Abstract
Bacteria have the exquisite ability to maintain a precise diameter, cell length and shape. The dimensions of bacteria size and shape are a classical metric in the distinction of bacterial species. Much of what we know about the particular morphology of any given species is the result of investigations of planktonic cultures. As we explore deeper into the natural habitats of bacteria, it is increasingly clear that bacteria can alter their morphology in response to the environment in which they reside. Specific morphologies are also becoming recognized as advantageous for survival in hostile environments. This is of particular importance in the context of both colonization and infection in the host. There are multiple examples of bacterial pathogens that use morphological changes as a mechanism for evasion of host immune responses and continued persistence. This review will focus on two systems where specific morphological changes are essential for persistence in animal models of human disease. We will also offer insight into the mechanism underlying the morphological changes and how these morphotypes aid in persistence. Additional examples of morphological changes associated with survival will be presented.
Keywords: Urinary Tract Infection, Otitis Media, Morphology, Persistence
Introduction
The 7th edition of “Bergey's Manual of Determinative Bacteriology” states, “no organism can be classified before its morphological … characters have been determined through a detailed study”. Bergey then states that E. coli can be recognized as rods up to 3µm in length that exist in a coccoid form that occurs singly, in pairs or short chains. Similarly, Haemophilus is described as very small rods up to 2µm in length that occur singly, in pairs, short chains or long threads. Thus, in 1957, it was apparent that although bacteria could be identified primarily through a single morphological determinant, deviations from the classic description of a bacterium’s appearance occurred. Why bacteria exhibit these different morphologies was not apparent at that time. Only now are we beginning to understand why a single strain of bacteria can exhibit multiple morphotypes. Critically, many species of bacteria use changes in morphology to aid in survival in hostile environments. These mechanisms can be of particular importance as certain morphotypes are optimized for survival of innate immune effectors as well as invasion into epithelial cells. Such invasion events could provide bacteria with an environment rich in nutrients and a refuge from immune pressures and so provide temporary or long-term respite from nutrient limitation and innate immune components. Further, it is becoming clear that differentiation as a method of survival, both in the environment and in the host as part of disease progression, is an increasingly common approach. It is thus imperative that the mechanisms behind protective differentiation be fully understood.
Intracellular bacterial communities: the paradigm of uropathogenic Escherichia coli
The initial observations that allude to an intracellular developmental pathway for uropathogenic Escherichia coli (UPEC) are revealed from electron microscopic images that demonstrated bacteria within bladder umbrella cells of infected mouse bladders (Mulvey et al., 1998). UPEC growth within the umbrella cells creates a bulge, or pod, on the luminal surface (Anderson et al., 2003). Pods are not evident in bladders inoculated with non-uropathogenic E. coli strains, suggesting the formation of pods relates to infectious lifestyles. Within the urinary tract, the intracellular niche is advantageous for UPEC. Urine is nutrient poor, and the absence of urease activity reduces the access to carbon sources in the urine. In contrast, the cytoplasm of the bladder umbrella cell is a rich nutritional environment. The epithelial membrane also provides a physical barrier against the activity of phagocytes and antibody-mediated clearance mechanisms. Within the epithelium, antimicrobial agents can damage UPEC, but UPEC survives through the induction of stress responses such as the DNA damage repair response (Justice et al., 2006, Li et al., 2010, Gawel & Seed, 2011).
