Abstract
BACKGROUND
SOX2 is a member of SOX (SRY-related HMG box) family of transcription factors.
METHODS
in this study, we examined the expression of SOX2 in murine and human prostatic specimens by immunohistochemistry.
RESULTS
we found that SOX2 was expressed in murine prostates during budding morphogenesis and in neuroendocrine (NE) prostate cancer (PCa) murine models. Expression of SOX2 was also examined in human prostatic tissue. We found that SOX2 was expressed in 26 of 30 benign prostate hyperplasia (BPH) specimens. In these BPH samples, expression of SOX2 was limited to basal epithelial cells. In contrast, 24 of 25 primary PCa specimens were negative for SOX2. The only positive primary PCa was the prostatic NE tumor, which also showed co-expression of synaptophysin. Additionally, the expression of SOX2 was detected in all prostatic NE tumor xenograft lines. Furthermore, we have examined the expression of SOX2 on a set of tissue microarrays consisting of metastatic PCa tissues. Expression of SOX2 was detected in at least one metastatic site in 15 of 24 patients with metastatic castration-resistant PCa; and the expression of SOX2 was correlated with synaptophysin.
CONCLUSIONS
SOX2 was expressed in developing prostates, basal cells of BPH, as well as prostatic NE tumors.
Keywords: SOX2, Prostate, benign prostatic hyperplasia, prostate cancer, neuroendocrine, metastasis
Introduction
The SRY-related high mobility group (HMG) box (SOX) family of transcription factors is instrumental for diverse developmental processes and in determining cell fate (1, 2). In particular, SOX2 seems to be essential for sustaining pluripotency and neural induction (2). In combination with Oct4, KLF4, and c-Myc, SOX2 can successfully reprogram somatic cells to become induced pluripotent stem cells (3), suggesting an important role for SOX2 in controlling the pluripotency of stem/progenitor cells. SOX2 has also been implicated in branching morphogenesis and epithelial cell differentiation during early lung development, since aberrant expression of SOX2 disrupts normal lung branching morphogenesis, resulting in reduced number of airways (4, 5). Mice over-expressing SOX2 exhibit an increased number of basal cells (marked by the expression of p63) and neuroendocrine (NE) cells in the respiratory mucosa (4), and lung carcinomas are developed in about half of these mice, indicating that SOX2 over-expression is oncogenic (6).
Expression of SOX2 has been reported in carcinomas arising in several organs including prostate (7–10). A recent study shows that SOX2 is expressed in both benign and malignant prostate tissue, but only in a small subpopulation of cells (<10%) (7). SOX2 is also detected in castration-resistant PCa metastasis samples (10). Several studies have highlighted the functional implication of SOX2 in tumor progression as a reduction of SOX2 levels has been shown to decrease proliferation and invasion while concomitantly increasing differentiation of cancer cells (11–15). In PCa, silencing SOX2 by shRNA decreases both cell proliferation and the invasive capacity of PCa cells (8, 16), and ectopic expression of SOX2 promotes PCa cell growth (9, 10, 16).
SOX2 expression is associated with neuroendocrine (NE) tumors. In skin, SOX2 is expressed in cutaneous neuroendocrine (NE) carcinoma (Merkel cell carcinoma) in addition to a subset of melanomas (17). SOX2 is also detected in NE carcinomas of lung (18), consistent with the observation that SOX2 over-expressing mice have an expansion of NE cells in the respiratory mucosa and develop lung carcinomas. However, the expression of SOX2 in NE PCa is yet to be examined.
NE PCa, or small cell carcinoma of the prostate, is a rare and highly aggressive subtype of PCa. Because it is androgen receptor (AR) negative, NE PCa is naturally androgen independent. Although the vast majority of primary human PCa are adenocarcinomas, immunohistochemical evidence of NE differentiation (NED) can be often found in human PCa, and PCa exhibiting NED is associated with poor prognosis (19, 20). Rapid autopsy of hormone-refractory metastatic PCa has shown NED in most cases (21), and patients that fail androgen deprivation therapy can develop NE cancer and adenocarcinoma with NED (22). However, although NE phenotype is associated with advanced stage PCa, NE and NED in prostate are understudied due to the scarcity of NE PCa specimens as advanced PCa is usually not biopsied.
A major interest of our laboratory has been NE tumors, largely due to the fact that numerous mouse PCa models readily develop or progress to NE tumors (23). Our focus has been identifying potential transcription factors that promote the NE phenotype. Because SOX2 can drive NE cell hyperplasia and carcinoma in the murine lung, we postulated that SOX2 may also play a similar role in the prostate. In order to begin to elucidate the relationship between SOX2 and the NE phenotype, we undertook a careful analysis of murine and human prostate to determine the expression pattern of SOX2 and whether SOX2 is co-expressed with NE markers. Specifically, we examined the expression of SOX2 during murine prostate development, in diseased prostate (benign prostatic hyperplasia [BPH], primary prostate adenocarcinoma, and NE PCa) and in metastatic PCa. We also studied the expression of SOX2 in prostate NE tumor murine models and analyzed the association of SOX2 expression with the NE phenotype in metastatic human PCa. We found SOX2 was expressed in embryonic prostates, BPH, and NE PCa.
Materials and methods
Mouse lines
All mice used in this study were housed and handled in accordance with the standard protocols approved by the Vanderbilt University Institutional Animal Care and Use Committee (IACUC). Seven to eight week old CD-1 mice (Harlan, Indianapolis) were used for timed mating. The day a vaginal copulation plug was observed was designated embryonic day 1. The 12T7 (24), 12T10 (25), and TRAMP (26) are transgenic mouse lines that develop PIN or neuroendocrine prostate cancer. NE10 (27) is a prostatic NE tumor xenograft line.
Human samples
Primary prostate specimens were obtained from the Department of Pathology, Vanderbilt University Medical Center with approval from the Institutional Review Board. The UWTMA22 and UWTMA46 are tissue microarrays constructed at the University of Washington. UWTMA22 consists of metastatic castration resistant PCa tissues from 24 patients; UWTMA46 consists of 24 LuCaP xenograft tumors derived from 19 patients, 4 of which are prostatic NE tumors derived from 3 patients.
