Abstract
Background
Sonothrombolysis is safe and may increase the likelihood of early recanalization in acute ischemic stroke (AIS) patients
Aims
In preparation of a phase III clinical trial, we contrast the likelihood of achieving a sustained recanalization and functional independence in a post-hoc subgroup analysis of patients randomized to transcranial Doppler monitoring plus intravenous (IV) tPA (sonothrombolysis) compared to IV tPA alone in the CLOTBUST trial
Methods
We analyzed the data from all randomized AIS patients with pre-treatment NIHSS scores ≥10 points and proximal intracranial occlusions in the CLOTBUST trial. We compared sustained complete recanalization rate (TIBI flow grades 4-5) and functional independence (modified Rankin Scale [mRS] 0-1) at 90 days. Safety was evaluated by the rate of symptomatic intracranial hemorrhage (ICH) within 72 hours of stroke-onset
Results
Of 126 patients, a total of 85 AIS patients met our inclusion criteria: mean age 71±11years, 56% men, median NIHSS 17 (interquartile range 14-20). Of these patients, 41 (48%) and 44 (52%) were randomized to IV tPA alone and sonothrombolysis, respectively. More patients achieved sustained complete recanalization in the sonothrombolysis than in the IV tPA alone group (38.6% vs. 17.1%; p=0.032). Functional independence at 90 days was more frequently achieved in the sonothrombolysis than in the IV tPA alone group (37.2% vs. 15.8%; p=0.045). Symptomatic ICH rate was similar in both groups (4.9% vs. 4.6%; p=1.00)
Conclusions
Our results point to a signal of efficacy and provide information to guide the subsequent phase III randomized trial of sonothrombolysis in patients with severe ischemic strokes
Keywords: sonothrombolysis, severe stroke, proximal occlusion, clinical outcome
Introduction
Intravenous (IV) thrombolytic therapy with tissue plasminogen activator (tPA) is associated with functional independence and reduced mortality following acute ischemic stroke [1,2]. However, patients with severe stroke (National Institutes of Health Stroke Scale [NIHSS] score of ≥ 10 points) predominantly have proximal and large arterial occlusions and reperfusion benefit of IV tPA may be limited [3-6]. Hence, these patients frequently experience persistent arterial occlusion or re-occlusion after completion of IV tPA infusion, decreasing the likelihood for long-term survival and good functional outcome [7-9]. On the contrary, results from the third International Stroke Trial (IST-3) suggest that patients with severe strokes have great potential for clinical recovery following thrombolytic therapy with IV tPA [10]. This reinforces ongoing efforts to explore adjuvant treatment strategies that might improve recanalization rate, reperfusion and clinical outcome in this patient population [11,12]
Sonothrombolysis, the adjuvant exposure of an intracranial occlusive thrombus to high-frequency ultrasound, is a promising strategy to augment the reperfusion benefit of concomitant IV thrombolytic therapy with tPA. Meta-analyses have concordantly demonstrated that sonothrombolysis with or without gaseous microbubbles is safe, triples the likelihood of tPA-induced recanalization and doubles the likelihood of achieving functional independence in unselected acute ischemic stroke populations when compared with IV tPA alone [13-15]. However, no effort has been done yet to particularly investigate the effect of sonothrombolysis in patients with severe stroke.
Aims
In preparation for a phase III pivotal trial of sonothrombolysis (CLOTBUST-ER NCT#01098981) [16], we performed a post-hoc subgroup analysis of the CLOTBUST trial to determine whether the group of patients with severe strokes could have a better chance for recovery if tPA-associated recanalization is amplified with ultrasound. We explored this question to see whether there is a signal of efficacy to provide information to guide the design of a phase III trial of sonothrombolysis in acute ischemic stroke patients.
Methods
We selected patients with severe stroke (pre-treatment NIHSS scores ≥10 points) and proximal intracranial occlusions on pre-treatment transcranial Doppler (TCD) defined as Thrombolysis in Brain Ischemia (TIBI) flow grades 0-3 from the previously published phase II randomized controlled CLOTBUST (Combined Lysis of Thrombus in Brain Ischemia) trial [17]. The CLOTBUST trial utilized commercially available hand-held TCD technology at diagnostic 2-MHz ultrasound frequencies to test safety and signal of efficacy of sonothrombolysis. Specifically, acute ischemic stroke patients were randomized into two treatment groups: the sonothrombolysis group received IV thrombolytic therapy with tPA in a standard dose (0.9 mg/kg) initiated within 3 hours from stroke-onset in combination with 2 hours of continuous TCD ultrasound monitoring (i.e., IV tPA + 2-MHz ultrasound). Patients in the control group received standard intravenous tPA with 2 hours of continuous placebo monitoring (i.e., IV tPA alone). In both groups, follow-up TCD measurements were performed to assess vessel patency at 30, 60, 90 and 120 minutes after the tPA bolus. Further details of the CLOTBUST study design are published elsewhere [18].
