cell function and multi-organ insulin sensitivity, irrespective of gut hormone responses. Together with reductions in hs-CRP, our data extend previous clinical work and demonstrate that bariatric surgery effectively promotes diabetes remission by lowering adiposopathy (Figure 3). Future work is necessary to address whether targeting adiposopathy by decreasing body fat and/or increasing adiponectin following bariatric surgery leads to better diabetes remission rates in obese adults, as adipose tissue appears intimately involved in the cross-talk between skeletal muscle, liver, and pancreatic glucose homeostasis.
Figure 3.
Summary of potential mechanisms underlying type 2 diabetes remission post-bariatric surgery. SG = sleeve gastrectomy. RYGB = Roux-en-Y Gastric Bypass. GIP = gastric inhibitory polypeptide. GLP-1 = glucagon-like polypeptide-1. TNF-α = tumor necrosis factor-α. hs-CRP = high sensitivity C-reactive protein.
Supplementary Material
ACKNOWLEDGEMENTS
SKM, JB, and SRK share responsibility for the integrity of analysis. All authors contributed to data collection and organization. SKM wrote the manuscript and all authors provided edits. Sarah Neale performed blood analysis from the Cleveland Clinic Preventive Research Lab. Dr. Richard Watanabee, PhD performed C-peptide deconvolution measures of ISR. The Cleveland Clinic Coordinating Center for Clinical Research provided the database and statistical analysis. We thank the CRU, bariatric surgery nursing staff, and participants for their outstanding efforts. This research was supported by Ethicon endo-surgery EESIIS 19900 (PRS), American Diabetes Association clinical translational award 1-11-26 CT (SRK), NIH RO1-DK089547 (PRS, SRK, JPK), and National Institutes of Health National Center for Research Resources, 1UL1RR024989, Cleveland, OH. SKM was supported by NIH T32 DK007319 grant.
Dr. Schauer obtained research grants from Ethicon Endo-surgery, NIH, and Bard-Davol; educational grants from Stryker Endoscopy, Gore, Baxter, Covidien, Allergan: honoraria from Ethicon Endo-surgery as scientific advisory board member, consultant and speaker. He has been a consultant/advisory board member for RemedyMD, StrykerEndoscopy, Bard-Davol, Gore, Barosense, Surgiquest, Carefusion. Dr. Kirwan receives grant funding from NIH, Nestle Inc., and ScottCare. Dr. Kashyap obtained research grants from Ethicon Endo-surgery, NIH, and American Diabetes Association. Dr. Brethauer receives honoraria from Ethicon Endo-Surgery as scientific advisory board member, consultant, and speaker and honoraria from Covidien for speaking. Dr. Bhatt discloses the following relationships - Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute; Harvard Clinical Research Institute; Mayo Clinic; Population Health Research Institute; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Associate Editor); Journal of the American College of Cardiology (Section Editor, Pharmacology); Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda. Dr. Nissen has consulted with Orexigen and Vivus.
Footnotes
CONFLICT OF INTEREST
All remaining authors report no conflict of interest.
REFERENCES
- 1.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes – 3 year outcomes. N Engl J Med. 2014 doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic roux-en-Y gastric bypass and sleeve gastrectomy: A randomized, prospective trial. Obesity Surg. 2012;22:740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after roux-en-Y gastric bypass and sleeve gastrectomy: A prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 5.Ikramuddin S, Korner J, Lee WJ, Connett J, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, Ahmed L, Vella A, Chuang L, Bessler M, Sarr MG, Swain JM, Laqua P, Jensen MD, Bantle JP. Roux-en-Y gastric bypass vs. intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The diabetes surgery study randomized clinical trial. JAMA. 2013;309:2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: An IDF statement for obese type 2 diabetes. Surg Obes Relat Dis. 2011;7:433–447. doi: 10.1016/j.soard.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, Kellum JM, Maher JW. Analysis of factors associated with durable remission of diabetes after roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254–259. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Brethauer SA, Aminian A, Romero Talamás H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M, Chand B, Schauer PR. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–636. doi: 10.1097/SLA.0b013e3182a5034b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237:751–756. doi: 10.1097/01.SLA.0000071560.76194.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after roux-en-Y gastric bypass surgery for obesity. Obesity Surg. 2010;20:1245–1250. doi: 10.1007/s11695-010-0198-8. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: The key to diabetes prevention. J Clin Endocrinol Metab. 2011;96:2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 15.Malin SK, Finnegan S, Fealy CE, Filion J, Rocco MB, Kirwan JP. β-Cell dysfunction is associated with metabolic syndrome severity in adults. Metab Syndr Relat Disord. 2013;12:79–85. doi: 10.1089/met.2013.