Abstract
Aims
Dipeptidyl-peptidase-4 inhibitors (DPP-4i) have been implicated with an increased pancreatic cancer risk. We therefore compared pancreatic cancer incidence and diagnostic work-up among initiators of DPP-4i versus sulfonylureas (SU) and thiazolidinediones (TZD).
Methods
Medicare claims data were examined in a new-user active-comparator cohort study. Patients >65 years with no prescriptions for DPP-4i, SU or TZD at baseline were included if they had at least two claims for the same drug within 180 days. Using an as-treated approach and propensity score-adjusted Cox models, we estimated hazard ratios (HR) and 95% confidence intervals (CI) for pancreatic cancer. Diagnostic work-up was compared using risk ratios (RR).
RESULTS
In the DPP-4i vs SU comparison, there were 18,179 DPP4i initiators of which 26 developed pancreatic cancer (follow-up time interquartile range 5–18 months). In the DPP-4i vs TZD comparison there were 29,366 DPP-4i initiators and 52 developed pancreatic cancer. The hazard of pancreatic cancer with DPP-4i was lower relative to SU (HR=0.6, CI 0.4–0.9) and similar to TZD (HR=1.0, CI 0.7–1.4). Excluding first 6 months of follow-up to reduce the potential for reverse causality did not alter results. Probability of diagnostic work-up post-initiation among DPP-4i initiators (79.3%) was similar to TZD (74.1%) (RR=1.06, CI 1.05–1.07) and SU (74.6%) (RR=1.06, CI1.05–1.07). The probability of diagnostic workup pre-index was ~80% for all cohorts.
Conclusion
Though limited by sample size and the observed duration of treatment in the US, our well-controlled population based study suggests no increased short-term pancreatic cancer risk with DPP-4i relative to SU or TZD.
Introduction
Dipeptidyl-peptidase-4 inhibitors (DPP-4i) were introduced in the United States in 2006 to improve glycemic control in adults with type 2 diabetes. Sitagliptin was the first in class, followed by saxagliptin (2008), linagliptin (2011) and alogliptin (2012).[1] There is considerable interest in these drugs due to their tolerability (apart from nasopharyngitis), body-weight neutrality and ease of use [1,2], but only limited data are available on their safety. In 2009, the Food and Drug Administration (FDA) issued a safety communication regarding post-marketing reports of acute pancreatitis in patients using sitagliptin or sitagliptin/metformin.[3] Subsequently, manufacturers of these drugs revised the labels to include information regarding reports of acute pancreatitis, recommending that their use be promptly discontinued if pancreatitis was suspected while using these products.[3–5] In 2011, an analysis of the FDA Adverse Events Reporting System (FAERS) demonstrated increased rates of pancreatitis and pancreatic cancer with incretin-mimetics compared to other antihyperglycemic therapies. Pancreatic cancer rate with sitagliptin was found to be 2.7 times the rate in the control group, raising concern about a potential adverse effect.[6] The FAERS analysis has been criticized mainly due to the limitations of the FAERS database; including the lack of denominator, disproportionate reporting, confounding and inconsistencies in exposure and outcome ascertainment.[7,8] In March 2013, Butler et al [9] examined pancreata from brain-dead organ donors and found increased pancreatic mass, exocrine cell proliferation and dysplasia in organ donors treated with incretin-mimetics (7 sitagliptin, 1 exenatide) compared with diabetic patients on other antihyperglycemic agents and non-diabetic controls. The authors suggested that these observations are compatible with an increased pancreatic cancer risk in those treated with incretin-mimetics.[9] However, this study is limited by small numbers (n=34), poor matching on baseline characteristics and absence of information about treatment duration.[10] Following this, the FDA issued a drug safety communication announcing that it is evaluating such reports but that it had “not reached any new conclusions about safety risks with incretin-mimetics”.[11] Recently two trials (SAVOR-TIMI 53 and EXAMINE) evaluating the cardiovascular effects of DPP-4i were reported. [12,13] The SAVOR-TIMI compared saxagliptin versus placebo over median 2.1 years follow-up and evaluated pancreatic cancer as a safety outcome but found no indication for an increased risk (5 events with saxagliptin versus 12 with placebo).[12] The EXAMINE trial comparing alogliptin versus placebo found no reports of pancreatic cancer over about 1.5 years of median follow-up in 5380 patients.[13]
There have been many pharmacoepidemiologic studies examining acute pancreatitis with DPP-4i [14–16], but none on pancreatic cancer. We therefore compared the pancreatic cancer incidence after initiation of DPP-4i versus sulfonylureas (SU) and thiazolidinediones (TZD) using 2006–2011 Medicare claims data which reflect the diabetes burden and treatment in older adults. We conducted this study despite the limited timeframe of available Medicare Part D data on dispensed drugs because of the imperative of conducting well-controlled studies in light of the hypothesis generated in relatively uncontrolled studies as treatment decisions are being made on a daily basis. While not intended to be definitive, the data presented are the first to examine a well-defined high-risk population, using the state-of-the-art new-user active-comparator study design, rigorous confounding control, and various sensitivity analyses.
Methods
The study was reviewed and approved by the University of North Carolina Chapel Hill Institutional Review Board (IRB # 12-1466). Before scrutinizing the data or conducting analyses, the study protocol was registered in the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) electronic register of studies (http://www.encepp.eu/encepp/viewResource.htm?id=3411).