Time-lapse fluorescence video microscopy reveals the growth of UPEC intracellular bacterial communities (IBCs) within live bladder explants (Justice et al., 2004). This novel approach permits dissection of discrete developmental intermediates of the UPEC intracellular life cycle. In the first branch of the developmental and differentiation pathway, internalized UPEC are non-motile, bacillary in morphology and form loosely organized colonies in the bladder umbrella cell cytoplasm (early IBC)(Figure 1). This mechanism of growth as a community is in marked contrast to the lifestyles of other previously described cytoplasmic pathogens (e.g. Listeria, Shigella) that harness the host actin to grow as single motile bacteria. The majority of the intracellular UPEC within each pod transition into a developmental phase characterized by a reduction in the rate of growth and an increase in organization into a biofilm-like structure with coccoid bacteria filling the host cytoplasm (mid-IBC). During the mid-IBC phase, a minority of the bacteria within each IBC proceeds through a second developmental branch, involving differentiation into a filamentous morphology (Figure 1), described in greater detail below. In addition, each IBC arises from a single invasion event (Schwartz et al., 2011), suggesting morphological changes represent a different phase of the developmental program. Therefore, the coccoid morphology is not the result of overgrowth from parallel invasion events of UPEC that exhibit different morphologies. In the third phase, peripheral bacteria of the IBC become motile and rod-like, dissociate from the community, and egress from the host cell with motility characteristic of flagellar movement. The released bacteria may attach to neighboring umbrella cells and initiate a new round of invasion (second-generation IBC). Alternatively, if urothelium is denuded in areas of umbrella cell exfoliation, the newly released UPEC may invade underlying transitional urothelial cells and establish quiescent intracellular reservoirs (Justice et al., 2004, Mysorekar & Hultgren, 2006). These reservoirs consist of 1–4 bacteria in membrane-bound compartments that contain the lysosomal protein, Lamp-1, and are considered resistant to host immunity, antibiotic treatment and serve as the reservoir for recurrent infection (Mulvey et al., 2001, Schilling et al., 2002, Justice et al., 2004, Mysorekar & Hultgren, 2006).
Figure 1.
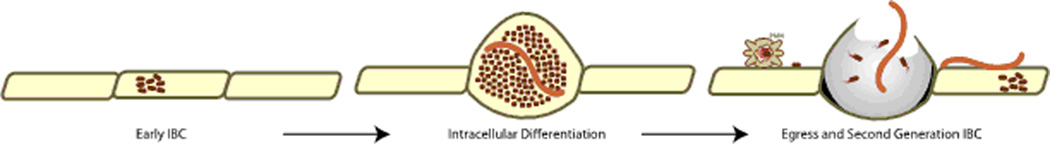
UPEC intracellular differentiation pathway. UPEC (maroon rods) invade into the cytoplasm of the bladder epithelial cell to initiate the intracellular developmental and differentiation pathway (early IBC). The epithelial cell becomes engorged with UPEC in the coccoid (dark maroon) and filamentous (light maroon) morphologies to form the pod (intracellular differentiation). The pod ultimately lyses, releasing rod and filamentous morphotypes that invade into neighboring naïve cells for persistence (egress and second generation IBC).
The majority of human clinical UPEC isolates from women with urinary tract infection exhibit IBC formation following transurethral inoculation into mice bladders (Garofalo et al., 2007), suggesting the intracellular developmental lifestyle is not restricted to a single UPEC strain. Further, prototypic cystitis or pyelonephritis UPEC isolates support IBC formation in five genetically distinct inbred mouse strains (Garofalo et al., 2007). IBC formation is observed to similar degrees in immunocompetent and immunocompromised hosts (Justice et al., 2004, Garofalo et al., 2007), suggesting that host immune pressure does not drive the developmental process with regards to the transient conversion through a coccoid morphology. Moreover, IBC-like structures are observed in the urine of women and children that present with symptoms of urinary tract infection (0 et al., 2007, Robino et al., 2013). Taken together, the intracellular lifestyle of UPEC occurs in multiple isolates and multiple mouse strains, suggesting that the developmental and differentiation pathway is a conserved program.
Evidence for an IBC pathway of other uropathogens as well as in human urine samples is accumulating (Table 1). Klebsiella pneumonia, another causative agent of urinary tract infection, the IBC pathway is present in both human urine samples as well as in the mouse model of urinary tract infection (Rosen et al., 2007, Rosen et al., 2008, Rosen et al., 2008). Human urine samples from patients suffering UTI with Enterococcus faecalis also contain IBC-like structures (Horsley et al., 2013). The IBC lifestyle appears to be a common lifestyle of both Gram-negative and Gram-positive urease-negative bacterial pathogens in the urinary tract.
Table 1.
IBCs formed by E. coli in mammary epithelium
The bladder epithelium is not the only tissue that supports IBC formation by E. coli. Similar to the contamination of the urinary tract by fecal matter in humans initiates infection, the mammary glands of bovines can be contaminated from fecal sources of E. coli. Intramammary introduction of E. coli leads to invasion and IBC formation within alveolar epithelial cells in a mouse model (Gonen et al., 2007, Mintz et al., 2013). In this model system, IBCs commonly develop in an immunocompromised host, suggesting bacterial clearance by a functional immune response occurs at this privileged site. It is therefore plausible that physiological stress dampens host immune responses and promotes susceptibility to IBC formation and persistence of E. coli in mammary tissues.