Validation of SOX2 antibody
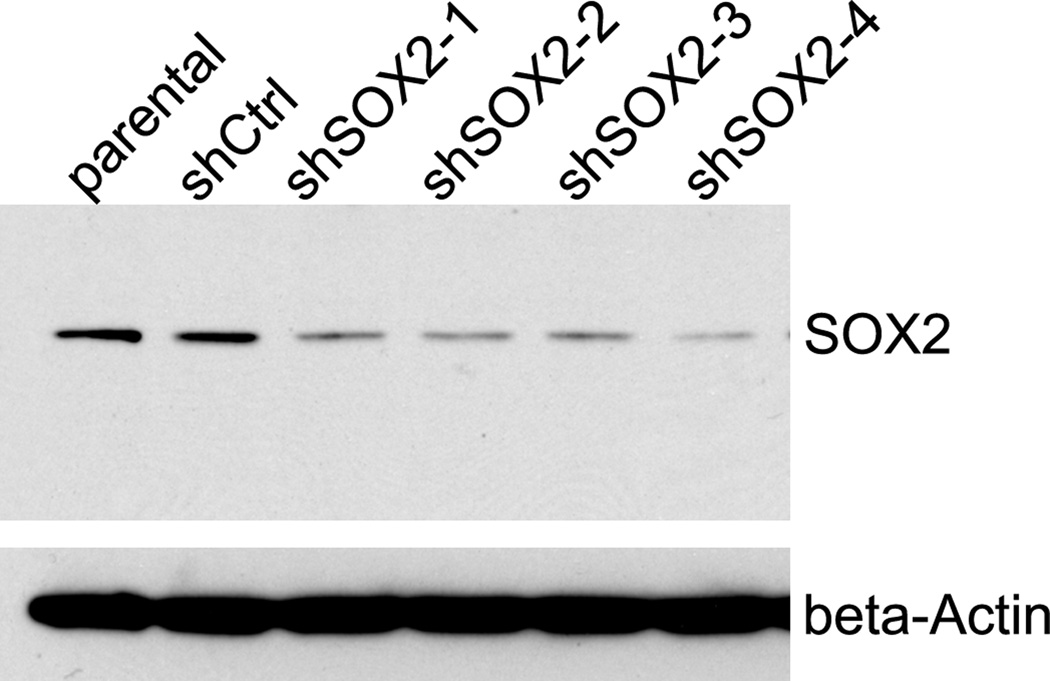
The specificity of SOX2 antibody was validated by western blot using cell lysate collected from control PC3 cells and PC3/shSOX2 cells (figure 1). The shSOX2 constructs were purchased from Origene (Rockville, MD). For retroviral infection, the shSOX2 or control retroviral vector plasmids were transfected into Phoenix packaging cells. The cell culture media were collected 24 hours later and used for infecting PC3 cells. The infection procedure was repeated twice, and 10ug/ml puromycin was used for selection of PC3 cells that have control or shSOX2 construct stably integrated.
Figure 1. Western blot analysis.
The level of SOX2 was knocked down in PC3 cells and protein lysate was prepared from parental PC3 cells and PC3 cells that have stably integrated with control shRNA, or with variant shSOX2 construct. Beta-Actin was used as loading control.
Immunohistochemistry (IHC) and immunofluorescence (IF) staining
IHC was conducted as described previously (28). Prostates were fixed in 10% buffered formalin overnight and processed to paraffin. IHC stains were preformed following routine deparaffinization and rehydration of 5 µm sections. Antigen retrieval was performed by microwaving slides for 20 min in boiling antigen unmasking solution (Vector Laboratories, Burlingame, CA). Endogenous peroxidase activity was blocked with DAKO Peroxidase Blocking Reagent (DAKO, Carpinteria, CA) for 15 min. Sections were incubated with primary antibodies overnight at 4°C in a humidified chamber. Antibodies used were: Foxa2 and p63 from Santa Cruz Biotechnology (Santa Cruz, CA), synaptophysin from BD Transduction Laboratories (San Jose, CA), and SOX2 from Abcam (Cambridge, MA) or Cell Signaling (Danvers, MA). Specific antibody binding was detected using the Vectastain Elite ABC peroxidase kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol with the DAKO DAB -Chromogen System (DAKO, Carpinteria, CA). Sections were counterstained with hematoxylin, dehydrated, and cover-slipped. For immunofluorescence staining, tissue sections were blocked with PBS containing 5% normal donkey serum (Vector Laboratories, Burlingame, CA) for 1 hr, and incubated overnight at 4°C with primary antibodies. After washing in PBS, sections were incubated with fluorescence conjugated secondary antibody (Molecular Probes, Eugene, OR) for1 hr (diluted 1:200 in blocking buffer,). Sections were washed in PBS and cover-slipped using mounting solution with DAPI (Vector Laboratories, Burlingame, CA).
Western blot
Protein lysates were prepared from prostate specimens as described previously (29). Twenty µg total protein was loaded for electrophoresis. After transfer, membranes were blocked in 5% milk for 1 hr, incubated overnight at 4°C with primary antibodies, and incubated with horseradish peroxidase conjugated secondary antibodies (GE Healthcare, Pittsburgh, PA) for 1 hr. ECL-Plus detection system (PerkinElmer, Waltham, MA) was used to visualize the reaction. Rabbit anti-SOX2 antibodies were from Abcam (Cambridge, MA) or Cell Signaling (Danvers, MA), and β-actin from Sigma (St Louis, MO).
Statistical analysis of IHC data
SAS 9.3 (SAS Institute, Cary, NC) was used for statistical analysis. The percentage of specific cell types in primary BPH and PCa specimens showing nuclear staining for SOX2 was recorded. Expression of SOX2 and synaptophysin in metastatic CRPC specimens were evaluated for both percentage of cells stained and intensity of nuclear (SOX2) or cytoplasmic (synaptophysin) staining. Intensity values were categorized as 0 negative, 1+ weak, 2+ moderate, and 3+ strong. The total score was calculated from intensity value and percentage of staining (intensity × percentage). Associations between SOX2 and synaptophysin expression was examined using Spearman’s rank test. All tests with a P value of <0.05 were considered statistically significant.