Study population
We conducted a new-user active-comparator cohort study using a 20% random sample of Medicare beneficiaries >65 years with fee-for-service Part A (hospital coverage), B (outpatient care) and D (dispensed prescription drugs) enrollment in at least one month during a calendar year from January 1, 2007 (2006 for Part A and B) to December 31, 2011. Medicare is the largest public health insurance program in the US, covering >98% of adults 65 years or older.[17] This data contains information about demographics, enrollment, diagnoses, procedures and prescription drugs for each enrollee.[17]
From this population, we identified two new-user active-comparator cohort pairs (supplementary figures 1,2) mimicking a clinical treatment decision: 1. initiators of DPP-4i versus SU (not exposed to DPP-4i or SU in the previous 6 months) and 2. initiators of DPP-4i versus TZD (not exposed to DPP-4i or TZD in the previous 6 months). Prevalent users in the 6 months before initiation were excluded (example, in the DPP-4i vs SU comparison, patients could be on any antihyperglycemic drugs other than DPP-4i and SU in the 6 months pre-initiation). A DPP-4i initiator with no previous prescription of SU and TZD would be eligible for inclusion in both comparisons (DPP-4i vs SU and DPP-4i vs TZD). Since the drugs of interest are indicated for diabetes management, patients were not required to have a diabetes claim for cohort inclusion. Drug initiation was defined as the first prescription of the drug with the index date defined as the date of dispensing. Patients needed to have at least 6 months of continuous Part D enrollment and at least 12 months parts A and B enrollment pre-index. To ensure that patients were actually started on the drugs, we restricted our cohorts to patients with a second prescription for the same drug dispensed within 6 months after the index prescription and follow-up started from the second fill date. Finally, using a sensitive definition of ICD-9-CM codes and procedure codes (Supplemental table 1), we excluded patients with evidence of cancer or cancer-related procedures any time before the start of follow-up.
Outcome
The outcome was incident pancreatic cancer defined as at least two inpatient or outpatient claims with ICD-9-CM codes 157.xx within two months.[18] This definition has been shown to have high specificity (minimize false positives, yield unbiased relative risk estimates) for other cancers in a Medicare population.[18] We analyzed the data using both as-treated (preferred in studies of adverse outcomes) and an intent-to-treat approach (preferred here because the induction period for pancreatic cancer is thought to be long). In the as-treated analysis, patients were followed up from the second prescription until: the outcome, discontinuation (no new prescription for the initiated drug, within days-supply plus a 180 days grace period to allow for dose adjustment/irregular use), switching or augmentation with the comparator drug, death, end of enrollment, or December 31, 2011. In the intent-to-treat approach patients were not censored when they stopped/switched/augmented therapy, but were followed until the outcome occurred, death, end of enrollment, or December 31, 2011. Patients with a diagnosis of any non-pancreatic cancer (except non-melanoma skin cancer) during follow-up were censored at that point since diagnostic-work-up or treatment of other cancers may affect the incidence of pancreatic cancer.
Confounding control and analysis
We estimated propensity scores using a number of baseline variables. Comorbidities and health care utilization were assessed during the 12 months pre-index and use of other drugs was assessed during the 6 months pre-index. Using these variables, we predicted the probability for initiating DPP-4i versus SU and DPP-4i versus TZD for each patient (the propensity score) using two separate logistic regression models.[19] We implemented the estimated propensity scores using weights that led to the “standardization” of covariates in the SU and TZD groups to the covariate distribution observed in DPP-4i initiators. This was achieved by assigning a weight of 1 to the treated (DPP-4i) and a weight of (propensity score/(1-propensity score)) to SU and TZD.[20] This weighting creates pseudo-populations of SU and TZD initiators with similar covariate distribution as in DPP-4i initiators. This covariate balance across groups allows us to estimate the unconfounded treatment effect in a population of patients similar to those actually initiating DPP-4i.[20, 21] Our weighted analysis thus answers the question “what would have happened to patients who initiated DPP-4i if they had initiated SU or TZD, instead”.[22]
After checking covariate balance in the pseudo-populations we computed weighted Kaplan Meier plots to check the proportional hazards assumption. We then fit Cox proportional hazards models in the weighted pseudo-populations with treatment as the only independent variable to compare pancreatic cancer incidence among initiators of DPP-4i vs SU and DPP-4i vs TZD.
Diagnostic procedures
Studies assessing cancer risk after a new diabetes diagnosis have raised concerns about differential cancer detection biasing the association between diabetes and cancer.[23, 24] The potential for detection bias also presents methodological challenges in studies assessing cancer risk with antihyperglycemic drugs. It is possible that patients initiating DPP-4i may undergo increased diagnostic screening just before and after drug initiation, which may lead to increased discovery of pancreatic cancer in the DPP-4i group relative to SU/TZD. To address this we compared the use of diagnostic procedures (Supplemental table 2) in 6 months before and after the index date among initiators of DPP-4i versus SU and TZD using risk ratios and 95% confidence intervals.
Sensitivity analyses
We conducted sensitivity analyses using a 6 month induction period (excluding first 6 months of follow-up). This was done in order to reduce the potential for a spurious drug-pancreatic cancer association due to pre-clinical pancreatic cancer leading to hyperglycemia and initiation of antihyperglycemic therapy (reversed causality).[25, 26] Bias resulting from reversed causality would be strongest immediately following treatment initiation. Second, we compared the pancreatic cancer incidence in DPP-4i initiators versus a combined comparison group of SU and TZD initiators.
Results
Tables 1 and 2 present the baseline covariates for each comparison. DPP-4i initiators had a mean age ~75 years and ~35% were men. Compared with DPP-4i initiators, SU initiators were more likely to be men, less likely to have connective tissue diseases, neuropathy or retinopathy and less likely to be on other antihyperglycemics, statins, angiotensin receptor blockers and beta-blockers during baseline (table 1). The SU initiators were also less likely to have had lipid testing and influenza vaccinations than the DPP-4i initiators. The TZD initiators were more likely to be men and non-white compared to the DPP-4i initiators (table 2). The prevalence of comorbidities and use of other antihyperglycemics, antihypertensives (except ACE inhibitors), statins was lower in TZD compared to the DPP-4i group. TZD initiators were also slightly less likely to get influenza vaccinations or lipid testing. In the column ‘effect of channeling’, we present the multivariable effect of these covariates on channeling between initiating DPP-4i versus comparators. After weighting (weighted SU/TZD columns, tables 1,2), all covariates in the weighted SU and TZD pseudo-populations are identical to the distribution of the DPP-4i initiators. This indicates that we were able to balance cohorts on all measured covariates which removes any confounding by these variables.
Table 1.
