Abstract
Chemotherapy has severe side-effects for normal rapidly proliferating organs, such as hair follicle, and causes massive apoptosis in hair matrix keratinocytes followed by hair loss. To define the molecular signature of hair follicle response to chemotherapy, human scalp hair follicles cultured ex vivo were treated with doxorubicin and global microarray analysis was performed 3 hours after treatment. Microarray data revealed changes in expression of 504 genes in doxorubicin-treated hair follicles versus the controls. Among these genes, upregulations of several tumor necrosis factor family of apoptotic receptors (FAS, TRAIL receptors 1/2), as well as of a large number of the keratin-associated protein genes were seen after doxorubicin treatment. Hair follicle apoptosis induced by doxorubicin was significantly inhibited by either TRAIL neutralizing antibody or caspase 8 inhibitor, thus suggesting a novel role for TRAIL receptor signaling in mediating doxorubicin-induced hair loss. These data demonstrate that the early phase of the hair follicle response to doxorubicin includes upregulation of apoptosis-associated markers, as well as substantial re-organization of the terminal differentiation programs in hair follicle keratinocytes. These data provide an important platform for further studies towards the design of novel approaches for management of chemotherapy-induced hair loss.
Keywords: skin, hair growth, doxorubicin, apoptosis
Introduction
Hair loss (alopecia) is a common side effect of many chemotherapeutic treatment protocols and is one of the most distressing aspects of cancer therapy. Because of the rapid proliferation of hair matrix keratinocytes during hair shaft production, the hair follicle represents a “by-stander” target for many chemotherapeutic agents (Botchkarev, 2003; Paus and Cotsarelis, 1999; Paus et al., 2013; Trueb, 2009). Despite significant advances in understanding the mechanisms of the hair follicle response to chemotherapeutic agents achieved within last decade (Bodo et al., 2007; Bodo et al., 2009; Botchkarev et al., 2000; Hendrix et al., 2005; Jimenez et al., 2008; Sharov et al., 2003; Sharov et al., 2004), no effective treatment is currently available to prevent or retard this devastating side-effect of anti-cancer therapy.
To study the effects of chemotherapeutic agents on the hair follicle, a number of experimental models have been proposed (Bodo et al., 2007; Jimenez and Yunis, 1992; Paus et al., 1994). In C57BL/6 mouse model for chemotherapy-induced hair loss (Lindner et al., 1997; Paus et al., 1994), cyclophosphamide administration causes rapid increase in the p53 protein in hair matrix keratinocytes followed by massive apoptosis, while genetic p53 ablation results in a complete resistance of the hair follicles to chemotherapy (Botchkarev et al., 2000). Fas (APO-1, CD95) as a p53 target is also involved in mediating apoptosis in the hair matrix keratinocytes and melanocytes during hair follicle exposure to cyclophosphamide (Lindner et al., 1997; Sharov et al., 2003; Sharov et al., 2004). In contrast to p53 and its target genes, members of the fibroblast growth factor (FGF) family including keratinocyte growth factor, as well as pharmacological modulator of the heat shock proteins geldanomycin show relative protective effects in the rodent models for chemotherapy-induced hair loss (Bodo et al., 2009; Danilenko et al., 1995; Jimenez et al., 2008).
During chemotherapy, the DNA damage response is regulated at several levels including involvement of the response sensors, transducers and effectors, which operate in a stage-dependent manner to induce apoptosis, cell-cycle arrest or senescence in target cells (reviewed in Jackson and Bartek, 2009; Paus et al., 2013). Analyses of the kinetics of the cellular response to chemotherapy reveal that anti-cancer drugs induce apoptosis in tumor cells within several hours after in vivo administration (Meyn et al., 1995a, b). Cyclophosphamide treatment in mice also induces apoptosis in rapidly proliferating hair matrix keratinocytes as soon as 24 hours after i/p administration (Botchkarev et al., 2000; Lindner et al., 1997; Sharov et al., 2003; Sharov et al., 2004).
In recently developed an ex vivo human model for chemotherapy-induced hair loss, it was shown that the cyclophosphamide derivative 4-hydroperoxycyclophosphamide induces apoptosis in isolated human hair follicles followed by their dystrophy in a manner that resembles the follicular response to cyclophosphamide in C57BL/6 mouse in vivo model (Bodo et al., 2007). In this model, microarray analyses of the relatively advanced stage (48 hours) of the hair follicle response to chemotherapy reveal the changes in expression of more than 400 genes including several growth factors (FGF-18, IL-8, etc.), apoptotic regulators (BAX, MDM2, etc.), adhesion/extracellular matrix-associated molecules (CTNND2, GPC6, etc.), suggesting their involvement in chemotherapy-induced hair loss (Bodo et al., 2007).