IBCs formed by nontypeable Haemophilus influenzae in the middle ear epithelium
Since the initial description of IBCs formed by UPEC, evidence is accumulating for IBCs in other infectious systems (Table 1). Although the nutritional status of the pathogen is not a key mediator in the establishment of IBCs in the systems presented thus far, nutritional status contributes significantly to IBC formation and morphological changes in some organisms (Table 1). Within the host, availability of essential nutrients (i.e. iron, zinc, manganese) is sequestered to prevent microbial outgrowth. This nutritional immunity is due to the near universal requirement for specific host-derived nutrients for bacterial growth (Hood & Skaar, 2012, Cassat & Skaar, 2013). Nontypeable Haemophilus influenzae (NTHI), a causative agent of upper and lower respiratory infections, must obtain heme-iron from the environment. Although classically considered an extracellular, opportunistic pathogen, there is increasing evidence for intracellular niches for NTHI in vitro (Clementi & Murphy, 2011). Further, the presence of NTHI within adenoids and bronchial epithelium suggests that an invasive phenotype may coincide with the chronic nature associated with NTHI-mediated diseases. The chronicity of NTHI infections including recalcitrance to antibiotic therapy, persistence in the presence of bactericidal antibodies and culture-negative clinical analysis are suggestive of the development of intracellular bacterial reservoirs within host cells (Hall-Stoodley & Stoodley, 2009, Clementi & Murphy, 2011). Loss of the SapA periplasmic binding protein, responsible for uptake of essential heme-iron, produces distinct differences in the capacity to invade polarized normal human bronchial cell cultures (Mason et al., 2011). In contrast to the parental strain where the internalized bacteria appear to proceed through the lysosomal pathway, the sapA mutant is not contained within a membranous compartment but colonizes the host cytoplasm reminiscent of the cytoplasmic location of UPEC IBCs, and in most cases retain bacterial density and membrane integrity (Raffel et al., 2013). These data suggest that physiological status, in response to fluctuations in the availability of the essential nutrient heme, may promote intracellular survival of NTHI.
Essential nutrients at privileged sites are normally sequestered. However, the production of inflammatory products (e.g. edema and fluid) provides sources of essential nutrients. Thus, bacteria experience fluctuations in availability of micronutrients early during infection. The ramifications of the fluctuations in the availability of heme-iron on NTHI pathogenesis are now emerging. These fluctuations in heme-iron availability can be mimicked by growth within a defined medium in the absence or presence of heme-iron to generate heme-iron ‘restricted’ and ‘replete’ NTHI, respectively (Szelestey et al., 2013). Co-culture of restricted or replete NTHI with monolayers of primary middle ear epithelial cells, in the presence of heme-iron, demonstrates that NTHI transiently restricted of heme-iron are better adapted to survive inside epithelial cells. Moreover, heme-iron restricted NTHI are more persistent in the chinchilla model for human otitis media when compared to NTHI replete for heme-iron (Szelestey et al., 2013). Transiently restricted NTHI IBCs fill the entire volume of primary cultured middle ear epithelial cells, which bulge the cells and resemble UPEC pods (Szelestey et al., 2013). These data suggest that exposure of NTHI to host immune pressures, which include host sequestration of essential nutrients, promote epithelial cell invasion, intracellular development and survival.
Advantages for differentiation from bacillary to coccoid morphology
There are both theoretical and evidence-based observations that a coccoid morphology has significant benefits for survival. The small and symmetric size allows for better packing of viable bacterial cells within the restricted host cell volume. A single UPEC that invades a bladder epithelial cell can multiply to 105 viable organisms. Further, the decrease in bacterial size provides the greatest surface area for nutrient uptake (Baker et al., 1983). Although little is known regarding the signals and mechanisms for differentiation from a bacillary to a coccoid morphology within UPEC IBCs, in other systems, environmental stress is a key stimulator for differentiation into coccoid morphotypes. Vibrio cholerae coccoid morphotypes arise from starvation and are as infectious as the bacillary morphotype (Krebs & Taylor, 2011). Coccoid forms of Helicobacter result from antibiotics or acid stress and remain viable for up to 1 year in seawater while the bacillary form survives for less than 2 weeks under the same conditions (Shahamat et al., 1989). Moreover, in the marine environment, transition to a coccoid morphology protects bacteria from predation by unicellular protists (Pernthaler, 2005). The similarities in the phagocytosis mechanisms of protists and professional innate immune phagocytes suggest that coccoid morphotypes may be protected from phagocytosis during disease. Taken together, the coccoid transition can provide multiple advantages to the bacterium, for example continued growth in nutrient limited environments, mediating exposure to stress, stability in nutrient deplete conditions as well as a means to subvert killing by predation and complement (Dalia & Weiser, 2011).