Distribution of selected baseline characteristics in initiators of dipeptidyl-peptidase-4 inhibitors (DPP-4i) and sulfonylureas (SU) a
| DPP-4i (n = 18,179) |
SU (n = 63,746) |
Effect of channelingb | weighted SUc |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | Odds ratio |
95% CI | % | ||
| Age Mean (SD) | 75.28 (7.00) | 75.60 (7.28) | 0.997 | 0.951 | 1.045 | 75.53 (7.22) | ||
| 66 to 75 years old | 10428 | 57.36 | 35632 | 55.90 | - | - | - | 57.44 |
| 76 to 85 years old | 5916 | 32.54 | 20884 | 32.76 | - | - | - | 32.72 |
| 86 years and above | 1835 | 10.09 | 7230 | 11.34 | - | - | - | 9.84 |
| Male | 6566 | 36.12 | 25340 | 39.75 | 0.892 | 0.860 | 0.925 | 36.06 |
| White | 13486 | 74.18 | 48907 | 76.72 | 0.766 | 0.728 | 0.805 | 74.20 |
| Black | 1925 | 10.59 | 7694 | 12.07 | 0.669 | 0.624 | 0.717 | 10.67 |
| Other | 2768 | 15.23 | 7145 | 11.21 | Reference | 15.13 | ||
| Comorbiditiesd | ||||||||
| Connective tissue disease | 6354 | 34.95 | 19307 | 30.29 | 1.143 | 1.102 | 1.186 | 34.96 |
| Depression | 3089 | 16.99 | 10262 | 16.10 | 1.001 | 0.955 | 1.049 | 17.01 |
| Chronic obstructive pulmonary disease | 3516 | 19.34 | 12972 | 20.35 | 0.941 | 0.90 | 0.985 | 19.4 |
| Chronic kidney disease | 3381 | 18.6 | 10923 | 17.14 | 0.995 | 0.947 | 1.046 | 18.77 |
| Congestive heart failure | 4588 | 25.24 | 15939 | 25.00 | 1.015 | 0.969 | 1.064 | 25.37 |
| Diabetic neuropathy | 3751 | 20.63 | 9631 | 15.11 | 1.212 | 1.159 | 1.268 | 20.72 |
| Diabetic nephropathy | 1542 | 8.48 | 3961 | 6.21 | 1.131 | 1.056 | 1.213 | 8.60 |
| Diabetic retinopathy | 2974 | 16.36 | 7721 | 12.11 | 1.107 | 1.054 | 1.163 | 16.49 |
| Diabetic cataract | 51 | 0.28 | 111 | 0.17 | 1.111 | 0.788 | 1.567 | 0.28 |
| Gastrointestinal disorders | 158 | 0.87 | 550 | 0.86 | 1.031 | 0.859 | 1.237 | 0.86 |
| Alcohol use e | 192 | 1.06 | 804 | 1.26 | 0.975 | 0.828 | 1.148 | 1.06 |
| Tobacco use e | 48 | 0.26 | 142 | 0.22 | 1.135 | 0.811 | 1.588 | 0.27 |
| Pancreatitis | 211 | 1.16 | 690 | 1.08 | 1.072 | 0.914 | 1.257 | 1.18 |
| Medication usef | ||||||||
| Insulin | 3823 | 21.03 | 9232 | 14.48 | 1.495 | 1.428 | 1.566 | 21.20 |
| Metformin | 9386 | 51.63 | 27902 | 43.77 | 1.269 | 1.224 | 1.314 | 51.95 |
| Thiazolidinediones | 3983 | 21.91 | 7567 | 11.87 | 1.892 | 1.811 | 1.977 | 22.27 |
| Angiotensin converting enzyme inhibitors | 6068 | 33.38 | 21806 | 34.21 | 0.953 | 0.917 | 0.990 | 33.57 |
| Angiotensin receptor blockers | 5403 | 29.72 | 12393 | 19.44 | 1.471 | 1.412 | 1.532 | 29.91 |
| Statins | 11908 | 65.50 | 35028 | 54.95 | 1.251 | 1.205 | 1.298 | 65.64 |
| Loop diuretics | 4771 | 26.24 | 16443 | 25.79 | 0.931 | 0.891 | 0.974 | 26.3 |
| Other diuretics | 4625 | 25.44 | 16923 | 26.55 | 0.906 | 0.871 | 0.942 | 25.54 |
| Beta blockers | 8989 | 49.45 | 28689 | 45.01 | 1.096 | 1.057 | 1.135 | 49.49 |
| Calcium channel blockers | 6238 | 34.31 | 19790 | 31.05 | 1.055 | 1.017 | 1.095 | 34.35 |
| Healthcare utilizationd | ||||||||
| Blood tests | 1759 | 9.68 | 5104 | 8.01 | 1.111 | 1.048 | 1.178 | 9.73 |
| Lipid panel | 15719 | 86.47 | 49572 | 77.76 | 1.476 | 1.405 | 1.551 | 86.59 |
| Influenza vaccinations | 9997 | 54.99 | 31990 | 50.18 | 1.121 | 1.083 | 1.16 | 55.04 |
Initiation defined as no dispensed prescriptions for DPP-4i or SU during the 6 months before initiation and filling a second prescription of the same drug/drug class within 6 months after the first prescription
Channeling between initiation of DPP-4i versus initiation of SU; odds ratios from multivariable logistic regression model including all covariates presented in the table (i.e., the propensity score model); odds ratios >1.0 indicate more likely to be initiated on DPP-4i than SU
Pseudo-population of SU initiators weighted to the distribution of covariates of the DPP-4i initiators using the propensity score to balance covariates (and therefore control for confounding)
Measured in the 12 months before drug initiation
Tobacco use and alcohol use and abuse defined using ICD-9-CM codes may be underestimated.
Measured in the 6 months before drug initiation
Table 2.