However, the mechanisms underlying the early phase of the hair follicle response to chemotherapy remain unclear. In this manuscript, we use global microarray profiling approach to define the molecular signatures of the early phase in response of the hair follicles to chemotherapy treatment ex vivo. We show here that the early phase of the hair follicle response to chemotherapy is far more complex than it was previously appreciated and includes upregulation of not only apoptosis-associated genes, but also marked re-organization of the terminal differentiation programs in hair follicle keratinocytes. These data provide an important foundation for further research in identification of the mechanisms that trigger hair follicle response to DNA damage and development of novel approaches for management of hair loss induced by anti-cancer drugs.
Results
Doxorubicin treatment induces apoptosis-driven premature catagen development in human hair follicles cultured ex vivo
To further develop an ex vivo human model for chemotherapy-induced hair loss used previously for studying hair follicle response to cyclophosphamide (Bodo et al., 2007), we focused our studies on doxorubicin, a broadly used anti-cancer drug which treatment induces apoptosis in the hair follicles followed by massive hair loss in patients, as well as in the rodent models (Amoh et al., 2007; Amoh et al., 2005; Cece et al., 1996; Selleri et al., 2006). In contrast to cyclophosphamide, doxorubicin does not require enzymatic conversion in the liver to become active as anti-cancer drug (Fraiser et al., 1991; Siu et al., 1999), which we considered as an advantage that allows direct testing of its effects on the hair follicles cultured ex vivo.
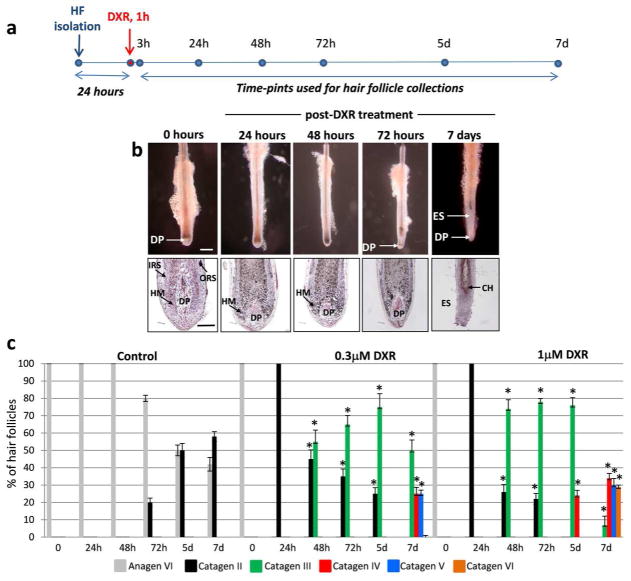
To define whether doxorubicin treatment is capable of inducing apoptosis and premature catagen development in human hair follicles cultured ex vivo, hair follicles isolated from the scalp of normal individuals were cultured for 24 hours as described previously (Bodo et al., 2007) followed by the treatment with different concentrations (0.3 or 1.0 μM) of doxorubicin for 1 hour (Fig. 1a). Doxorubicin concentrations for cultured hair follicles were selected according to recommendations published previously (Siu et al., 1999). Both doxorubicin concentrations tested induced rapid transition of normal anagen hair follicles into catagen II/III stages already 24 hours after treatment (Fig. 1b–c). Treatment of hair follicles with 1.0 μM doxorubicin induced more rapid catagen development compared to the group of follicles treated with 0.3 μM doxorubicin (Fig. 1c). After 5–7 days all hair follicle treated with both concentrations of doxorubicin entered catagen V-VI phase of the hair cycle, whereas about 67% of the follicles in the control group still remained in anagen VI/catagen II phases (Fig. 1b–c).
Figure 1. Doxorubicin induces premature catagen development in human hair follicles cultured ex vivo.
A: Scheme illustrating the experimental design of this study.
B: Morphological changes in HFs treated with 1 μM doxorubicin (DXR) for 1 hr at different time points after treatment. Scale bars: 300 μm and 100 μm
C: Dynamics of catagen development in HFs treated with 0.3 μM or 1 μM doxorubicin versus the controls (mean ± SD, * p<0.05, Student’s t-test).