Second branch of differentiation within UPEC IBCs: transition from bacillary to a filamentous morphology
Bacterial communities are composed of individual bacteria that although coordinated, contain subpopulations that may serve vitally different roles in the development and persistence of the community as a whole. UPEC IBCs are a striking example of this phenomenon. During experimental urinary tract infection, a subpopulation of UPEC proceed through a developmental pathway that involves the inhibition of septation and the production of filamentous morphotypes as much as 70 µm in length (Mulvey et al., 1998, Justice et al., 2004) (Figure 1). Filamentous morphotypes of UPEC, K. pneumoniae, Enterobacter aerogenes, and Proteus mirabilis are readily observed in human urine samples of patients with UTIs (Rosen et al., 2007). Therefore, differentiation into a filamentous morphology is common to multiple pathogens that cause urinary tract infections (Table 1).
Filamentation is a direct consequence of host immunity and is essential for persistence during infection
The appearance of significant populations of filamentous UPEC during experimental UTI provoked a line of investigation into the regulation of UPEC filamentation during UTI (Justice et al., 2006). Deletion of SulA, a cell division inhibitor associated with the DNA damage repair response (SOS response)(Kelley, 2006), results in the absence of filamentous morphotypes, and the mutant strain is sharply attenuated in the formation of secondary IBCs and quiescent intracellular reservoirs (Justice et al., 2006). Filamentous bacteria are observed in the bladders of mice with intact Toll-like receptor 4 signaling (TLR4), but are not detectable in the bladders of mice with hypomorphic tlr4 alleles (Justice et al., 2004, Justice et al., 2006). As such, SulA is dispensable in hosts defective in TLR4-signal transduction (Justice et al., 2006), which indicates that filamentation is required for persistence during urinary tract infections.
Fluctuations in heme-iron availability both promote NTHI intracellular survival and dramatically influence the architecture of bacterial biofilms grown on biotic and abiotic surfaces. In contrast to the mat-like extracellular biofilms that form with continuous exposure to heme-iron, extracellular biofilms that form following transient heme-iron restriction form an open, mesh-like architecture that consists of multinucleate filamentous morphotypes (Vogel et al., 2012, Szelestey et al., 2013). The architecture of biofilms following transient heme-iron restriction requires the activity of a SulA ortholog, suggesting that filamentation is an important component of the architectural attributes associated with fluctuations in heme-iron availability (Szelestey et al., 2013). Consistent with the role of SulA in the persistence of UPEC in the urinary tract, SulA is also essential for persistence of NTHI in the chinchilla model of otitis media (Szelestey et al., 2013). Although the mechanism of SulA activation in this system remains unclear, filamentation appears to be a response to the restoration of essential nutrients following transient restriction. Therefore, bacterial filamentation is a common trait of the pathogenic lifestyle and is essential for at least two independent infections caused by two different organisms.
Advantages for differentiation from bacillary to filamentous morphology
Filamentation is observed for a number of different pathogens (Table 1) and offers multiple strategies for survival. First, antimicrobial agents that target the septation are ineffective against the filamentous morphotype. In addition, recent evidence indicates that filamentous morphotypes evade killing by host phagocytes. This strategy is suggested by multiple observations. Filamentous UPEC are the predominant extracellular morphotype between rounds of IBC formation (Justice et al., 2004, Justice et al., 2006). Intact filamentous UPEC in immediate proximity of phagocytes engorged with UPEC rods are viable (Justice et al., 2004). Following phagocytosis of populations of mixed morphologies, there is a significant decrease in the bacillary population with a minimal decrease in the filamentous morphotypes (Horvath et al., 2011). This results in an enrichment of the filamentous forms when these mixed populations are exposed to both cultured and primary phagocytes (Horvath et al., 2011), providing quantitative evidence that the filamentous form is resistant to phagocytosis.