Distribution of selected baseline characteristics in initiators of dipeptidyl-peptidase-4 inhibitors (DPP-4i) and thiazolidinediones (TZD) a
| DPP-4i (n = 29,366) |
TZD (n = 26,332) |
Effect of channelingb | weighted TZDc |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | Odds ratio |
95% CI | % | ||
| Age Mean (SD) | 75.61 (7.10) | 74.64 (6.70) | 0.945 | 0.901 | 0.992 | 75.62 (7.50) | ||
| 66 to 75 years old | 16407 | 55.87 | 16100 | 61.14 | - | - | - | 55.60 |
| 76 to 85 years old | 9782 | 33.31 | 8130 | 30.87 | - | - | - | 33.65 |
| 86 years and above | 3177 | 10.82 | 2102 | 7.98 | - | - | - | 10.74 |
| Male | 10590 | 36.06 | 10609 | 40.29 | 0.884 | 0.852 | 0.916 | 35.94 |
| White | 22245 | 75.75 | 18628 | 70.74 | 1.321 | 1.259 | 1.387 | 75.77 |
| Black | 3059 | 10.42 | 3140 | 11.92 | 1.060 | 0.99 | 1.134 | 10.40 |
| Other | 4062 | 13.83 | 4564 | 17.33 | (reference) | 13.83 | ||
| Comorbiditiesd | ||||||||
| Connective tissue disease | 9966 | 33.94 | 7763 | 29.48 | 1.108 | 1.067 | 1.15 | 34.08 |
| Depression | 4709 | 16.04 | 3712 | 14.10 | 1.003 | 0.955 | 1.054 | 16.15 |
| Chronic obstructive pulmonary disease | 5595 | 19.05 | 3999 | 15.19 | 1.080 | 1.030 | 1.133 | 18.88 |
| Chronic kidney disease | 5790 | 19.72 | 4031 | 15.31 | 1.114 | 1.058 | 1.172 | 19.70 |
| Congestive heart failure | 7740 | 26.36 | 4373 | 16.61 | 1.430 | 1.361 | 1.502 | 26.41 |
| Diabetic neuropathy | 6478 | 22.06 | 4813 | 18.28 | 1.114 | 1.066 | 1.164 | 22.24 |
| Diabetic nephropathy | 2660 | 9.06 | 1954 | 7.42 | 1.040 | 0.971 | 1.114 | 9.12 |
| Diabetic retinopathy | 5260 | 17.91 | 4432 | 16.83 | 1.010 | 0.965 | 1.058 | 17.92 |
| Diabetic cataract | 83 | 0.28 | 73 | 0.28 | 0.988 | 0.716 | 1.364 | 0.28 |
| Gastrointestinal disorders | 256 | 0.87 | 208 | 0.79 | 1.006 | 0.834 | 1.215 | 0.86 |
| Alcohol use e | 316 | 1.08 | 258 | 0.98 | 1.132 | 0.954 | 1.342 | 1.10 |
| Tobacco use e | 78 | 0.27 | 59 | 0.22 | 1.086 | 0.769 | 1.534 | 0.26 |
| Pancreatitis | 318 | 1.08 | 243 | 0.92 | 1.071 | 0.902 | 1.273 | 1.08 |
| Medication usef | ||||||||
| Insulin | 5409 | 18.42 | 4445 | 16.88 | 0.977 | 0.932 | 1.024 | 18.51 |
| Metformin | 16805 | 57.23 | 14282 | 54.24 | 1.208 | 1.165 | 1.253 | 57.68 |
| Sulfonylureas | 13530 | 46.07 | 11352 | 43.11 | 1.051 | 1.015 | 1.089 | 46.25 |
| Angiotensin converting enzyme inhibitors | 10907 | 37.14 | 9899 | 37.59 | 0.949 | 0.914 | 0.986 | 37.20 |
| Angiotensin receptor blockers | 8184 | 27.87 | 5982 | 22.72 | 1.216 | 1.166 | 1.269 | 28.11 |
| Statins | 19331 | 65.83 | 15466 | 58.73 | 1.206 | 1.163 | 1.252 | 65.85 |
| Loop diuretics | 8294 | 28.24 | 5025 | 19.08 | 1.245 | 1.189 | 1.304 | 28.26 |
| Other diuretics | 7831 | 26.67 | 6861 | 26.06 | 0.99 | 0.952 | 1.03 | 26.48 |
| Beta blockers | 15350 | 52.27 | 11288 | 42.87 | 1.217 | 1.174 | 1.261 | 52.23 |
| Calcium channel blockers | 10334 | 35.19 | 8440 | 32.05 | 1.049 | 1.011 | 1.088 | 35.39 |
| Healthcare utilizationd | ||||||||
| Blood tests | 2675 | 9.11 | 2261 | 8.59 | 1.032 | 0.972 | 1.096 | 9.08 |
| Lipid panel | 25483 | 86.78 | 22105 | 83.95 | 1.196 | 1.138 | 1.258 | 87.01 |
| Influenza vaccinations | 16325 | 55.59 | 13427 | 50.99 | 1.112 | 1.074 | 1.151 | 55.79 |
Initiation defined as no dispensed prescriptions for DPP-4i or TZD during the 6 months before initiation and filling a second prescription of the same drug/drug class within 6 months after the first prescription
Channeling between initiation of DPP-4i versus initiation of TZD; odds ratios from multivariable logistic regression model including all covariates presented in the table (i.e., the propensity score model); odds ratios >1.0 indicate more likely to be initiated on DPP-4i than TZD
Pseudo-population of TZD initiators weighted to the distribution of covariates of the DPP-4i initiators using the propensity score to balance covariates (and therefore control for confounding)
Measured in the 12 months before drug initiation
Tobacco use and alcohol use and abuse defined using ICD-9-CM codes may be underestimated.