Abbreviations: CH-club hair; DP-dermal papilla, DXR-doxorubicin; ES-epithelial strand; HF-hair follicle; HM-hair matrix
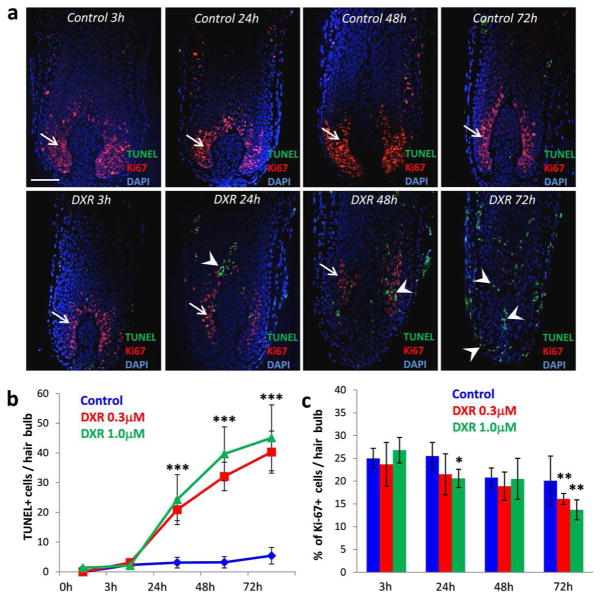
Analysis of the hair follicle apoptosis and cell proliferation performed by double immuno-visualization of apoptotic (TUNEL+) and proliferating (Ki-67+) cells showed appearance of numerous TUNEL+ cells and significant decrease of proliferating cells in the hair follicle matrix already 24 hours after doxorubicin treatment (Fig. 2a–c). Further increase of TUNEL+ cells was seen in catagen III/IV hair follicles 2–3 days after doxorubicin treatment (Fig. 2a, b). Consistently with morphological data (Fig. 1c), hair follicles treated with 1.0 μm of doxorubicin showed more rapid increase in the number of TUNEL+ cells and decrease of cell proliferation in the hair matrix compared to the group of follicles treated with 0.3 μM doxorubicin (Fig. 2b, c). However, control hair follicles showed appearance of single TUNEL+ cells during hair follicle progression to catagen II/III stages only 5 days after beginning of the experiment, while lack of TUNEL+ cells and numerous proliferating Ki-67+ cells were seen in the control follicles at the beginning of the study (Fig. 2a–c). These data suggest that similarly to 4-hdydroperoxycyclophosphamide (Bodo et al., 2007), doxorubicin treatment induces premature apoptosis-driven catagen development in human hair follicles cultured ex vivo, which is consistent with its effects on the hair follicles observed in the in vivo rodent models or in patients received anti-cancer therapy (Amoh et al., 2007; Amoh et al., 2005; Cece et al., 1996; Selleri et al., 2006).
Figure 2. Increase of apoptosis and decrease of cell proliferation in the hair follicles treated by doxorubicin.
A: Double immuno-detection of apoptotic (TUNEL+, green) and proliferating (Ki-67+, red) cells in the HFs treated with doxorubicin versus the control. Scale bar 100 μm.
B: Increase in the number of TUNEL+ cells in the HF s exposed to 0.3 μM or 1 μM doxorubicin (mean ± SD, *** p<0.001, Student’s t-test).
C: Decrease in the number of Ki-67+ cells in the HF s exposed to 0.3 μM or 1 μM doxorubicin (mean ± SD, * p<0.05, ** p<0.01, Student’s t-test).
Abbreviations: DXR-doxorubicin
Early phase response of the hair follicles to doxorubicin includes upregulation of pro-apoptotic FAS and TRAIL receptors and keratin-associated protein genes
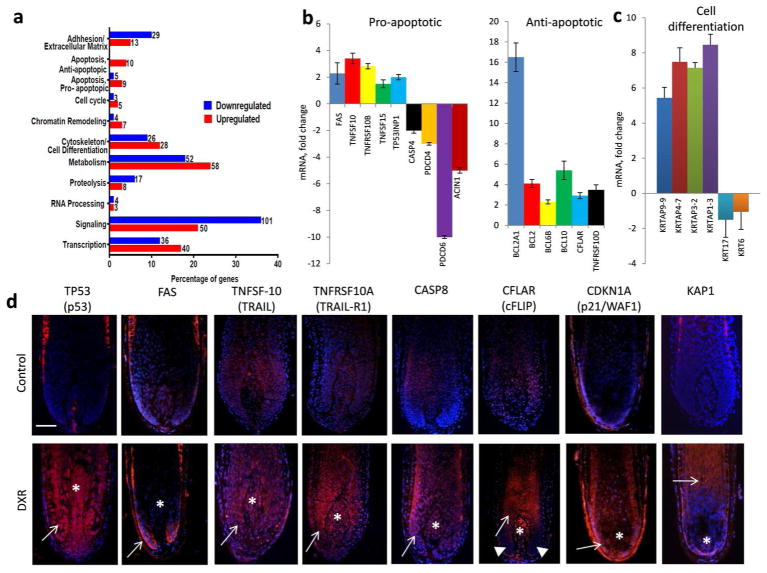
To define molecular mechanisms underlying the initiation of the hair follicle response to doxorubicin, hair bulbs of the follicles cultured ex vivo were harvested 3 hours after doxorubicin treatment and processed for RNA isolation followed by global microarray analyses with Affymetrix GeneChipSystem, as described previously (Fessing et al., 2010). Microarray data were validated by qRT-PCR and immunohistology and revealed 2-fold and higher changes in expression of 504 genes in doxorubicin-treated hair follicles versus the controls (Fig. 3a). Genes that show changes in expression in the hair follicles after doxorubicin treatment were grouped into several functional categories which encoded apoptosis/cell cycle regulators, adhesion/extracellular matrix molecules, cytoskeletal proteins, metabolic enzymes and signaling/transcription regulators (Fig. 3a, Suppl. Tables S1, S2).