Patients who were additionally treated with intra-arterial thrombolysis were excluded from our analysis since endovascular procedures are not allowed in the subsequent phase III trial. Each participating center had IRB approval and all subjects gave written informed consent to participate in the original trial.
We included the following outcome variables in our analysis. Sustained complete recanalization was defined as TIBI flow grade 4-5 assessed by TCD at 2 hours after tPA bolus. Re-occlusion on TCD was diagnosed as worsening of flow by at least one TIBI grade following complete recanalization within 2 hours from tPA bolus. Symptomatic intracranial hemorrhage (ICH) was assessed using ECASS-2 definition as imaging evidence of ICH with an increase of 4 points or more in the NIHSS score within 72 hours from stroke onset [19]. Functional independence was defined as modified Rankin scale (mRS) score of 0 to 1 at 3 months.
Statistical analysis
Statistical comparisons were performed between subgroups of patients using the χ2 test, Fisher's exact test, unpaired t test, and Mann-Whitney U test as indicated for dichotomous or continuous variables. Univariate and multivariate analysis was performed with the use of logistic regression assessing if the treatment groups were independently associated with complete vessel recanalization 2 hours after tPA bolus and functional independence at 3 months. Baseline variables that were significantly different between the two study groups were built into the adjusted models. Significance level was set at p<0.05. Odds ratios (OR) and frequencies are presented with corresponding 95% confidence intervals (CI). The statistical software package STATA (Version 12.1, StataCorp., College Station, TX) was used for statistical analysis.
Results
Of 126 randomized patients in the CLOTBUST trial, 105 had severe strokes and proximal occlusions of the middle cerebral artery. A total of 20/105 (19%) patients were additionally treated with intra-arterial thrombolysis and excluded from further analysis, leaving 85/105 (81%) patients for our subanalysis: mean age was 71±11years, 56% were men, median NIHSS score was 17 (interquartile range 14-20) points. Of these patients, 41/85 (48%) were assigned to the IV tPA arm and 44/85 (52%) to the sonothrombolysis arm. Data on 90-days mRS outcome was available in 81/85 patients (IV tPA: n=38; sonothrombolysis: n=43). Except for sex (men 44% vs. 68%, p=0.024) groups were balanced in terms of age, vascular risk factors, baseline stroke severity and TIBI flow grades, onset-to-treatment time and occlusion site (Table 1).
Table 1.
Baseline characteristics of the two study groups.
| Variable | IV tPA (n=41) | Sonothrombolysis (n=44) | P value |
|---|---|---|---|
| Sex, male, n (%) | 18 (44) | 30 (68) | 0.024 |
| Age, years (mean±SD) | 73±12 | 70±10 | 0.35 |
| Vascular risk factors, n (%) | |||
| Arterial hypertension | 22 (54) | 28 (64) | 0.35 |
| Nicotine | 7 (17) | 12 (27) | 0.26 |
| Diabetes mellitus | 12 (29) | 9 (20) | 0.35 |
| Atrial fibrillation | 11 (27) | 11 (25) | 0.85 |
| NIHSS, median (IQR) | 18 (14-20) | 17 (14-21) | 0.85 |
| TIBI, median (IQR) | 2 (1-3) | 2 (1-2) | 0.75 |
| M1 MCA occlusion, n (%) | 32 (78) | 35 (80) | 0.87 |
| M2 MCA occlusion, n (%) | 9 (22) | 9 (20) | 0.87 |
| ICA obstruction*, n (%) | 10 (24) | 15 (34) | 0.35 |
| Pretreatment SBP, mmHg (mean±SD) | 156±23 | 160±22 | 0.38 |
| Insonation depth, mm (mean±SD) | 51±8 | 50±7 | 0.45 |
| OTT time, min (mean±SD) | 135±32 | 144±30 | 0.19 |
IV indicates intravenous; tPA, tissue plasminogen activator; SD, standard deviation; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; TIBI, Thrombolysis In Brain Ischemia; MCA, middle cerebral artery; ICA, internal carotid artery; OTT, onset to treatment; SBP, systolic blood pressure