The role of shape is important in the resistance to phagocytosis of plastic polymer particles, marine bacteria and yeast (Cannon & Swanson, 1992, Pernthaler, 2005, Champion & Mitragotri, 2006, Justice et al., 2008). The mechanism by which filamentous shapes are not phagocytosed is unclear, but has been the subject of recent investigations. The uptake of bacillary shaped E. coli is independent of the orientation of initial interaction with macrophages (Moller et al., 2012). In contrast, phagocytosis of filamentous bacteria only occurs when macrophages can gain access to the pole (Champion & Mitragotri, 2006, Horvath et al., 2011, Moller et al., 2012, Prashar et al., 2013). If the pole of filamentous E. coli is engaged, there is no difference in the velocity of phagocytosis between filamentous E. coli and bacillary E. coli (Moller et al., 2012). Formation of the actin cup, essential for initiation of phagocytosis, is only observed when macrophages engage E. coli filaments at the pole, and not when macrophages engage the long axis (Moller et al., 2012, Champion & Mitragotri, 2006, Moller et al., 2012). Engagement of macrophages with the pole of filamentous Legionella pneumophilia results in a remodeling of the actin cup into a tubular structure (Prashar et al., 2013). The tubular cup appears as a long membranous invagination that extends around the circumference of the macrophage and requires actin treadmilling (Prashar et al., 2013). The tubular structures retain the ability to fuse with lysosomes, but the compartment does not become acidified due to leaking of hydrolases into the medium (Prashar et al., 2013). Filamentous morphotypes of L. pneumophilia are trafficked to the replicative supportive vacuole in a type IV secretion-dependent manner (Prashar et al., 2013). These filamentous forms initially continue to grow as filaments, but eventually exhibit septation to produce bacillary shaped bacteria that can escape the macrophage (Prashar et al., 2013). Filamentous L. pneumophilia also invade human lung epithelial cells using a unique “hook”, “membrane wrap” and “zipper” mechanism (Prashar et al., 2012). The filamentous L. pneumophilia invade into the epithelial cells, septate and continue to grow within the intracellular compartment (Prashar et al., 2012). Taken together, filamentous morphology provides a survival advantage in the prevention of phagocytosis and killing by macrophages.
Concluding Statements
Morphological differentiation is critical for survival of bacteria, both in the environment and in the host as part of the disease process. The role of differentiation in bacterial survival is exemplified by UPEC. Uropathogenic strains of E. coli form IBCs protected from the host’s immune system, and with ready access to nutrients present within the host cell. This intracellular lifestyle also confers an added benefit to UPEC through the development of a filamentous population of cells that exhibit increased resistance to phagocytosis. Differentiation-driven protection is now increasingly recognized as a generalized lifestyle, in other UTI pathogens as well as other genera of bacteria that cause diseases not related to the urinary tract. Investigations into NTHI showed that the nutritional status produces morphological changes that lead to the generation of intracellular communities and improved survival in a chinchilla model of OM. The mechanisms that generate these protective differentiation events requires additional investigation. Whether these are common mechanisms that produce protective filamentation in other bacteria has yet to be resolved. However, the importance of differentiation as a protective mechanism for both environmental bacteria as well as pathogens, drives our interest in determining what the mechanisms are underpinning this critical morphological differentiation.
Figure 2.
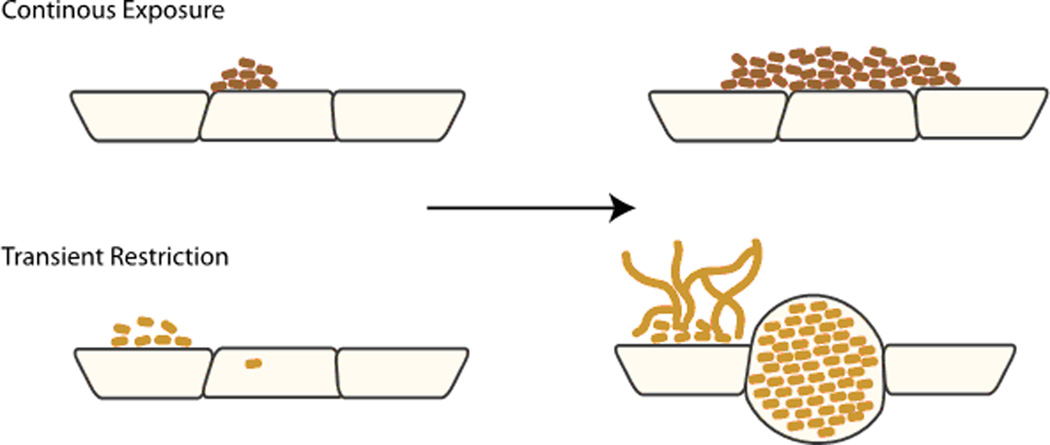
NTHI differentiation in response to fluctuations in nutritional availability. NTHI IBC formation is dependent upon the nutritional state at the time of infection. Continuously exposed to heme-iron (dark brown rods) form mat-like biofilms on the epithelial surface with limited intracellular development. In contrast, restricted for heme-iron (light brown rods) form a biofilm on the surface that has an open architecture containing filamentous NTHI. In addition, heme-iron restricted NTHI responded to nutritional limitation by forming IBCs within the epithelial cells.