Figure 3. Microarray, qRT-PCR and immunohistochemical profiling of human hair follicles 3–24 hours after doxorubicin treatment.
A: Microarray analysis of the global gene expression in HF collected 3 hours after 1 μm doxorubicin treatment versus control HFs: functional assignments of the genes with altered expression induced by DXR.
B, C: Validation of microarray results by qRT-PCR reveal changes in expression of the genes encoding distinct pro- and anti-apoptotic markers (B, left and right panels, respectively) and cell differentiation-associated markers (C) after doxorubicin treatment compared to controls.
D: Increase of expression of P53, FAS, TRAIL, TRAIL-R1, caspase-8, cFLIP, P21 and KAP1 in the epithelial (arrows) or mesenchymal (asterisks) portions of the hair bulb 24 hours after doxorubicin treatment. Note lack of cFLIP expression in hair matrix keratinocytes located at the bottom part of the hair bulb (arrowheads), while prominent cFLIP expression is seen in the differentiating hair shaft keratinocytes (arrow) and dermal papilla (asterisk). Scale bar 100 μm.
Genes that are involved in the control of apoptosis were sub-divided into the categories of pro- and anti-apoptotic regulators (Fig. 3b, c; Suppl. Tables S1, S2). Interestingly, among the group of genes encoded different pro-apoptotic regulators, several TNF-family apoptotic receptors and their ligands including FAS, TNFRSF10A, TNFRSF10B (TRAIL receptors 1 and 2, respectively), TNFSF10 (TRAIL) and TNFSF15 showed marked upregulation in doxorubicin-treated hair follicles versus the controls (Fig. 3b, d). Immunohistological and quantitative immunohistomorphometric analyses revealed that after doxorubicin treatment, markedly increased expressions of the TRAIL, TRAIL-R1, p53 and caspase-8 were seen in the hair follicle compartments enriched either in proliferating keratinocytes (distal hair matrix) or in post-mitotic differentiating cells (proximal part or precortex of the hair bulb) compared to controls (Fig. 3b, d; Suppl. Fig. S1 a, b).
However, microarray data validated by qRT-PCR also showed increased expression of anti-apoptotic markers (BCL2, BCL2A1, BCL6B, BCL10, TNFRSF10D, CFLAR) in doxorubicin-treated hair follicles compared to controls (Fig. 3b, d). This was not surprising due to the fact that hair follicles contain the significant number of cells that survive after chemotherapy, including the dermal papilla fibroblasts, post-mitotic differentiating keratinocytes, as well as epithelial progenitor cells protected from apoptosis (Paus et al, 2013). Quantitative immunohistomorphometric data revealed that one of the anti-apoptotic regulators cFLIP (CFLAR) that inhibit TNF receptor-mediated apoptosis and caspase-8 activity (Kavuri et al., 2011), is strongly upregulated in post-mitotic differentiating cells of the inner root sheath and hair shaft (precortex area), as well as in the dermal papilla, while its expression is detected at low levels in proliferating hair matrix keratinocytes located at the bottom part of the hair bulb (Fig. 3d; Suppl. Fig. S1 a, b). Because cFLIP expression decreased in differentiating hair bulb keratinocytes 48 and 72 hours after doxorubicin treatment (Suppl. Fig. S2), these data suggest that the balance of pro- and anti-apoptotic regulators at early time-points after chemotherapy (3–24 hours) is critical for the fate (apoptosis versus survival) of distinct hair follicle cell populations (proliferating versus differentiating keratinocytes).
In addition to changes in expression of apoptosis-related genes, two key genes that encode cell cycle inhibitors and promote cell differentiation in the hair follicle (Scheitz and Tumbar, 2013), such as cyclin-dependent kinase inhibitors CDKN1A (P21) and CDKN1C (P57), also showed marked upregulation in the hair follicle after doxorubicin treatment (Fig. 3d, Suppl. Tables S1, S2). Furthermore, doxorubicin treatment resulted in upregulation of expression of 15 genes encoding keratin-associated proteins (such as KRTAP9-9, KRTAP4-7, KRTAP3-2, KRTAP1-3 and others) and involved in execution of terminal differentiation program in hair matrix keratinocytes (Fig. 3c, d). Consistently with microarray and qRT-PCR data, hair follicles treated with doxorubicin showed earlier onset of expression of KAP1 in differentiating hair matrix keratinocytes compared to controls (Fig. 3d). The increased expression of the keratin-associated protein genes was accompanied by downregulation of the genes encoding selected keratins including KRT17, KRT16, KRTHA3B and KRT6A/6B after doxorubicin treatment, compared to controls (Suppl. Tables S1, S2). These data suggest that doxorubicin induces marked changes in the gene expression in hair matrix keratinocytes, which include not only pro-and anti-apoptotic genes, but also marked re-organization of cell differentiation program and premature activation of the keratin-associated protein genes.