concomitant greater or equal than 70% stenosis or complete occlusion of the proximal ICA
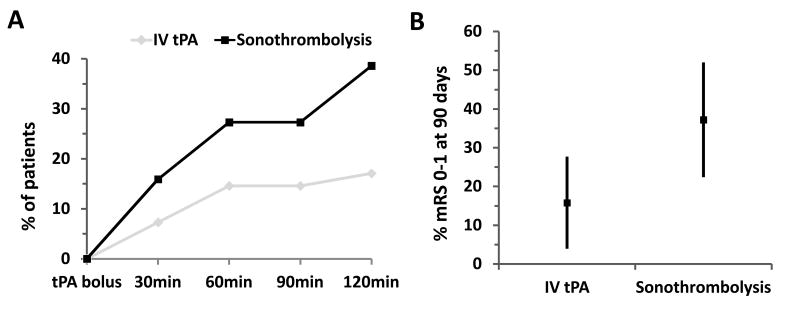
More patients achieved sustained complete recanalization in the sonothrombolysis than in the IV tPA group: 38.6% (17/44; 95%CI: 0.24-0.53) versus 17.1% (7/41; 95%CI: 0.05-0.29), p=0.032 Fisher's Exact Test (Figure 1, A). Sonothrombolysis was associated with a higher likelihood of complete recanalization (OR: 3.06; 95%CI: 1.11-8.44; p=0.03). After adjusting for significant baseline imbalances (i.e. sex), this association remained unchanged (OR: 3.65; 95%CI: 1.29-10.33; p=0.015). The rate of re-occlusion within 2 hours after tPA bolus did not differ between the sonothrombolysis and the IV tPA alone group (4.6% vs. 4.9%; p=1.0).
Figure 1.
(A) Course of complete recanalization during two hours after tPA bolus and (B) functional independence (%, 95%CI) at 90 days according to treatment group.
With a 21.4% absolute increase, patients in the sonothrombolysis group more frequently attained functional independence at 90 days than in the IV tPA alone group: 37.2% (16/43; 95%CI: 0.22-0.52) versus 15.8% (6/38; 95%CI: 0.04-0.28), p=0.045 Fisher's Exact Test (Figure 1, B). On univariate analysis, sonothrombolysis was associated with a higher odds of functional independence (OR: 3.16; 95%CI:1.09-9.20; p=0.035). This association was no longer significant after adjusting for sex (OR: 2.89, 95%CI: 0.97-8.59; p=0.057). Sonothrombolysis with diagnostic ultrasound frequency was associated with a similar symptomatic ICH rate when compared with IV thrombolysis alone: 4.6% (2/44; 95%CI: 0.02-0.11) versus 4.9% (2/41; 95%CI: 0.02-0.12), p=1.00 Fisher's Exact Test.
These data suggest that it may be reasonable to expect functional independence (the planned primary outcome for the anticipated trial) in 16% of patients with systemic thrombolysis, and that it may be reasonable to anticipate that treatment with sonothrombolysis could increase this proportion by 21% to a rate of 37%. With this assumption, the subsequent trial would require a continuity-corrected sample size of 202 patients (101 in each group) to provide 90% power. Further sample size justification of the CLOTBUST-ER trial and its methods are outlined elsewhere [16].
Discussion
Our results indicate that sonothrombolysis can augment tPA-associated recanalization in patients with severe stroke and proximal middle cerebral artery occlusion, and it may lead to better functional outcome in this group of patients compared with IV tPA alone [5-8]. Our analysis further adds to the available literature supporting the safety of monitoring with 2-MHz frequency transcranial ultrasound. Most notably, safety does not seem to be compromised in patients with presumably large cerebral ischemic lesions and greater stroke severity as these patients are naturally deemed at high risk for intracerebral hemorrhage after IV thrombolysis with tPA [20]. Our subgroup analysis showed that 21.4% more patients with severe strokes achieved functional independence (mRS 0-1) at 3 months, a rate slightly higher than the overall trend for benefit seen in all CLOTBUST patients [17]. These findings suggest that the most significant benefit of sonothrombolysis can be expected in patients with severe strokes since these patients can experience most dramatic clinical recovery following early recanalization of a proximal middle cerebral artery occlusion. This essentially parallels the results of the recent IST-3 trial that pointed to a significant trend towards pronounced treatment effects with IV tPA in patients with severe strokes [10].