Measured in the 6 months before drug initiation
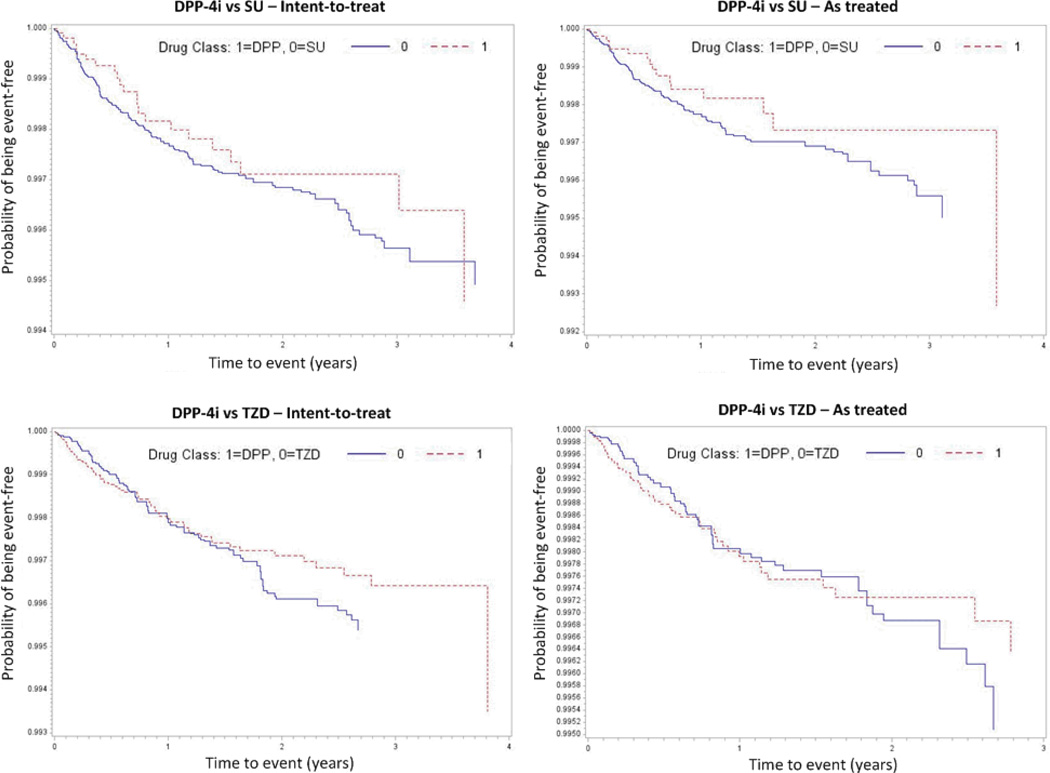
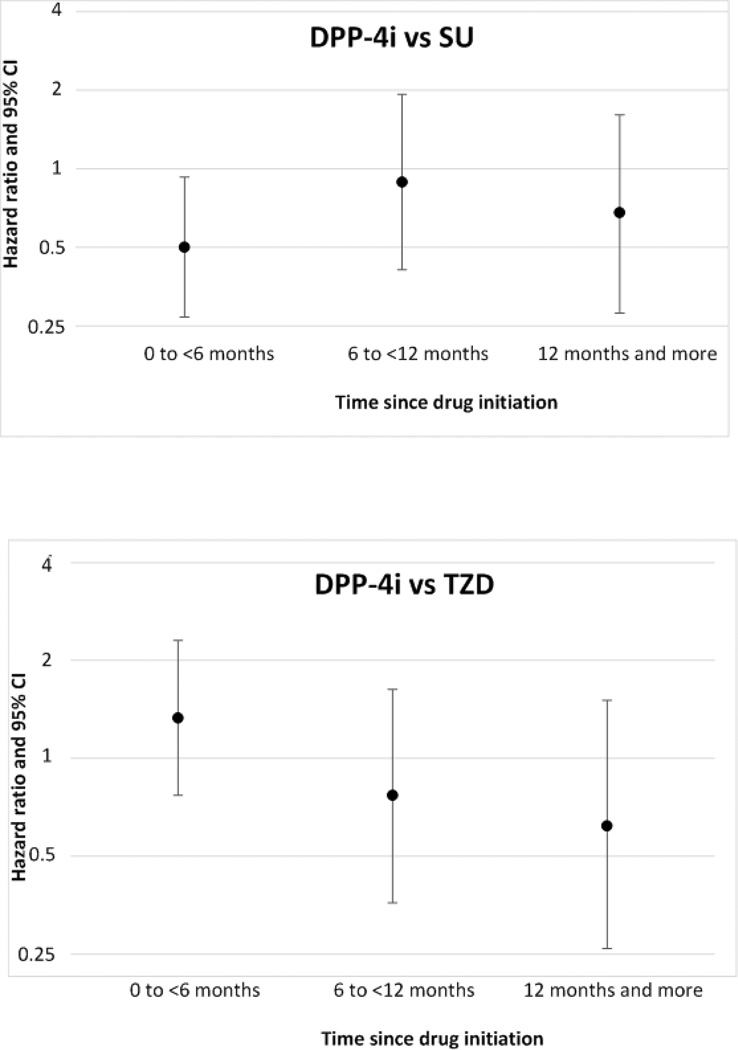
Table 3 presents incidence rates for pancreatic cancer per 100,000 person-years, time-to-event, and the crude and adjusted (weighted) hazard ratios comparing DPP-4i with SU and TZD. In the as-treated analysis, based on 26 events among 18,179 DPP-4i initiators and 177 events among 63,746 SU initiators, the adjusted HR was 0.62 (CI: 0.41, 0.94). There were 52 pancreatic cancers among 29,366 DPP-4i initiators and 54 events among 26,332 TZD initiators leading to an adjusted HR of 0.97 (CI: 0.65, 1.43). Overall these results indicate no increased short-term hazard of pancreatic cancer with DPP-4i relative to SU, nor with DPP-4i relative to TZD. Using the intent-to-treat approach (table 3), the adjusted HR was 0.68 (CI: 0.47, 1.00) for DPP-4i vs SU and 0.88 (CI: 0.62, 1.23) for DPP4i vs TZD. As shown in figure 1, similar relations were observed in those on drugs for a longer time. Figure 2 presents results stratified by duration of use since initiation. Based on a total of 53 events among 6,994 DPP-4i and 31,603 SU initiators on therapy for one year or more, we found no indication of an increased pancreatic cancer incidence with DPP-4i (HR=0.68, CI: 0.28, 1.61). In the DPP-4i versus TZD comparison, there were 11,768 DPP-4i and 12,690 TZD initiators on treatment for one year or more and the hazard ratio of pancreatic cancer based on 22 events was 0.62 (0.26, 1.51).
Table 3.