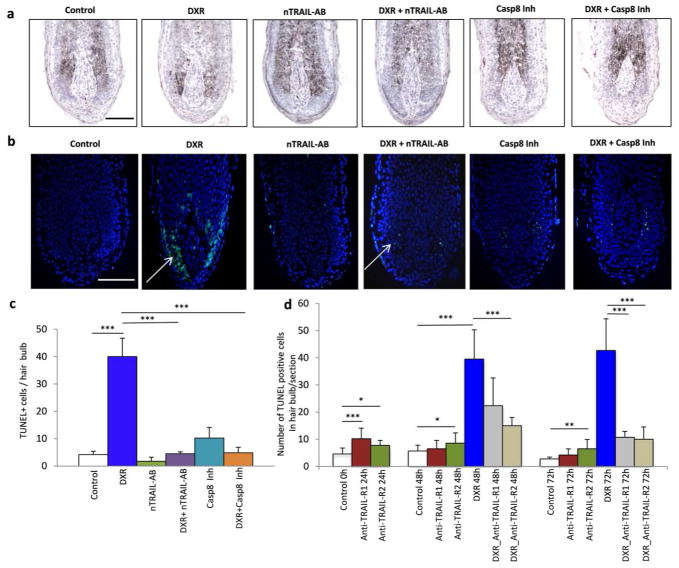
Inhibition of the TRAIL receptor signaling and caspase 8 results in decrease of doxorubicin-induced apoptosis in the hair follicles
To assess the impact of TRAIL-receptor mediated signaling in the control of apoptosis induced by doxorubicin in the hair follicles, hair follicles were treated with TRAIL-neutralizing antibody for 24 hours prior to and after doxorubicin treatment. TRAIL-neutralizing antibody has been shown previously to effectively inhibit apoptosis in different models (Cantarella et al., 2010; Murata et al., 2006). Treatment of the follicles with TRAIL-neutralizing antibody alone did not result in any significant changes in keratinocyte proliferation or apoptosis, while expression of TRAIL in the hair matrix was markedly decreased suggesting for inhibition of TRAIL activity (data not shown). However, hair follicles treated with combination of doxorubicin and TRAIL-neutralizing antibody showed marked decrease in the number of TUNEL+ cells compared to the follicles treated with doxorubicin alone suggesting that doxorubicin-induced apoptosis in hair matrix keratinocytes is mediated, at least in part, by the TRAIL receptor signaling (Fig. 4a–c). Furthermore, pre-treatment of the hair follicles with TRAIL-R1 and TRAIL-R2 antagonistic antibodies results in significant decrease in the number of TUNEL+ cells in the hair bulb after doxorubicin treatment (Fig. 4d), suggesting that both TRAIL-R1 and TRAIL-R2 are involved in mediating doxorubicin-induced apoptosis in hair matrix keratinocytes.
Figure 4. TRAIL- or TRAIL-R1/R2 antagonistic antibodies and caspase 8 inhibitor decrease apoptosis in the hair follicles treated by doxorubicin.
HFs were treated with TRIAL neutralising antibody (5 μg/ml), TRIALR1 or TRAIL-R2 antagonistic antibodies or with caspase 8 inhibitor (50 μm) for 24 hours prior to doxorubicin treatment.
A: Histomorphology of the HFs exposed for 48 hours to the different treatments.
B: Detection of apoptotic cells by TUNEL (green) reveals numerous apoptotic cells in the HF treated with doxorubicin (arrow). Scale bars: 100 μm.
C: Quantitative analysis of apoptosis shows dramatic elevation in TUNEL+ cells in doxorubicin-treated HF, whereas treatment with TRIAL neutralising antibody and caspase 8 inhibitor significantly reduced doxorubicin-induced apoptosis (mean ± SD, *** p<0.001 Student’s t-test).
D: Treatment with TRIALR1 or TRAIL-R2 antagonistic antibodies significantly reduced doxorubicin-induced apoptosis and number of TUNEL+ cell in the HFs (mean ± SD, * - p<0.05, ** - p<0.01, *** p<0.001; Student’s t-test).