Results
SOX2 is expressed during murine embryonic prostate development
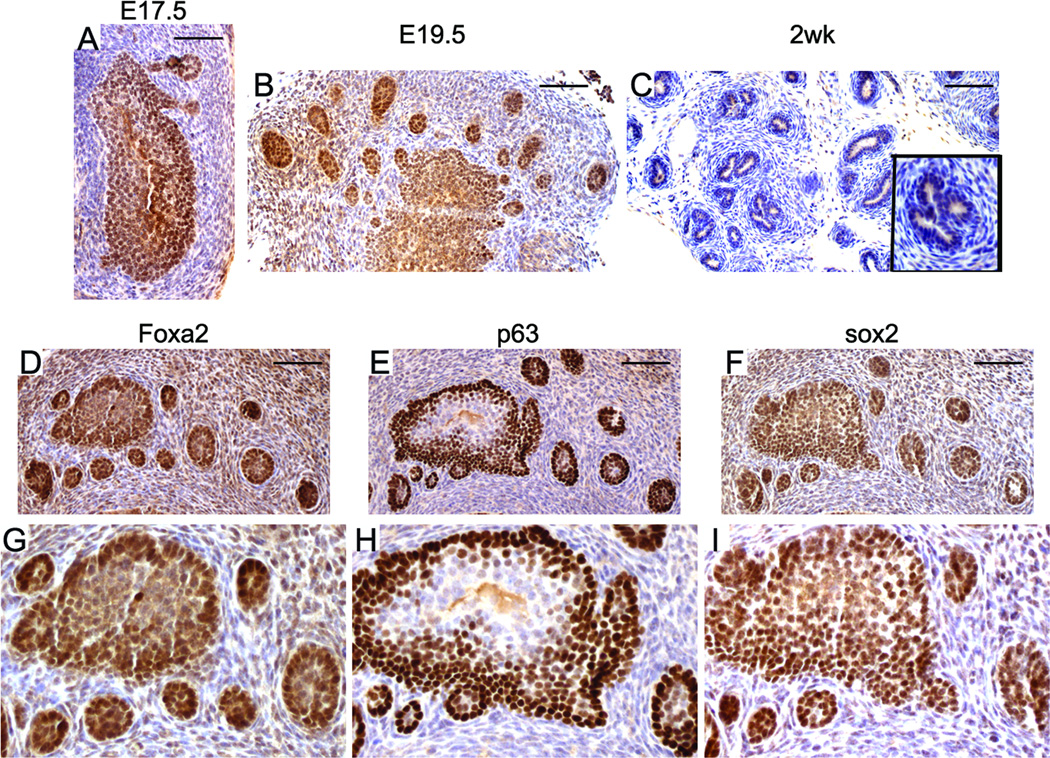
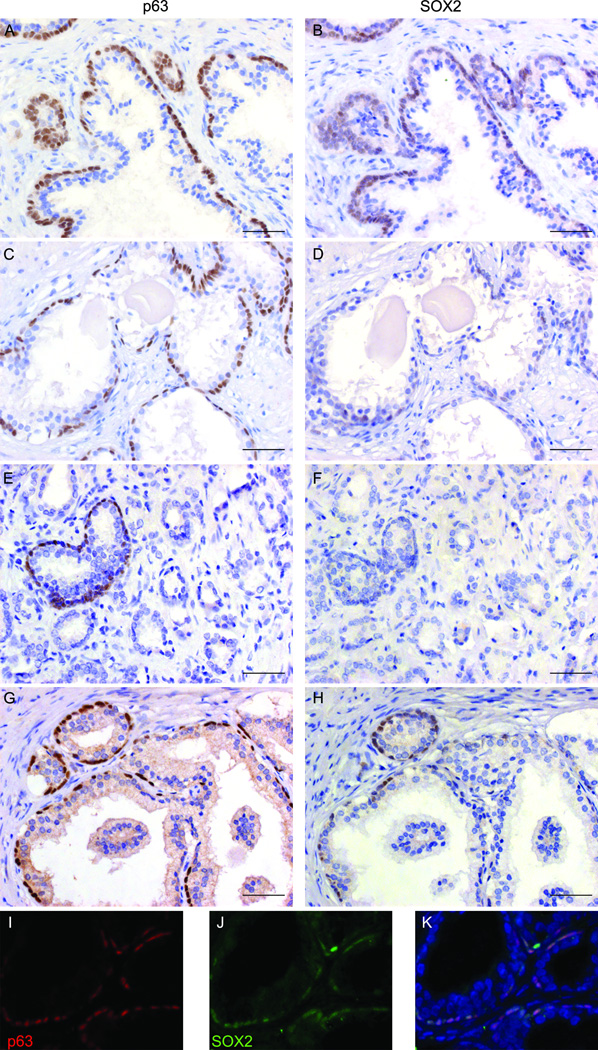
Prostate budding morphogenesis occurs around E17 to E18 (time varies among different mouse strains) when solid epithelial cords grow into the surrounding urogenital sinus mesenchyme to form the rudimentary prostate buds. SOX2 expression was detected during prostate budding morphogenesis (Fig. 2A & 2B). Nuclear SOX2 staining was observed in the urogenital sinus epithelial cells, with stronger nuclear staining in cells at the epithelial–mesenchymal interface. Some stromal cells show weak staining for SOX2. The expression pattern of SOX2 in the budding prostate is similar to that of Foxa2 and p63 (Fig. 2D–I)(30). At two weeks after birth (Fig. 2C) and in adults (Fig. 3A), nuclear SOX2 staining is essentially undetectable in luminal prostatic epithelial cells. Due to background cytoplasmic staining (Fig. 2C and Fig. 3A), it is not clear whether SOX2 is expressed in mouse prostate basal epithelial cells at these stages.
Figure 2. SOX2 expression during murine embryonic prostate development.