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appachi SK, Kashyap SR. 'Adiposopathy' and cardiovascular disease: The benefits of bariatric surgery. Curr Opin Cardiol. 2013;28:540–546. doi: 10.1097/HCO.0b013e3283642a33. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch FF, Pareja JC, Geloneze SR, Chaim E, Cazzo E, Geloneze B. Comparison of metabolic effects of surgical-induced massive weight loss in patients with long-term remission versus non-remission of type 2 diabetes. Obesity Surg. 2012;22:910–917. doi: 10.1007/s11695-012-0589-0. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap SR, Bhatt DL, Wolski K, Wantanabe RM, Abdul-Ghani MA, Abood B, Pothier CE, Brethauer SA, Nissen S, Gupta M, Kirwan JP, Schauer PR. Metabolic Effects of Bariatric Surgery in Patients with Moderate Obesity and Type 2 Diabetes: Analysis of a Randomized Control Trial Comparing Surgery vs. Intensive Medical Treatment. Diabetes Care. 2013;36:2175–2182. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, Nissen S, Brethauer SA, Schauer PR, Kirwan JP, Kashyap SR. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: Effects of bariatric surgery vs. standard medical therapy. Int J Obes. 2013;38:364–370. doi: 10.1038/ijo.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 21.Bays H, Blonde L, Rosenson R. Adiposopathy: How do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Exper Rev Cardiovasc Ther. 2006;4:871–895. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- 22.Swarbrick MH, Havel JP. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Pamuklar Z, Spagnoli A, Torquati A. Serum leptin levels are inversely correlated with omental gene expression of adiponectin and markedly decreased after gastric bypass surgery. Surg Endosc. 2012;26:1476–1480. doi: 10.1007/s00464-011-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragón-Alonso A, Pascual-Díaz M, Pérez-Paredes M, Lozano-Almela ML. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obesity Surg. 2012;22:950–955. doi: 10.1007/s11695-012-0643-y. [DOI] [PubMed] [Google Scholar]
- 25.Kelly KR, Haus JM, Solomon TPJ, Patrick-Melin A, Cook M, Rocco M, Barkoukis H, Kirwan JP. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SR. Inflammatory markers and bariatric surgery: A meta-analysis. Inflammation Res. 2012;61:789–807. doi: 10.1007/s00011-012-0473-3. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 28.Turner AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 29.Hagemann D, Holst J, Gethmann A, Banasch M, Schmidt W, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept. 2007;143:64–68. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: A comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:683–690. doi: 10.1016/j.soard.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes mellitus after gastric bypass surgery. Diabetes Care. 2013;36:2062–2069. doi: 10.2337/dc12-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003;312:1118–1122. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto M, Ohara-Imaizumi M, Kubota N, Hashimoto S, Eto K, Kanno T, Kubota T, Wakui M, Nagai R, Noda M, Nagamatsu S, Kadowaki T. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827–835. doi: 10.1007/s00125-008-0944-9. [DOI] [PubMed] [Google Scholar]
- 35.Staiger K, Stefan N, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Machicao F, Kellerer M, Stumvoll M, Fritsche A, Häring HU. Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J Clin Endocrinol Metab. 2005;90:6707–6713. doi: 10.1210/jc.2005-0467. [DOI] [PubMed] [Google Scholar]
- 36.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: Relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 37.Guldstrand M, Ahrén B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endo Metab. 2003;284:E557–E565. doi: 10.1152/ajpendo.00325.2002. [DOI] [PubMed] [Google Scholar]
- 38.Patane G, Caporaello N, Marchetti P, Parrino C, Sudano D, Marselli L, Vigneri R, Frittitta L. Adiponectin increases glucose-induced insulin secretion through the activation of lipid oxidation. Acta Diabetol. 2013;50:851–857. doi: 10.1007/s00592-013-0458-x. [DOI] [PubMed] [Google Scholar]
- 39.Li JF, Lai DD, Ni B, Sun KX. Comparison of laparoscopic Roux-en Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes: a meta-analysis of randomized controlled trials. Can J Surg. 2013;56:E158–E164. doi: 10.1503/cjs.026912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en Y Gastric Bypass and Sleeve Gastrectomy: Mechanisms of Diabetes Remission and Role of Gut Hormones. J Clin Endo Metab. 2013;98:4391–4399. doi: 10.1210/jc.2013-2538. [DOI] [PubMed] [Google Scholar]
- 41.Habegger KM, Heppner KM, Amburgy SE, Ottaway N, Holland J, Raver C, Bartley E, Muller TD, Pfluger PT, Berger J, Toure M, Benoit SC, Dimarchi RD, Perez-Tilve D, D'Alessio DA, Seeley RJ, Tschop MH. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes. 2014;63:505–513. doi: 10.2337/db13-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutia R, Brakoniecki K, Bunker P, Paultre F, Homel P, Carpentier AC, McGinty J, Laferrere B. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63(4):1214–1223. doi: 10.2337/db13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.