Pancreatic cancer incidence among initiators a of dipeptidyl-peptidase-4 inhibitors (DPP-4i), sulfonylureas (SU) and thiazolidinediones (TZD)
| Comparison | Drug | Number of new- users |
Events | time to event in years interquartile range (median) |
Total person- years |
Incidence (per 100,000 person years) |
Unadjusted HR (95% CI) b |
Adjusted HR (95%CI) c |
|---|---|---|---|---|---|---|---|---|
| As treated analysis | ||||||||
| DPP-4i vs SU | DPP-4i d | 18,179 | 26 | 0.36 – 1.45 (0.75) | 18,813 | 138.20 | 0.63 (0.39, 0.91) | 0.62 (0.41, 0.94) |
| SU | 63,746 | 177 | 0.55 – 1.85 (0.99) | 80,768 | 219.15 | 1.00 (reference) | 1.00 (reference) | |
| DPP-4i vs TZD | DPP-4i d | 29,366 | 52 | 0.43 – 1.50 (0.79) | 31,333 | 165.96 | 0.97 (0.66, 1.42) | 0.97 (0.65, 1.43) |
| TZD | 26,332 | 54 | 0.57 – 1.71 (0.96) | 32,261 | 167.38 | 1.00 (reference) | 1.00 (reference) | |
| Intent to treat analysis | ||||||||
| DPP-4i vs SU | DPP-4i | 18179 | 35 | 0.48 – 2.25 (1.16) | 25,902 | 135.12 | 0.64 (0.45, 0.91) | 0.68 (0.47, 1.00) |
| SU | 63746 | 213 | 0.66 – 2.51 (1.48) | 103,652 | 205.50 | 1.00 (reference) | 1.00 (reference) | |
| DPP-4i vs TZD | DPP-4i | 29,366 | 63 | 0.52 – 2.22 (1.18) | 41675 | 151.17 | 0.93 (0.66, 1.30) | 0.88 (0.62, 1.23) |
| TZD | 26,332 | 75 | 0.93 – 2.73 (1.81) | 48925 | 153.29 | 1.00 (reference) | 1.00 (reference) | |
Initiation defined as no dispensed prescriptions for the drugs being compared during the 6 months before initiation and filling a second prescription of the same drug/drug class within 6 months after the first prescription
Hazard ratios and their 95 % confidence intervals from Cox proportional hazards models for pancreatic cancer with baseline treatment as the only independent covariate
Hazard ratios adjusted for variables in Table 1 and 2 using propensity score weighting (standardized to DPP-4i population)
Number of DPP-4i initiators different in both cohorts because for the DPP-4i vs SU comparison, patients could be on any diabetes medication (including TZD) except for DPP-4i and SU during the washout period. Similarly for the DPP-4i versus TZD comparison, patients could be on any other drugs except DPP-4i and TZD.
Figure 1. Kaplan-Meier plots of time to event for pancreatic cancer with dipeptidyl-peptidase-4 inhibitors (DPP-4i), sulfonylureas (SU) and thiazolidinediones (TZD).
For the graphs titled ‘DPP vs SU’ red dotted line = DPP-4i and blue solid line = SU For graphs titled ‘DPP vs TZD’, red dotted line = DPP-4i and blue solid line = TZD
Figure 2. Hazard ratios and 95% CI for pancreatic cancer stratified by time since drug initiation.
To reduce the potential of reverse causality, we repeated the as-treated analyses excluding the first 6 months of follow-up. In the DPP-4i vs SU comparison, this yielded 12,332 DPP-4i and 49,265 SU initiators and a total of 100 pancreatic cancers resulting in an adjusted hazard ratio of 0.73 (CI: 0.40, 1.32). In the DPP-4i vs TZD comparison, there were 21,020 DPP-4i and 21,562 TZD initiators and 51 pancreatic cancers with an adjusted hazard ratio of 0.71 (CI: 0.40, 1.25).
We also compared DPP-4i initiators with a combined group of SU or TZD initiators such that no patient had a prescription of any of these three drugs in the 6 months pre-initiation. Using an as-treated approach, this yielded 17,166 DPP-4i initiators and 69,729 initiators of SU/TZD and an adjusted hazard ratio of 0.71 (CI: 0.47, 1.08) indicating no increased pancreatic cancer incidence with DPP-4i relative to SU and TZD combined.
We did not find any difference in the risk of diagnostic work-up in the 6 months before and after drug initiation (Supplemental table 3). We performed this analysis to address the potential for increased diagnostic work-up in DPP-4i that could bias towards a higher incidence due to diagnosing some preclinical pancreatic cancers. In the 6 months post-index, the risk of diagnostic work-up was between 74.1 to 79.3% in all groups. The probability of diagnostic work-up among initiators of DPP-4i was similar to SU (RR: 1.06; CI: 1.05,1.07) and TZD initiators (RR: 1.06; CI: 1.05,1.07). In the 6 months pre-index, the risk of diagnostic work-up was similar in all groups (81.0–86.8%).
Conclusions
We found no evidence of increased short-term pancreatic cancer incidence with DPP-4i versus SU or TZD in our new-user active-comparator cohort study based on a 20% random sample of all currently available Medicare claims. Our study is limited by the short treatment duration, both as a function of actual treatment dynamics (as-treated analysis – follow-up IQR 5–18 months, median 10 months) and the availability of data (intent-to-treat - IQR 6–26 months, median 14 months). However, we required the patients to have two prescriptions of the same drug and started follow-up from the second prescription. The mean (median) time between the two prescriptions was approximately 1.5 (1) months (Supplemental table 4) indicating that the patients were on treatment for a slightly longer period than reported. Our approach of excluding patients with any cancer between the first and the second prescription can affect generalizability of results to all patients initiating these drugs, but the number of patients excluded was very small (<100 in each group) so that this is negligible. Short follow-up is likely to be an issue for the next few years since none of the clinical trials are planned for >5 years and real-world treatment patterns do not allow long-term follow-up of patients. Given the likely long induction period for pancreatic cancer it may be impossible to detect differences in cancer initiation between DPP-4i and comparators in our study. However our approach of synchronizing patients on diabetes severity and baseline pancreatic cancer risk likely makes the distribution of early stage preclinical cancers similar in DPP-4i versus comparators and we should thus be able to detect a difference in cancer promotion.
A recent meta-analysis among diabetic patients reported increased odds of pancreatic cancer with SU use versus non-use (although there was considerable heterogeneity across all studies that could not be explained by study design, setting or location), and no difference in odds with TZD use versus non-use.[27] Our observation of a slightly lower adjusted hazard of pancreatic cancer with DPP-4i versus SU could be a function of only 26 events in the DPP-4i group and also a potentially increased pancreatic cancer risk with SU which could make DPP-4i appear protective. Therefore these results should be interpreted with caution and additional data are needed to investigate this further. For each comparison, the results did not change among long term drug users as shown in figures 1 and 2.