Our study is in concordance with other previous reports documenting neurological improvement after systemic thrombolysis augmented by mechanical and pharmacological treatments [11, 12]. The rationale why we decided to analyze patients with severe stroke severity is based on the fact that these patients most commonly (> 80%) have an underlying proximal major occlusion [3, 4], and hence, only modest benefits with IV tPA therapy [5]. Also, even though the continuous exposure of cerebral ischemic tissues to ultrasound seems to have additional effects [21], it benefits patients mostly by improving early recanalization of a large thrombo-embolic occlusion. Rapid arterial recanalization and restoration of perfusion belong to the most important independent predictors of good functional outcome after acute ischemic stroke [22, 23]. In our analysis, complete recanalization rate after 2 hours from initiation of sonothrombolysis was twice that of IV tPA alone, and compares favorably with tPA-induced recanalization rates in the literature [24]. Yet it needs further study whether such upfront treatment effect can be ultimately translated into good clinical outcomes. In fact, improved recanalization rates thought to represent potential advantages of endovascular approaches did not meet such expectations [25-27] likely due to significant time delays in endovascular treatment initiation associated with poor outcomes [28]. Sonothrombolysis can be deployed with minimal time delays compared to endovascular approaches. Moreover, while the majority of severe stroke patients still present to the closest emergency rooms largely lacking skilled neuro-endovascular expertise, amplification of the only approved systemic therapy with ultrasound could be initiated in the pre-hospital setting and continued during patient transport [29]. Pre-hospital assessment of the intracranial vasculature using a portable ultrasound device has been shown to be feasible and reliable in acute stroke patients when compared with neurovascular imaging on admission [30].
Our results are limited by the relatively small number of patients and the use of exclusive data from the CLOTBUST trial, which is several years old. During this decade general stroke management and outcomes have improved. Also, as this was a post-hoc analysis our subgroup differences should be interpreted cautiously and cannot be generalized to other sonothrombolysis approaches (e.g. low-frequency ultrasound, transcranial color-coded duplex) as well as occlusions other than middle cerebral artery territory. Furthermore, as the benefit of IV tPA diminishes with increasing time to treatment, it should be acknowledged that our exploratory results on good functional outcome refer only to tPA delivery in the 3 hour time window [23]. Our subsequent CLOTBUST-Hands-Free phase II trial, however, included acute ischemic stroke patients with any intracranial occlusion site treated within 4.5 hours from symptom onset and showed promising results in terms of recanalization and functional outcome [31]. The CLOTBUST-ER trial allows enrollment of both anterior and posterior circulation stroke patients and tPA delivery according to national labels within 4.5 hours from stroke onset. Lastly, we cannot comment on the potential efficacy of sonothrombolysis according to thrombus length since this specific information was not collected in the original CLOTBUST trial. Strengths of our analysis include the set of randomized patients with severe stroke subjected to sonothrombolysis and sample homogeneity in terms of patient characteristics and procedure (e.g., duration of insonation, time points of recanalization assessment).
Conclusions
Sonothrombolysis with 2-MHz pulsed wave ultrasound is a safe adjuvant treatment to IV tPA in patients with severe ischemic strokes attributable to middle cerebral artery occlusion. Based on the observed absolute increase in the three-month functional independence rate with sonothrombolysis compared to IV tPA, our data point to a signal of efficacy and provide information to guide the subsequent phase III randomized controlled trial of sonothrombolysis in these patients (CLOTBUST-ER NCT#01098981) [16].
Acknowledgments
Disclosures/Conflicts of Interests: Drs. Barlinn and Barreto were supported through National Institute of Neurological Disorders and Stroke (NINDS) Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) grant (PI—James Grotta, MD, University of Texas-Houston), project CLOTBUST Hands Free, phase I/II studies of an operator-independent device for sonothrombolysis in stroke. Drs. Alexandrov and Barreto serve as consultants to Cerevast Therapeutics, Inc. Dr. Alexandrov holds an US patent 6733450 “Therapeutic Method and Apparatus for Use of Sonication to Enhance Perfusion of Tissues,” assignee—Texas Board of Regents. Drs. Tsivgoulis and Mikulik have been supported by European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123). Mr. Alleman is the Vice President of Operations at Cerevast Therapeutics, Inc. Dr. Schellinger serves as consultant to Cerevast Therapeutics, Inc and received travel grants and speaker fees from Boehringer Ingelheim. Dr. Schellinger is a member of the ECASS 4 EXTEND steering committee and of the advisory board of Boehringer Ingelheim.