Nuclear SOX2 staining was detected in the prostatic buds at E17.5 and E19.5 (A&B). At 2 weeks after birth, the nuclear staining of SOX2 was hardly detected in prostate (C, inset is a higher magnification picture). The prostatic buds co-expressed Foxa2 (D), p63 (E), and SOX2 (F). G, H, and I are higher magnification picture of D, E, and F, respectively. Scale bars represent 25 µm.
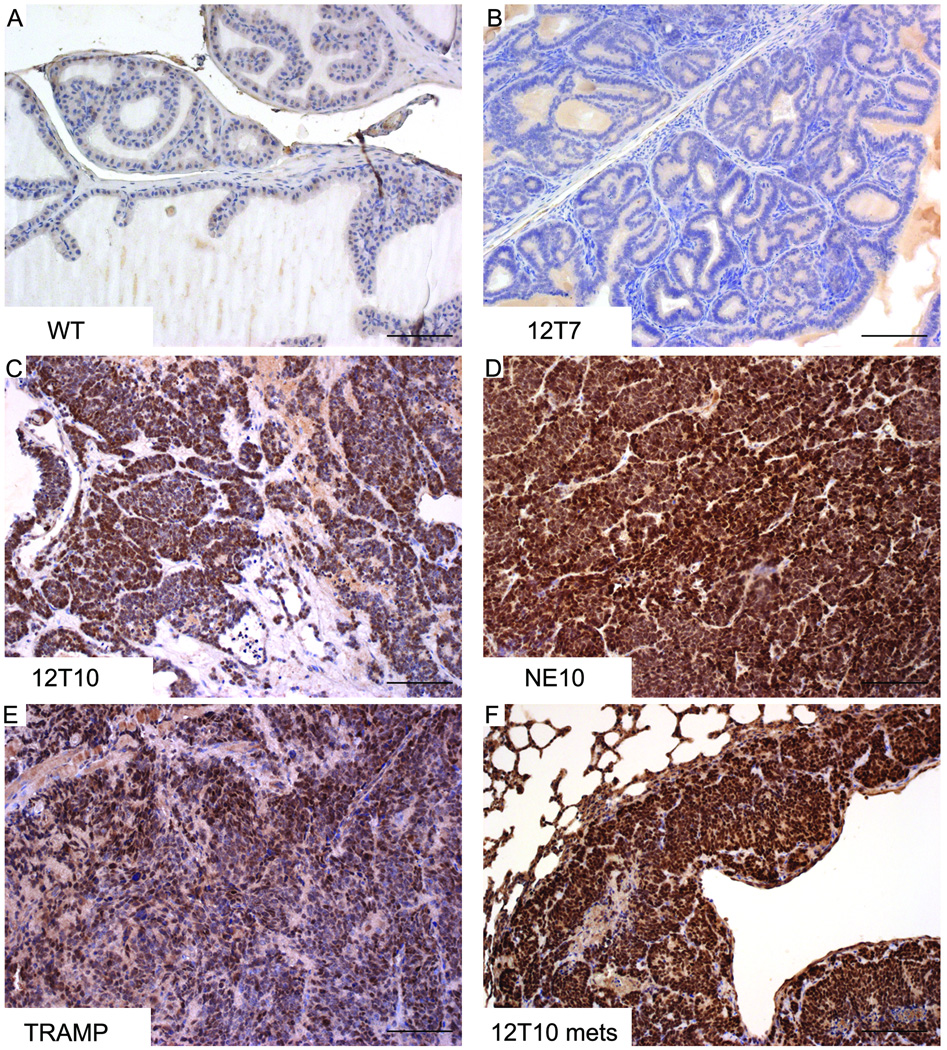
Figure 3. SOX2 expression in NE PCa murine models.
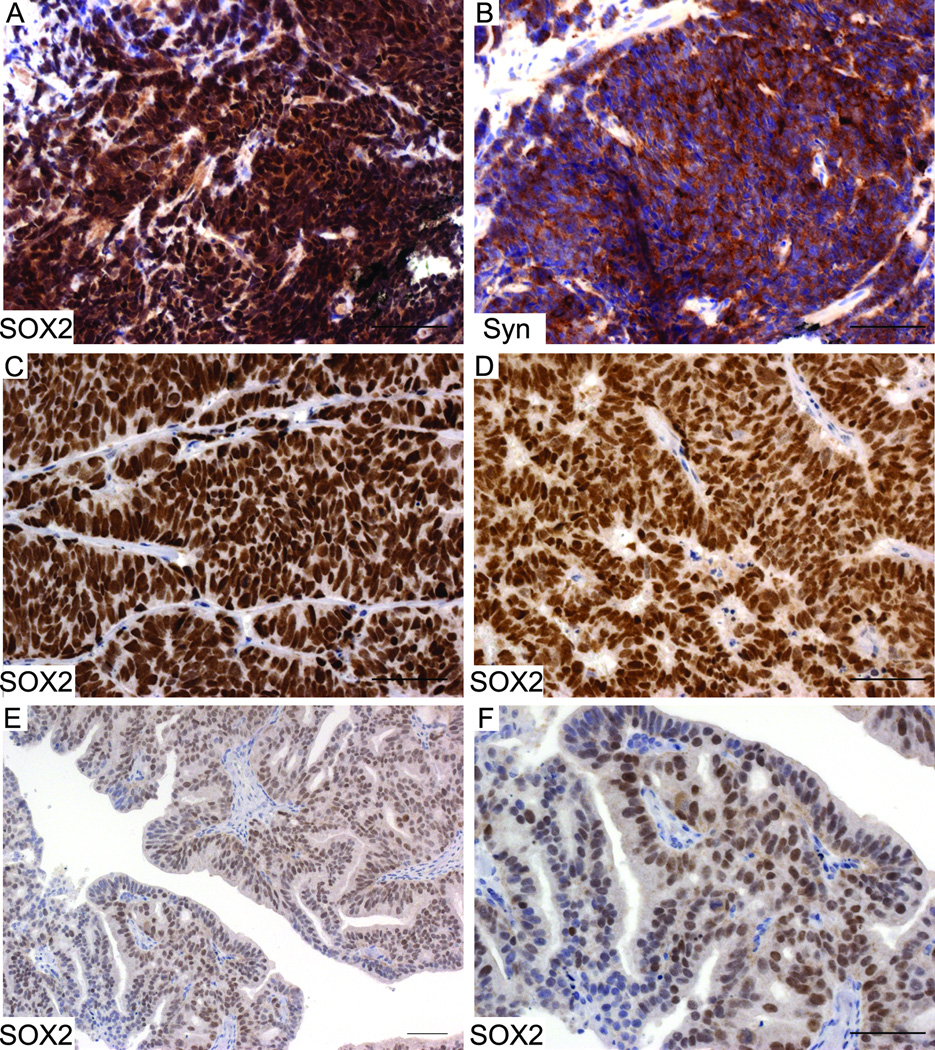
IHC staining was performed on prostate sections derived from wild type (WT, A), 12T7 (B), 12T10 (C), NE10 (D), and TRAMP (E) mice, or on NE PCa lung metastasis in 12T10 mouse (F). Strong nuclear SOX2 staining was detected in prostate NE tumors (12T10, NE10, TRAMP, and NE PCa metastasis), but not in wild type or 12T7 mouse prostates. Scale bars represent 50 µm.