Acknowledgements
KMM and SSJ are supported by National Institute of Health Grant R01 DC013313.
Footnotes
The authors have no conflict of interest to declare.
References
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- Baker RM, Singleton FL, Hood MA. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983;46:930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. Hyperosmotic stress response of Campylobacter jejuni. J Bacteriol. 2012;194:6116–6130. doi: 10.1128/JB.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. J Cell Sci. 1992;101(Pt 4):907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Madiraju MV, Fol M, Lofton H, Maloney E, Reynolds R, Rajagopalan M. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J Bacteriol. 2006;188:1856–1865. doi: 10.1128/JB.188.5.1856-1865.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi CF, Murphy TF. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Frontiers in cellular and infection microbiology. 2011;1:1. doi: 10.3389/fcimb.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia AB, Weiser JN. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe. 2011;10:486–496. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, Hultgren SJ. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawel D, Seed PC. Urinary tract infection drives genome instability in uropathogenic Escherichia coli and necessitates translesion synthesis DNA polymerase IV for virulence. Virulence. 2011;2:222–232. doi: 10.4161/viru.2.3.16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen E, Vallon-Eberhard A, Elazar S, Harmelin A, Brenner O, Rosenshine I, Jung S, Shpigel NY. Toll-like receptor 4 is needed to restrict the invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine model of acute mastitis. Cell Microbiol. 2007;9:2826–2838. doi: 10.1111/j.1462-5822.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, Kupelian A, Rohn JL. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS One. 2013;8:e83637. doi: 10.1371/journal.pone.0083637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DJ, Jr., Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, Justice SS. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 2011;13:426–437. doi: 10.1016/j.micinf.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice SS, Hunstad DA, Seed PC, Hultgren SJ. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc Natl Acad Sci U S A. 2006;103:19884–19889. doi: 10.1073/pnas.0606329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol. 2008;6:162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. From the cover: Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WL. Lex marks the spot: the virulent side of SOS and a closer look at the LexA regulon. Mol Microbiol. 2006;62:1228–1238. doi: 10.1111/j.1365-2958.2006.05444.x. [DOI] [PubMed] [Google Scholar]
- Krebs SJ, Taylor RK. Nutrient-dependent, rapid transition of Vibrio cholerae to coccoid morphology and expression of the toxin co-regulated pilus in this form. Microbiology. 2011;157:2942–2953. doi: 10.1099/mic.0.048561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt KA, Poulsen LK, Molin S. Identification of coccoid Escherichia coli BJ4 cells in the large intestine of streptomycin-treated mice. Infect Immun. 1993;61:5029–5034. doi: 10.1128/iai.61.12.5029-5034.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Smith P, Horvath DJ, Jr., Romesberg F, Justice SS. SOS regulatory elements are essential for UPEC pathogenesis. Microbes Infect. 2010;12:662–668. doi: 10.1016/j.micinf.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Mason KM, Raffel FK, Ray WC, Bakaletz LO. Heme utilization by nontypeable Haemophilus influenzae is essential and dependent on Sap transporter function. J Bacteriol. 2011;193:2527–2535. doi: 10.1128/JB.01313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz M, Mintz D, Ilia-Ezra R, Shpigel NY. Pam3CSK4/TLR2 signaling elicits neutrophil recruitment and restricts invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine mastitis model. Vet Immunol Immunopathol. 2013;152:168–175. doi: 10.1016/j.vetimm.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Moller J, Luehmann T, Hall H, Vogel V. The race to the pole: how high-aspect ratio shape and heterogeneous environments limit phagocytosis of filamentous Escherichia coli bacteria by macrophages. Nano Lett. 2012;12:2901–2905. doi: 10.1021/nl3004896. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Takade A, Miyamoto H, Taniguchi H, Yoshida S. Morphological variety of intracellular microcolonies of Legionella species in Vero cells. Microbiol Immunol. 2001;45:557–562. doi: 10.1111/j.1348-0421.2001.tb02658.x. [DOI] [PubMed] [Google Scholar]