Our results were robust to changes in analysis approaches and varying induction periods. We found little evidence for differential diagnostic work-up in DPP-4i initiators, indicating that differential outcome detection bias is less of a concern in our study.
Our results are contrary to those in the aforementioned FAERS analysis and the histologic study on human pancreata which suggested an increased pancreatic cancer risk with DPP-4i.[6,9] The limitations of these studies clearly warranted further investigation using large population-based healthcare data and state-of-the-art non-experimental methods.
A strength of our study is the use of a new-user active-comparator cohort design which is analogous to a head-to-head clinical trial [28] and mimics the most relevant clinical decision (‘which treatment to initiate’ rather than ‘treatment or not’). It allows synchronizing follow-up in all cohorts which is the basis for sensitivity analyses of induction periods.[28] Specifically, we identified initiators of DPP-4i or comparators after a 6 month washout. Potential confounders were measured before drug initiation thereby avoiding the problem of controlling for covariates potentially affected by prevalent treatment.[28] The covariate balance achieved by our propensity score weighting reassures us about absence of confounding by these covariates. Using active comparators helped balance the groups on diabetes severity, baseline pancreatic cancer risk (particularly important since diabetes is a risk factor for pancreatic cancer) and addressed confounding by indication and frailty. Given that many plausible predictors of treatment choice were already balanced by using an active comparator (before propensity score implementation), we may be more inclined to make the assumption that unmeasured confounding is not a major concern in our study, although we can never be certain about this point.
Compared to SU and TZD, DPP-4i initiators were more likely to get statins, influenza vaccines or blood lipids tested suggesting that DPP-4i initiators are more likely to follow guidelines of disease prevention, i.e., healthy users.[29] However, we successfully balanced the cohorts of DPP-4i and SU and TZD initiators on all these factors using weighting and this would tend to reduce imbalances of unmeasured factors associated with measured healthy user behaviors.[30]
Our study should be interpreted in the context of its limitations. As outlined above, the main limitation is short duration of follow-up and the results should be cautiously accepted. The follow-up time in the DPP-4i group was slightly shorter than the follow-up for comparators (supplemental table 5), but Cox models do not require equal person-time to be valid. The relatively constant slope of the Kaplan-Meier plots provide some reassurance that cancer risk in DPP-4i initiators are not increasing in the second year; however no implications can be made at this time about longer periods of exposure. Our study also had limited number of outcomes and number of DPP-4i initiators. DPP-4i were introduced in the US only in 2006 while SU and TZD have been on the market for a longer time, which might explain the small number of DPP-4i initiators as many potential candidates were excluded because of prior exposure to SU/TZD. Limited number of outcomes may be attributed to the fact that pancreatic cancer is a rare disease.[31] However, the median age at pancreatic cancer diagnosis is 71 years and the Medicare data is likely to have the highest power to study this outcome compared to other claims data from equivalent years. Based on the pancreatic cancer rates in the general US population reported by National Cancer Institute’s Surveillance Epidemiology and End Results (SEER), we calculated the expected number of events for our study population and compared it to the observed events in our data.[32] The ratio of observed to expected events was between 2–3 implying that that our study population had more than twice the number of pancreatic cancer events than the general US population, consistent with the fact that diabetic individuals have a 2-fold increased pancreatic cancer risk compared with the general population.[33, 34] In separate analyses (data not shown), we attempted to examine incident pancreatic cancer with the GLP-1 receptor agonists. However, the sample size was even more restricted, precluding robust analysis. Since GLP-1 receptor agonists and DPP-4i potentiate incretin action through different mechanisms and the exact spectrum of adverse events is uncertain, we did not combine these into a single analysis.[8]
There is a possibility of reverse causality affecting our study. Patients with preclinical pancreatic cancer may have worsening of their diabetes leading to initiation of antihyperglycemic treatments. However, our results did not change even after excluding the first 6 months of follow-up. We would have liked to extend this to 12 months or longer but were limited by treatment dynamics and data availability.
Finally, we could not adequately control for potential risk factors for pancreatic cancer like smoking, body mass index (BMI) since they not well-measured in Medicare claims.[35–37] However, we used chronic obstructive pulmonary disease as a proxy for smoking and balanced the comparison groups on this variable. We could not statistically adjust for BMI. However, BMI is only weakly associated with pancreatic cancer and therefore not adjusting for it is not expected to affect our results.[37]
In summary, we did not find an increased short-term pancreatic cancer risk after initiation of DPP-4i versus SU or TZD in older diabetic patients. This study does not establish the safety of DPP-4i – but uses real-world treatment patterns to present the first alternate view of the situation in contrast to the reports of increased risk from non-population based studies with similar or even lesser exposure and events. Given the short follow-up, ourstudy could not assess long term risk and therefore physicians should cautiously interpret the results while using them to guide clinical decisions. Further research should continue to assess the risk as more data become available as recently suggested.[38–41] Meanwhile, our results will help prudent clinicians and patients to make reasonable treatment decisions based on the currently available evidence.
Supplementary Material
Acknowledgements
The project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR000083 and a Gillings Innovation Laboratory award from the UNC Gillings School of Global Public Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest: M.G. and C.G. are doctoral students at UNC Chapel Hill. T.S. receives investigator-initiated research funding and support as principal investigator (R01AG023178) and co-investigator (R01AG042845) from the National Institute on Aging at the National Institutes of Health. He also receives research funding as Principal Investigator of the UNC-DEcIDE center from the Agency for Healthcare Research and Quality and from the Patient Centered Outcomes Research Institute. T.S. does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology (currentmembers: GlaxoSmithKline, UCB BioSciences, and Merck) and from unrestricted research grants from pharmaceutical companies (Merck, Sanofi, Amgen) to UNC. V.P. receives salary support from investigator initiated grants from Merck and Amgen. M.M. previously received salary support from a research grant from Pfizer. J.B. is supported by the NIH (UL1TR000083 and R01HL110380). He is an investigator and/or consultant without any direct financial benefit to him under contracts between his employer and the following companies: Amylin Pharmaceuticals, Inc., Andromeda, Astellas, Astra_Zeneca, Bayhill Therapeutics, Inc., Boehringer Ingelheim GmbH & Co. KG, Bristol-Myers Squibb Company, Catabasis, Cebix, Inc., CureDM, Diartis Pharmaceuticals, Elcelyx Therapeutics, Inc., Eli Lilly and Company, Exsulin, Genentech, GI Dynamics, GlaxoSmithKline, Halozyme Therapeutics, F. Hoffmann-La Roche, Ltd., Intarcia Therapeutics, Johnson & Johnson, Lexicon, LipoScience, Macrogenics, Medtronic, Merck, Metabolic Solutions Development Co., Metabolon, Inc., Metavention, Novan, Novo Nordisk A/S, Orexigen Therapeutics, Inc., Osiris Therapeutics, Inc., Pfizer, Inc., PhaseBio Pharmaceuticals Inc, Quest Diagnostics, Rhythm Pharmaceuticals, Sanofi, Spherix, Inc., Takeda, ToleRx, Transpharma Medical Ltd., TransTech Pharma, Veritas, Verva.