Because activation of TRAIL receptor pathway is accompanied by the recruitment of the adaptor protein FADD and pro-caspase 8 to the intracellular death domain of the receptors followed by pro-caspase 8 cleavage and activation of the effector caspases (Bremer et al., 2006), the effects of caspase 8 inhibitor on doxorubicin-induced apoptosis in the hair follicle were tested. Similarly to the TRAIL-neutralizing antibody, treatment of the hair follicles with caspase 8 inhibitor resulted in significant decrease in the number of TUNEL+ cells in doxorubicin-treated hair follicles compared to the follicles treated with doxorubicin alone (Fig. 4 a–c). These data suggest that apoptosis induced by doxorubicin in the hair follicles is mediated, at least in part, by the TRAIL receptor 1/2 signaling involving an activation of caspase 8 as its downstream effector.
Discussion
Chemotherapy has severe side effects for normal rapidly proliferating tissues, such as hair follicle, leading to massive apoptosis in hair matrix keratinocytes followed by hair loss (Botchkarev, 2003; Hendrix et al., 2005; Paus and Cotsarelis, 1999; Paus et al., 2013). Because of the ethical issues in obtaining scalp biopsies from patients treated with chemotherapy, only limited information is available about the molecular events underlying the response of human hair follicles to chemotherapy (Paus et al., 2013). Significant advantages in this area were achieved by using isolated human hair follicles cultured ex vivo and exposed to chemotherapy (Bodo et al., 2007).
Here, we used an ex vivo human model for chemotherapy-induced hair loss (Bodo et al., 2007) and global microarray profiling to study changes in the molecular signature of human hair follicles during the early stages of their response to doxorubicin, a broadly used anti-cancer drug (Tacar et al, 2013). Cytotoxic effects of doxorubicin are based on its binding to and inhibiting activity of the DNA-associated enzymes (topoisomerases I/II), on intercalation with DNA base pairs, as well as on targeting multiple apoptotic regulatory molecules, such as Bcl2/Bax, caspases, AMP-activated protein kinase, etc. (Tacar et al, 2013). Consistently with the data obtained from tumor cells, we demonstrate here that already 3 hours after doxorubicin treatment hair follicles show marked changes in the expression levels of the genes encoding several components of the apoptotic/cell cycle machinery, cytoskeleton/cell differentiation markers, signaling/transcription regulators, etc. (Suppl. Tables S1, S2).
In general, these data are consistent with data obtained in the same model that show changes in expression of quite similar groups of genes in the hair follicles 48 hours after 4-hydroperoxycyclophosphamide treatment (Bodo et al., 2007). Indeed, several key regulators of apoptosis, cell differentiation and signaling/transcription, such as TNFRSF10B, CDKN1A, FGF18, or ID4, showed similar changes in their expression in the hair follicles treated with either doxorubicin (Suppl. Tables S1, S2) or 4- hydroperoxycyclophosphamide (Bodo et al., 2007). However, the differences between two datasets are also evident, and most likely, reflect the distinct mechanisms of the DNA damage response activated by doxorubicin versus 4-hydroperoxycyclophosphamide, as well as the distinct time-points in the response to the treatment (early versus more advanced) selected for analyses (Bodo et al., 2007).
Interestingly, among the genes encoding pro-apoptotic regulators, several members of the TNF family of apoptotic receptors (FAS, TRAIL receptors 1 and 2) show upregulation in their expression after doxorubicin treatment. FAS as a direct p53 target gene is involved in the control of apoptosis induced by chemotherapy in many organs including hair follicle (Friesen et al., 1996; Muller et al., 1997; Botchkarev et al., 2000; Sharov et al., 2003; Sharov et al., 2004). Similarly to FAS receptor, signaling through TRAIL receptor 1/2 activated by the TRAIL ligand induces apoptosis in normal and transformed keratinocytes (Kavuri et al., 2011; Leverkus et al., 2000). Expression of the TRAIL receptors 1/2 in tumor cells is increased after doxorubicin treatment (Wu et al., 2007). Apoptotic signaling through the FAS and TRAIL receptors requires their interaction with the intracellular adaptor molecule FADD, followed by activation of procaspase-8, its recruitment into the death inducing signaling complex (DISC) and activation of caspase-3 along the common final pathway of apoptosis (reviewed in Botchkareva et al., 2006; Curtin and Cotter, 2003; Peter and Krammer, 2003).
We show here that similarly to tumor cells, doxorubicin treatment results in upregulation of the TRAIL receptors 1 and 2 expressions in the hair matrix keratinocytes, while treatment of the hair follicles with TRAIL-neutralizing antibodies or TRAIL-R1/R2 antagonistic antibodies markedly reduces a number of TUNEL+ cells in the hair follicles (Fig. 3b, c; Fig. 4). Because TRAIL and TRAIL-R1 are direct p53 target genes (Kuribayashi et al., 2008; Liu et al., 2004), TRAIL-receptor mediated apoptosis is likely to be a part of the p53-regulated apoptotic program triggered by doxorubicin in hair matrix keratinocytes. However, because in some cases the increase of TRAIL-R1/2 expression in tumor cells after chemotherapy occurs also in p53-independent manner (Cheng et al., 2012), further studies are required to define the contribution of p53 to upregulation of TRAIL receptors in the hair follicles after doxorubicin treatment. In addition, genetically engineered mice with ablation of the TRAIL-R1 or TRAIL-R2 might be used to further dissect the role of these receptors in the control of chemotherapy-induced apoptosis in hair matrix keratinocytes.