SOX2 is expressed in NE PCa murine models
In the adult, wild type prostate luminal epithelial cells were negative for SOX2 (Fig. 3A). Similarly, nuclear SOX2 staining was not observed in the 12T7 prostate PIN model, although some luminal epithelia cells exhibit weak cytoplasmic staining (Fig. 3B), which was considered to be background. In contrast, strong nuclear staining for SOX2 was observed in the 12T10, NE10, and TRAMP NE tumors (Figs. 3C–E). Expression of SOX2 was also detected in prostatic NE tumor metastasis (Fig. 3F).
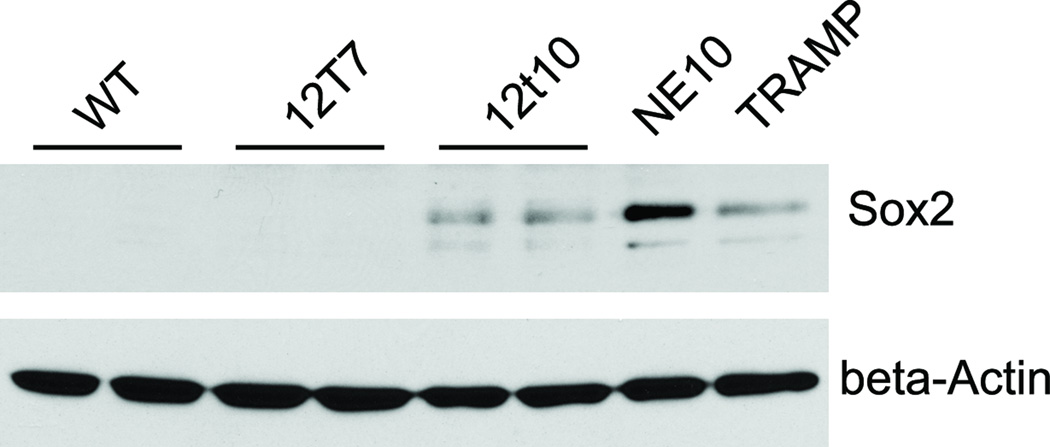
Expression of SOX2 in murine NE PCa was confirmed by western blot. SOX2 expression was detected in 12T10, NE10, and TRAMP tumor lysates, but not in prostates derived from wild type or 12T7 mice (Fig. 4). The lack of SOX2 immunoreactivity on western blots of normal or PIN mouse prostates supports the conclusion that the cytoplasmic IHC staining seen in some sections was indeed non-specific background staining.
Figure 4. Western blot analysis.
Protein samples were prepared from prostates derived from wild type (WT), 12T7, 12T10, NE10, and TRAMP mice. The expression of SOX2 was detected in 12T10, NE10, and TRAMP prostate NE tumors, but not in PIN (12T7) or wild type prostates. NE10 tumor exhibits the highest expression level of SOX2.
Expression of SOX2 in human prostate cancer cell lines
SOX2 expression was examined in established benign or cancerous prostatic cell lines by western blot (Fig. 5). SOX2 was strongly expressed in DU145 and PC3 cells, with lower amounts in CWR22Rv1 cells. DU145 and PC3 are AR negative cell lines and their growth is independent of the presence of androgen while CWR22Rv1 is AR positive, but grows as a castrate-resistant cancer cell line (31). Low SOX2 expression was detected in RWPE1, LNCaP, LAPC4, and CWR22Pc cell lines. Compared with the three cell lines that have high/moderate level of SOX2, these cell lines are generally less aggressive in vivo and are androgen responsive (32). SOX2 was essentially undetectable in NHPrE and BHPrE cells, two newly established human prostate epithelial cell lines derived from normal and benign prostate tissue, respectively (33). Each cell line will form normal prostate glandular structure when recombined with inductive embryonic urogenital sinus mesenchyme (UGM) and grafted under the renal capsule (33).
Figure 5. SOX2 expression in established prostate cell lines.
Protein samples were prepared from various prostatic cell lines. DU145 and PC3 exhibited the strongest expression of SOX2. Moderate level of SOX2 was observed in 22Rv1 cells. RWPE1, LNCaP, LAPC4, or CWR22Pc cells exhibited low SOX2 expression. The expression of SOX2 was hardly detected in NHPrE and BHPrE cells.
Expression of SOX2 in benign and malignant human prostate cancer specimens
The expression of SOX2 in benign and malignant human prostatic tissues has been assessed in a recent study where focal expression of SOX2 was detected in 90% of benign tissues, 65% of HGPIN samples, and 52% of PCa (7). Here, we examined the expression of SOX2 in BPH, primary prostate adenocarcinoma, prostate NE cancer (small cell carcinoma), and castration-resistant metastatic tumors. All the human specimens examined in this study were listed in Table 1. Most (26 of 30; 87%) BPH specimens showed positive SOX2 staining in prostatic basal cells (Fig. 5B), which co-express the basal cell marker p63 (Fig. 6A & Figs. 6I–6K). The other four BPH cases showed little or no SOX2 expression in the basal cell layer despite the presence of p63-positive basal cells (Fig 6C&D). Most (22 of 24; 92%) primary prostate adenocarcinoma specimens were negative for SOX2 staining (Fig. 6F). Although some benign prostatic acini or ducts within the tumors still expressed p63 (Fig. 6E), they did not exhibit SOX2 immunoreactivity (Fig. 6F), suggesting that loss of SOX2 expression is not simply a reflection of an absent basal cell layer. In the two SOX2 positive prostate adenocarcinoma cases, SOX2 expression was only detected in adjacent benign areas but not in cancer areas (Figs. 6G&H).