Footnotes
Disclosures -- Final decisions regarding design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and submission of the manuscript were the sole responsibilities of the authors.
Authors’ Contributions: M.G, J.B., C.G., M.M. and T.S. participated in study conception and design. M.G., T.S., V.P. and J.B. participated in the acquisition of the data. M.G., T.S., V.P., M.M. and J.B. participated in the analysis and interpretation of the data. M.G., T.S. and J.B. wrote the first draft of the manuscript. All authors reviewed and provided comments on the manuscript. M.G. is the guarantor of this work; M.G. and V.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Reference List
- 1.Gallwitz B. Emerging DPP-4 inhibitors: focus on linagliptin for type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:1–9. doi: 10.2147/DMSO.S23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Information for Healthcare Professionals - Acute pancreatitis and sitagliptin (marketed as Januvia and Janumet) [Accessed on 20th April 2013]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm183764.htm.
- 4.Highlights of prescribing information: Januvia. [Accessed on 20th April 2013]; http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021995s019lbl.pdf.
- 5.Highlights of prescribing information: Janumet XR. [Accessed on 20th April 2013]; http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202270s003lbl.pdf.
- 6.Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA 2011 Adverse Event Reporting System. [Accessed April 10, 2013]; http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. 4-26-2013.
- 8.Drucker DJ, Sherman SI, Bergenstal RM, et al. The safety of incretin-based therapies--review of the scientific evidence. J Clin Endocrinol Metab. 2011;96(7):2027–2031. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked Expansion of Exocrine and Endocrine Pancreas with Incretin Therapy in Humans with increased Exocrine Pancreas Dysplasia and the potential for Glucagon-producing Neuroendocrine Tumors. Diabetes. 2013 Jul;62(7):2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn SE. Incretin Therapy and Islet Pathology - A Time for Caution. Diabetes. 2013 Jul;62(7):2178–2180. doi: 10.2337/db13-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. [Accessed April 20th, 2013]; http://www.fda.gov/Drugs/DrugSafety/DrugSafetyPodcasts/ucm344232.htm.
- 12.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013 Oct 3;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 13.White William B, Cannon Christopher P, Heller Simon R. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 14.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33(11):2349–2354. doi: 10.2337/dc10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dore DD, Seeger JD, Arnold CK. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25(4):1019–1027. doi: 10.1185/03007990902820519. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Chang HY, Richards TM, et al. Glucagonlike Peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173(7):534–539. doi: 10.1001/jamainternmed.2013.2720. [DOI] [PubMed] [Google Scholar]
- 17.Strengths and Limitations of CMS Administrative Data in Research. [Accessed on 20th April 2013]; http://www.resdac.org/resconnect/articles/156. [Google Scholar]
- 18.Setoguchi S, Solomon DH, Glynn RJ, et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum Paul R, Rubin Donald B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 20.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 21.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 22.Sturmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf. 2006;15(10):698–709. doi: 10.1002/pds.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowker SL, Richardson K, Marra CA, et al. Risk of breast cancer after onset of type 2 diabetes: evidence of detection bias in postmenopausal women. Diabetes Care. 2011;34(12):2542–2544. doi: 10.2337/dc11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson JA, Bowker SL, Richardson K, et al. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011;54(9):2263–2271. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara A, Lewis JD, Quesenberry CP, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34(4):923–929. doi: 10.2337/dc10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huxley R, Ansary-Moghaddam A, Berrington de GA, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):510–519. doi: 10.1038/ajg.2013.7. [DOI] [PubMed] [Google Scholar]
- 28.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 29.Sturmer T, Jonsson FM, Poole C, et al. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology. 2011;22(3):298–301. doi: 10.1097/EDE.0b013e318212640c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther. 2007;82(2):143–156. doi: 10.1038/sj.clpt.6100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SEER Stat Fact Sheets: Pancreas. [Available 15th April, 2013]; http://seer.cancer.gov/statfacts/html/pancreas.html#incidence-mortality.
- 32.Surveillance Epidemiology and End results (SEER) Statistics Stratified by Age. [Accessed April 10, 2013]; http://seer.cancer.gov/faststats/selections.php?series=age.
- 33.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 34.Larsson SC, Permert J, Hakansson N, et al. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93(11):1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156(19):2255–2260. [PubMed] [Google Scholar]
- 36.Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170(4):403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 38.Bailey CJ. Interpreting Adverse Signals in Diabetes Drug Development Programs. Diabetes Care. 2013 Jul;36(7):2098–2106. doi: 10.2337/dc13-0182. Epub 2013 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brass EP. Assessing the Benefit-Risk for New Drugs: Are the FDA's Endocrinologic and Metabolic Drugs Advisory Committee and the Division of Metabolism and Endocrinology Products in sync? Diabetes Care. 2013 Jul;36(7):1823–1826. doi: 10.2337/dc13-0891. Epub 2013 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cefalu WT, Rosenstock J, Henry RR, et al. Signals and Noise in Drug Safety Analyses: The incretin therapy debate provides the rationale for revamping epidemiologic pharmacovigilance. Diabetes Care. 2013 Jul;36(7):1804–1806. doi: 10.2337/dc13-0895. Epub 2013 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan AG1, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med. 2014 Feb 27;370(9):794–797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.