However, doxorubicin treatment also increases expression of a number of anti-apoptotic genes in the hair follicle, which is consistent with the data showing that distinct hair follicle cell populations (selected outer root sheath and hair matrix keratinocytes, dermal papilla fibroblasts) characterized by high expression of anti-apoptotic proteins are programmed to survive after chemotherapy and contribute to subsequent hair follicle regeneration (Bodo et al., 2007; Lindner et al, 1997; Paus et al., 2013). One of such proteins, cFLIP is capable of inhibiting caspase-8 activation and apoptosis in keratinocytes mediated by the TRAIL- or FAS receptors (Kavuri et al., 2011). Since cFLIP is strongly upregulated in the post-mitotic differentiating keratinocytes and is not expressed in proliferating hair matrix keratinocytes, these data confirm the previously proposed model suggesting that the balance of pro- and anti-apoptotic factors play a critical role in the control of cell fate decision (apoptosis versus survival) in the distinct hair follicle cell populations after chemotherapy (Bodo et al., 2007; Lindner et al, 1997; Paus et al., 2013).
Our data also reveal marked upregulation of 15 genes encoding keratin-associated proteins (or KAPs) in the hair follicles after doxorubicin treatment (Fig. 3b, c). In normal human hair follicles, KAPs are expressed in differentiating keratinocytes of the hair matrix, cortex and cuticle (Rogers et al., 2006), while doxorubicin-treatment leads to pre-mature activation of the KAP genes, as evident by the KAP1 expression in post-mitotic hair matrix keratinocytes (Fig. 3c). Since recent data reveal that KAPs interacts with hair-specific keratins (Matsunaga et al., 2013), it appears to be interesting to check whether pre-mature activation of keratin-associated protein genes and early onset of expression of the KAPs in the hair follicles after doxorubicin treatment play a role in alterations in the hair shaft structure and breakage during chemotherapy-associated anagen effluvium (Paus et al., 2013).
Interestingly, increase of KAP1 expression in the hair matrix keratinocytes after doxorubicin treatment coincides with upregulation of P21 cyclin-dependent kinase inhibitor, a key p53 target gene that promotes mitotic exit and terminal differentiation in hair follicle keratinocytes (Lee et al., 2013; Mitsui et al., 2001). In addition, microarray analyses reveal marked increase of expression of the related CDKN1C (P57) gene in doxorubicin-treated follicles (Suppl. Table S1). Thus, it would be interesting to test a hypothesis whether P21 and P57 contribute to marked upregulation of the keratin-associated protein genes in the hair follicle after doxorubicin treatment, or other mechanisms are involved in mediating these effects of doxorubicin.
Taken together, these data provide previously unreported insights into the mechanisms underlying the early events in the complex changes induced in human hair follicles by doxorubicin and reveal a new role for TRAIL receptor signaling in the control of chemotherapy-induced hair loss. These data also serve as an important platform for the development of approaches for the prevention or diminishing of the toxic effects of chemotherapy on the hair follicles and for search of the drug-specific hair-protective paradigms for cancer patients.
Materials and Methods
Hair follicle culture and pharmacological experiments
This study was approved by Institutional Review Board Committee of Boston University to ensure subject protection and adherence to the Declaration of Helsinki Principles. Patient consent for experiments was not required because human scalp skin samples were obtained from anonymous donors (five different female individuals) undergoing face-lift cosmetic surgery procedures. The hair follicles were microdissected from skin samples and cultured in Williams E medium supplemented with 10 μg/ml insulin, 10 ng/ml hydrocortisone, 2mM L-glutamine, and antibiotic/antimycotic mixture as described previously (Philpott et al., 1994). Individual hair follicles were cultured in 0.5 ml of supplemented Williams E medium at 37°C in a 5%CO2. After 24 hours of culture, Doxorubicin HCl (DXR; 0.3 or 1.0 μM; Thermo Fisher Scientific, Waltham, MA, USA) was added to the microdissected anagen HFs for 1 hour, and culture medium was replaced with freshly prepared supplemented Williams E medium after completion of the DXR treatment. Control hair follicles were treated with the PBS instead of doxorubicin. For pharmacological modulation of apoptosis induced by doxorubicin, hair follicles were treated with TRAIL neutralizing antibody (5 μg/ml, Abcam, Cambridge, MA) or Caspase 8 inhibitor (50uM, Santa Cruz Biotech Inc., Dallas, TX), TRAIL-R1 and TRAIL-R2 mAbs (clones HS101 and HS201 respectively, 10ug/ml, Enzo Biochem. Inc, Farmingdale, NY) and corresponding isotype-matched immunoglobulin for 24 hours prior to DXR exposure. For each time point, 20–25 hair follicles from five individuals were studied.