Table 1.
Human prostate specimens used in this study
| Cohort | Histology | Patients | SOX2+ | SOX2− |
|---|---|---|---|---|
| BPH | BPH | 30 | 26 | 4 |
| Primary PCa | adenocarcinoma | 24 | 2* | 22 |
| neuroendocrine | 1 | 1 | 0 | |
| UWTMA22 (Metastasis) | synaptophysin+ | 10 | 8 | 2 |
| synaptophysin− | 14 | 7 | 7 | |
| UWTMA46 (LuCaP) | adenocarcinoma | 16 | 2 | 14 |
| neuroendocrine | 3 | 3 | 0 | |
SOX2 was only detected in benign areas.
Figure 6. Expression of SOX2 in human BPH and primary prostate adenocarcinoma specimens.
Immunohistochemical staining of SOX2 or p63 was performed on serial sections derived from BPH (A–D) or PCa (E–H) tissues. Panels A&B represented the 26 of 30 BPH cases that displayed positive SOX2 staining in basal cell layer; panels C&D represented the 4 of 30 BPH cases that showed little or no SOX2 staining but were still positive for p63. Panels E&F represented the 22 of 24 primary PCa cases that lost SOX2 expression. Some benign areas in these PCa specimens were positive for p63 but negative for SOX2 staining. Panels G&H represented cancer-adjacent normal areas in the 2 of 24 PCa cases where both p63 and SOX2 were expressed. Panels I-K are images from dual immunofluorescence staining performed on sections derived from BPH specimens. SOX2 (in green) was co-expressed with basal cell marker-p63 (in red). DAPI was used for counter-staining. Scale bars represent 25 µm.
Since SOX2 was associated with NE tumor phenotype in skin (17) and lung (18) and that SOX2 was expressed in murine models of prostatic NE tumors, we examined the expression of SOX2 in human prostatic NE tumors. A NE tumor biopsy specimen examined exhibited strong nuclear SOX2 expression (Fig. 7A), along with co-expression of synaptophysin (Fig. 7B). We also examined the expression of SOX2 on a set of prostate tissue microarrays (TMAs) UWTMA46, a collection of LuCaP xenografts. All of the NE tumor specimens derived from three patients were positive for SOX2 (Figs. 7C&D). In the non-NE xenografts derived from two patients that were positive for SOX2, one case showed nuclear SOX2 staining in almost half epithelial cells (Figs. 7E&F, median value 47.5%), whereas the other case showed positive SOX2 staining in only 5% of epithelial cells (data not shown). The remaining non-NE PCa xenografts derived from other 14 patients were negative for SOX2.
Figure 7. SOX2 expression was detected in human NE PCa specimens.
A&B: IHC staining of SOX2 and NE markers (synaptophysin) was performed on serial sections derived from NE PCa needle biopsies. Both SOX2 and synaptophysin were highly expressed on these sections. The expression of SOX2 was also examined on a set of tissue microarrays consisting of LuCaP xenografts derived from 19 patients. Panels C&D represented positive SOX2 staining on all the NE PCa cases. Panels E&F represented the one case that was adenocarcinoma but showed positive SOX2 staining. Panel F is a higher magnification picture of E. Scale bars represent 25 µm.
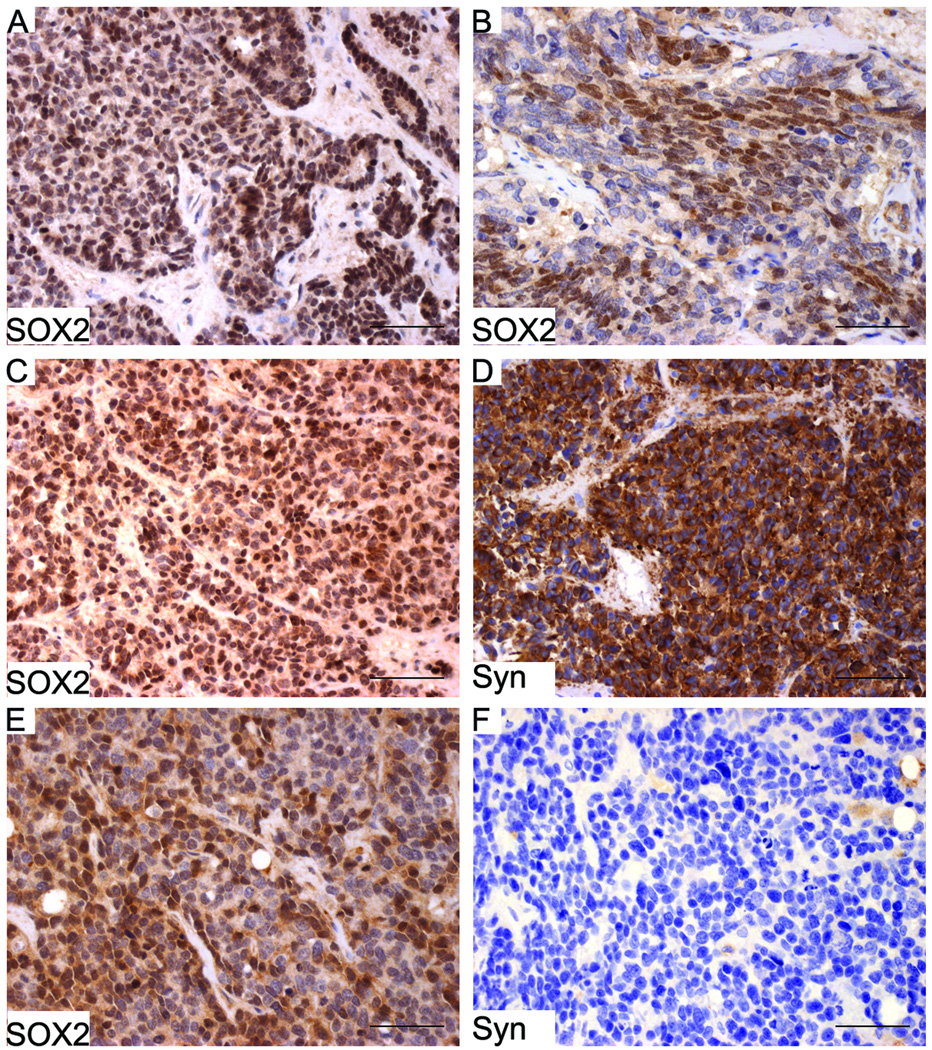
We have also examined the expression of SOX2 on UWTMA22, a set of prostate TMAs that consists of castration resistant PCa bone and soft tissue metastases. Of 24 patients, 15 (63%) were positive for SOX2 in at least one metastatic site (Table 1 and Fig. 8), despite the observation that primary adenocarcinomas of the prostate exhibit loss of SOX2 expression. Serial sections from the UWTMA22 were also stained for synaptophysin by IHC and compared to SOX2 expression (Fig. 8). The expression of SOX2 was correlated with synaptophysin positive staining (Spearman’s test, p=0.037; Rho=0.143 [Table 2]).