Footnotes
Clinical Trial Registration—URL: www.strokecenter.org/trials/. Trial name: CLOTBUST Phase II.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121–2125. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 4.Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44:1153–7. doi: 10.1161/STROKEAHA.111.000604. [DOI] [PubMed] [Google Scholar]
- 5.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–54. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 6.Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–7. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 8.Paliwal PR, Ahmad A, Shen L, et al. Persistence of hyperdense middle cerebral artery sign on follow-up CT scan after intravenous thrombolysis is associated with poor outcome. Cerebrovasc Dis. 2012;33:446–52. doi: 10.1159/000336863. [DOI] [PubMed] [Google Scholar]
- 9.Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–40. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]):a randomised controlled trial. Lancet. 2012;379:2352–63. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bavarsad Shahripour R, Alexandrov AV. Ancillary approaches to plasminogen activators. Ann N Y Acad Sci. 2012;1268:113–9. doi: 10.1111/j.1749-6632.2012.06688.x. [DOI] [PubMed] [Google Scholar]
- 12.Barreto AD, Alexandrov AV. Adjunctive and alternative approaches to current reperfusion therapy. Stroke. 2012;43:591–598. doi: 10.1161/STROKEAHA.111.617902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsivgoulis G, Eggers J, Ribo M, et al. Safety and efficacy of ultrasound- enhanced thrombolysis:A comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke. 2010;41:280–287. doi: 10.1161/STROKEAHA.109.563304. [DOI] [PubMed] [Google Scholar]
- 14.Ricci S, Dinia L, Del Sette M, et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2012;10:CD008348. doi: 10.1002/14651858.CD008348.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Saqqur M, Tsivgoulis G, Nicoli F, et al. The role of sonolysis and sonothrombolysis in acute ischemic stroke:A systematic review and meta- analysis of randomized controlled trials and case-control studies. J Neuroimaging. 2013 Apr 22; doi: 10.1111/jon.12026. [DOI] [PubMed]
- 16.Phase 3, Randomized, Placebo-Controlled, Double-Blinded Trial of the Combined Lysis of Thrombus With Ultrasound and Systemic Tissue Plasminogen Activator (tPA) for Emergent Revascularization in Acute Ischemic Stroke (CLOTBUST-ER) [Accessed December 28, 2013];The Internet Stroke Center. http://www.strokecenter.org/trials/clinicalstudies/a-randomized-activecontrolled-double-blinded-trial-of-the-combined-lysis-of-thrombus-withultrasound-and-systemic-tissue-plasminogen-activator-tpa-for-emergentrevascularization-clotbuster-in-acute-ischemic-stroke.
- 17.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov AV, Wojner AW, Grotta JC, et al. CLOTBUST:design of a randomized trial of ultrasound-enhanced thrombolysis for acute ischemic stroke. J Neuroimaging. 2004;14:108–12. [PubMed] [Google Scholar]
- 19.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic. Stroke. 2001;32:1330–5. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 20.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 21.Alexandrov AV, Barlinn K, Strong R, Alexandrov AW, Aronowski J. Low-power 2-MHz pulsed-wave transcranial ultrasound reduces ischemic brain damage in rats. Transl Stroke Res. doi: 10.1007/s12975-011-0080-6. [DOI] [PubMed] [Google Scholar]
- 22.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 23.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–8. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real- world experience and a call for action. Stroke. 2010;41:2254–8. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 25.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–72. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hölscher T, Dunford JV, Schlachetzki F, et al. Prehospital stroke diagnosis and treatment in ambulances and helicopters-a concept paper. Am J Emerg Med. 2013;31:743–7. doi: 10.1016/j.ajem.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Schlachetzki F, Herzberg M, Hölscher T, et al. Transcranial ultrasound from diagnosis to early stroke treatment: part 2: prehospital neurosonography in patients with acute stroke: the Regensburg stroke mobile project. Cerebrovasc Dis. 2012;33:262–71. doi: 10.1159/000334667. [DOI] [PubMed] [Google Scholar]
- 31.Barreto AD, Alexandrov AV, Shen L, et al. CLOTBUST-Hands Free:pilot safety study of a novel operator-independent ultrasound device in patients with acute ischemic stroke. Stroke. 2013;44:3376–81. doi: 10.1161/STROKEAHA.113.002713. [DOI] [PubMed] [Google Scholar]