- Oh JD, Karam SM, Gordon JI. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc Natl Acad Sci U S A. 2005;102:5186–5191. doi: 10.1073/pnas.0407657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler J. Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol. 2005;3:537–546. doi: 10.1038/nrmicro1180. [DOI] [PubMed] [Google Scholar]
- Ponnusamy D, Clinkenbeard KD. Yersinia pestis intracellular parasitism of macrophages from hosts exhibiting high and low severity of plague. PLoS One. 2012;7:e42211. doi: 10.1371/journal.pone.0042211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar A, Bhatia S, Gigliozzi D, Martin T, Duncan C, Guyard C, Terebiznik MR. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J Cell Biol. 2013;203:1081–1097. doi: 10.1083/jcb.201304095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar A, Bhatia S, Tabatabaeiyazdi Z, Duncan C, Garduno RA, Tang P, Low DE, Guyard C, Terebiznik MR. Mechanism of invasion of lung epithelial cells by filamentous Legionella pneumophila. Cell Microbiol. 2012;14:1632–1655. doi: 10.1111/j.1462-5822.2012.01828.x. [DOI] [PubMed] [Google Scholar]
- Raffel FK, Szelestey BR, Beatty WL, Mason KM. The Haemophilus influenzae Sap transporter mediates bacterium-epithelial cell homeostasis. Infect Immun. 2013;81:43–54. doi: 10.1128/IAI.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Vignoli R. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathogens and disease. 2013;68:78–81. doi: 10.1111/2049-632X.12047. [DOI] [PubMed] [Google Scholar]
- Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS medicine. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DA, Pinkner JS, Walker JN, Elam JS, Jones JM, Hultgren SJ. Molecular variations in Klebsiella pneumoniae and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect Immun. 2008;76:3346–3356. doi: 10.1128/IAI.00340-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DA, Pinkner JS, Jones JM, Walker JN, Clegg S, Hultgren SJ. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun. 2008;76:3337–3345. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger CM, Finlay BB. Macrophages inhibit Salmonella typhimurium replication through MEK/ERK kinase and phagocyte NADPH oxidase activities. J Biol Chem. 2002;277:18753–18762. doi: 10.1074/jbc.M110649200. [DOI] [PubMed] [Google Scholar]
- Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci U S A. 2004;101:2422–2427. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti V, Ammann TW, Thurnheer T, Bagheri HC, Belibasakis GN. Phenotypic diversity of multicellular filamentation in oral streptococci. PLoS One. 2013;8:e76221. doi: 10.1371/journal.pone.0076221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahamat M, Paszko-Kolva C, Yamamoto H, Mai U, Pearson A, Colwell R. Ecological studies of Campylobacter pylori. Klinicheskaia Wochenschr. 1989;67:62–63. [Google Scholar]
- Szabados F, Kleine B, Anders A, Kaase M, Sakinc T, Schmitz I, Gatermann S. Staphylococcus saprophyticus ATCC 15305 is internalized into human urinary bladder carcinoma cell line 5637. FEMS Microbiol Lett. 2008;285:163–169. doi: 10.1111/j.1574-6968.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- Szelestey BR, Heimlich DR, Raffel FK, Justice SS, Mason KM. Haemophilus responses to nutritional immunity: epigenetic and morphological contribution to biofilm architecture, invasion, persistence and disease severity. PLoS Pathog. 2013;9:e1003709. doi: 10.1371/journal.ppat.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AR, Szelestey BR, Raffel FK, Sharpe SW, Gearinger RL, Justice SS, Mason KM. SapF-mediated heme-iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Frontiers in cellular and infection microbiology. 2012;2:42. doi: 10.3389/fcimb.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P, Waterfield NR, Crossman L, et al. Comparative genomics of the emerging human pathogen Photorhabdus asymbiotica with the insect pathogen Photorhabdus luminescens. BMC Genomics. 2009;10:302. doi: 10.1186/1471-2164-10-302. [DOI] [PMC free article] [PubMed] [Google Scholar]