Figure 8. SOX2 expression in metastatic PCa.
The expression of SOX2 was examined on a set of tissue microarrays (UWTMA22) that consist of metastatic castration-resistant PCa tissues. The expression of SOX2 was detected in both soft tissue (A, liver metastasis) and bone metastasis (B), in both NE (C) and non-NE (E) PCa metastasis. On serial sections C and D, both SOX2 and synaptophysin were expressed; however, on serial sections E and F, SOX2 but not synaptophysin was expressed. Scale bars represent 25 µm.
Table 2.
Correlation of expression of SOX2 and Synaptophysin
| Synaptophysin | |||||||
|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | P value | Rho value | ||
| SOX2 | Score 0 | 121 (76.1) | 20 (76.9) | 7 (50.0) | 9 (56.2) | 0.037 | 0.143 |
| Score 1 | 10 (6.3) | 3 (11.5) | 3 (21.4) | 2 (12.5) | |||
| Score 2 | 15 (9.4) | 3 (11.5) | 3 (21.4) | 5 (31.3) | |||
| Score 3 | 13 (8.2) | 0 (0.0) | 1 (7.1) | 0 (0.0) | |||
Discussion
In this study, we characterized the expression of SOX2 in murine and human prostatic tissue. Our data demonstrated that SOX2 was expressed during embryonic prostate budding morphogenesis. In the adult prostate, SOX2 expression was primarily detected in prostatic basal epithelial and NE cells. Therefore, in the prostate SOX2 is largely an embryonic and NE marker, which is turned off in the luminal compartment following birth.
In the adult human prostate, SOX2 expression was limited to the basal compartment. The expression of SOX2 was lost in 24 primary prostate adenocarcinomas, which agrees with the notion that prostates lose basal cells during cancer progression. Interestingly, in these primary PCa samples, we noticed some p63 positive but SOX2 negative areas, suggesting that loss of SOX2 expression in basal cells occurs before p63 is lost in PCa. Our finding that SOX2 is lost in prostate adenocarcinoma differs from a previous report which showed SOX2 is detected in 51% prostate carcinoma cases. One possible reason for the differences in SOX2 expression in these two cohorts could be because of different treatment regimens used. For example, androgen has been shown to inhibit the expression of SOX2 (10), thus androgen deprivation therapy may induce the expression of SOX2 in patients who have undertaken antiandrogens. The loss of SOX2 in adenocarcinomas, however, may be transient since SOX2 was detected in 15 of 24 metastatic hormone refractory PCa patients. The observation that SOX2 is expressed in PCa metastases is consistent with a recent study where SOX2 was detected in majority of castration resistant metastatic PCa specimens (10), as well as our characterization of SOX2 expression in established prostate cell lines which suggests an association with castrate-resistance as well.
We also showed that the expression of SOX2 was detected in NE tumors of prostate. SOX2 was expressed in human NE and NE tumor murine models examined. In human pathology specimens, SOX2 was expressed in primary NE PCa needle biopsy specimens and in all the NE tumor xenografts. In the metastatic PCa specimens, the expression of SOX2 was correlated with NE marker, synaptophysin. Because NE PCa are AR-negative, they do not respond to hormonal therapy. Currently, there is no effective therapy for treating NE PCa and most patients with NE PCa survive less than one year (34). Identifying genes and pathways that are active in NE PCa can improve our understanding on prostatic NE tumor biology and provide potential therapeutic targets. Studies have shown that signaling pathways active in stem cells and in embryonic prostate development are often activated in prostate NE tumors (30, 35–38). Our finding that SOX2, a “stemness’ gene, is expressed in embryonic prostates and in prostate NE tumors, provides another piece of evidence supporting the connection of NE phenotype with stem cell features. Moreover, characterizing the expression pattern of SOX2 in well characterized NE mouse models allows us to further interrogate the molecular underpinnings of NE PCa.
The observation that the majority of adenocarcinomas lose SOX2 expression, while increased SOX2 expression is associated with castrate-resistant metastatic prostate cancer, suggests that those patients who express SOX2 may be more likely to progress to castrate resistant disease. Moreover, the expression of SOX2 in NE tumor and tumors which have undergone NED, support the notion that SOX2 may support progression to castrate resistance via a NED pathway. Indeed, a recent study found that ectopic expression of SOX2 drives castration-resistant PCa growth, indicating that SOX2 is not only expressed in castration-resistant PCa, but also an important driver in promoting PCa progression (10). Coupled with this observation, our findings support exploring the predictive value of SOX2 for PCa with a larger patient cohort.
In conclusion, SOX2 was expressed during murine prostate development. In human prostate specimens, SOX2 expression was detected in basal cells. The expression of SOX2 was lost in primary prostate adenocarcinoma, but was detected in castration-resistant metastatic PCa and in prostatic NE tumors.
Acknowledgements
We would like to thank the patients and their families who were willing to participate in the Prostate Cancer Donor Program and the physicians and rapid autopsy team at the University of Washington. This research was supported by: NIH to RM (5R01 DK055748-14 and 4R01 CA076142-14); DOD to RM (PC074022); DOD to XY (PC111074); NIH to SWH (5R01 DK067049); Pacific Northwest Prostate Cancer SPORE (P50CA97186); PO1 NIH grant (PO1CA085859); and Richard M. LUCAS Foundation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1.Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–355. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 2.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3(2):167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317(1):296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, et al. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE. 2009;4(12):e8248. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS ONE. 2010;5(6):e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugolkov AV, Eisengart LJ, Luan C, Yang XJ. Expression analysis of putative stem cell markers in human benign and malignant prostate. Prostate. 2011;71(1):18–25. doi: 10.1002/pros.21217. [DOI] [PubMed] [Google Scholar]
- 8.Bae KM, Su Z, Frye C, McClellan S, Allan RW, Andrejewski JT, et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. Journal of Urology. 2010;183(5):2045–2053. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, et al. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol. 2011;3(4):230–238. doi: 10.1093/jmcb/mjr002. [DOI] [PubMed] [Google Scholar]
- 10.Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PloS one. 2013;8(1):e53701. doi: 10.1371/journal.pone.0053701. Epub 2013/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L, et al. SOX2 Gene Regulates the Transcriptional Network of Oncogenes and Affects Tumorigenesis of Human Lung Cancer Cells. PLoS ONE. 2012;7(5):e36326. doi: 10.1371/journal.pone.0036326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolzenburg S, Rots MG, Beltran AS, Rivenbark AG, Yuan X, Qian H, et al. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, et al. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene. 2012;31(18):2270–2282. doi: 10.1038/onc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang R, Liao D, Cheng T, Zhou H, Shi Q, Chuang TS, et al. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer. 2011;104(9):1410–1417. doi: 10.1038/bjc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girouard SD, Laga AC, Mihm MC, Scolyer RA, Thompson JF, Zhan Q, et al. SOX2 contributes to melanoma cell invasion. Lab Invest. 2012;92(3):362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin F, Lin P, Zhao D, Chen Y, Xiao L, Qin W, et al. Sox2 targets cyclinE, p27 and survivin to regulate androgen-independent human prostate cancer cell proliferation and apoptosis. Cell Prolif. 2012;45(3):207–216. doi: 10.1111/j.1365-2184.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laga AC, Lai CY, Zhan Q, Huang SJ, Velazquez EF, Yang Q, et al. Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology. Am J Pathol. 2010;176(2):903–913. doi: 10.2353/ajpath.2010.090495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sholl LM, Long KB, Hornick JL. Sox2 expression in pulmonary non-small cell and neuroendocrine carcinomas. Appl Immunohistochem Mol Morphol. 2010;18(1):55–61. doi: 10.1097/PAI.0b013e3181b16b88. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32(1):65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- 20.Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006;30(6):705–712. doi: 10.1097/00000478-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64(24):9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 22.Mosquera JM, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15(1):1–10. doi: 10.1593/neo.121550. Epub 2013/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabowska MM, Degraff DJ, Yu X, Jin RJ, Chen Z, Borowsky AD, et al. Mouse models of prostate cancer: picking the best model for the question. Cancer metastasis reviews. 2014 doi: 10.1007/s10555-013-9487-8. Epub 2014/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, et al. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78(6):319–334. [PubMed] [Google Scholar]
- 25.Masumori N, Thomas TZ, Case T, Paul M, Kasper S, Chaurand P, et al. A probasin-large T antigen transgenic mouse line develops prostate adeno and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61:2239–2249. [PubMed] [Google Scholar]
- 26.Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, et al. Dissociation of Epithelial and Neuroendocrine Carcinoma Lineages in the Transgenic Adenocarcinoma of Mouse Prostate Model of Prostate Cancer. Am J Pathol. 2008;172(1):236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masumori N, Tsuchiya K, Tu WH, Lee C, Kasper S, Tsukamoto T, et al. An allograft model of androgen independent prostatic neuroendocrine carcinoma derived from a large probasin promoter-T antigen transgenic mouse line. J Urol. 2004;171:439–442. doi: 10.1097/01.ju.0000099826.63103.94. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Wang YQ, Jiang M, Bierie BB, Hayward SW, Shen MM, et al. Activated beta-catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. The Prostate. 2009;69(3):249–262. doi: 10.1002/pros.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-Catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30(16):1868–1879. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62(4):339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- 31.Sramkoski RM, Pretlow TG, 2nd, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, et al. A new human prostate carcinoma cell line, 22Rv1. In vitro cellular & developmental biology Animal. 1999;35(7):403–409. doi: 10.1007/s11626-999-0115-4. Epub 1999/08/26. [DOI] [PubMed] [Google Scholar]
- 32.Dagvadorj A, Tan SH, Liao Z, Cavalli LR, Haddad BR, Nevalainen MT. Androgen-regulated and highly tumorigenic human prostate cancer cell line established from a transplantable primary CWR22 tumor. Clin Cancer Res. 2008;14(19):6062–6072. doi: 10.1158/1078-0432.CCR-08-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, et al. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28(2):344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. 2012;30(36):e386–e389. doi: 10.1200/JCO.2011.41.5166. Epub 2012/11/22. [DOI] [PubMed] [Google Scholar]
- 35.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. 2006;66:1013–1029. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 36.Simon RA, di Sant'Agnese PA, Huang LS, Xu H, Yao JL, Yang Q, et al. CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum Pathol. 2009;40(2):252–258. doi: 10.1016/j.humpath.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, et al. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009;69(7):787–798. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 38.Sotomayor P, Godoy A, Smith GJ, Huss WJ. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate. 2009;69(4):401–410. doi: 10.1002/pros.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]