Microarray and qRT-PCR analyses
For microarray analysis, hair bulbs were dissected and processed for RNA isolation 3 hours after completion of the DXR treatment. Total RNA was isolated from the hair follicle bulbs using TriIzol® Reagent (Invitrogen, San Diego, CA). All experiments were performed using at least three replicates, and total RNA isolated from three experimental and control samples was pooled and processed for microarray analyses using one sample of pooled RNA per experimental and control group. All microarray analyses were performed at the Microarray Core Facility at Boston University School of Medicine using Human Genome U133A 2.0 array (Affimetrix Inc. Santa Clara, CA). After statistical analysis and initial filtering the microarray data the changes in gene expression after doxorubicin treatment equal or higher 2 fold with at least one signal equal or higher than 80 fluorescence units for the gene was considered significant (Fessing et al., 2010). For quantitative RT-PCR analysis, equal amounts of total RNA was used as a template for cDNA synthesis using SupperScript III First-Strand Synthesis System and random primers (Life Technologies, San Diego, CA). PCR primers were designed using Beacon Designer software (Premier Biosoft International, Paolo Alto, CA) and are listed in Suppl. Table S3. Real-time PCR was performed using iCycler Thermal Cycler (Bio-Rad Corp., Hercules, CA). Differences between samples and controls were calculated using Gene Expression Macro program (Bio-Rad Corp., Hercules, CA) based on the ΔΔCt equitation method, as described previously (Fessing et al., 2011; Sharov et al., 2005; Sharov et al., 2006).
Immunohistochemistry and quantitative immunohistomorphometry
For qRT-PCR, histomorphology and immunohistochemical analyses, hair follicles were collected at 3, 24, 48, 72 hours and 7 days after doxorubicin treatment. For immunohistochemical analyses, 8 μm longitudinal cryosections of the hair follicles performed through the central part of the dermal papilla were used, as described previously (Botchkareva et al., 2007). The cryosections were incubated with primary antisera (see Suppl. Table S4) overnight at room temperature, followed by application of corresponding TRITC- or Alexa Fluor® 555 Conjugate -labelled secondary antibody (Invitrogen, UK; diluted 1:200) for 45 min at 37°C. Incubation steps were interspersed by four washes with phosphate buffer-saline (PBS, 5 min each), as described previously (Botchkarev et al., 1999; Muller-Rover et al., 1998; Siebenhaar et al., 2007). For double immunofluorescence detection of TUNEL-positive cells and Ki-67 immunoreactivity, ApopTag® Fluorescein Direct In Situ Apoptosis Detection Kit (EMD MilliporeCorp, Billerica, MA) and anti Ki-67 antibody were used.
Alkaline phosphatase staining was used for identification and quantification of the hair follicles at distinct stages of catagen, as described previously (Hendrix et al., 2005). Ki67+ cells were calculated below the distal end of the dermal papilla and were expressed as percentage to total number of DAPI+ cells in the hair matrix, while TUNEL+ cells were calculated per hair bulb, according to recommendations published previously (Bodo et al., 2007). In total, 20–25 hair follicles were assessed per time-point in each experimental and control groups. Image preparation and analysis were performed using a fluorescent microscope (Nikon, Tokyo, Japan) in combination with DS-C1 digital camera and ACT-2U image analysis software (Nikon, Tokyo, Japan).
Immunofluorescence intensity was determined using ImageJ software (NIH, Bethesda, USA), as described previously (Ramot et al., 2010). In brief, red fluorescent signal was collected from experimental tissues in RGB format using the same exposure conditions. To measure the fluorescence intensity at each pixel, the RGB images were converted to 8-bit grayscale format. Regions of interest of distinct size (hair matrix – 170 μm2, dermal papilla - 180 μm2, and pre-cortex - 200 μm2) within the control and DXR-treated hair follicles were selected, and the mean values of intensity (the sum of gray values of all the pixels in the selection divided by the number of pixels) was calculated for each selected areas. In total, 20–25 hair follicles were assessed per time-point for each marker (TRAIL, TRAIL-R1, cFLIP) in the DXR-treated and control groups, and the differences between mean values obtained from the control and DXR-treated HFs were calculated using Student’s t-test.
Supplementary Material
Acknowledgments
This study was supported in part from the NIAMS grant to V.A.B. (AR049778) and from the NIH KO1 Award (AR056771) to A.A.S. Authors thank Drs. J. Schweizer, L. Langbein and M. Rogers for providing anti-KAP1 antibody.
Abbreviations
- DXR
doxorubicin
- KAP
keratin-associated protein
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis inducing ligand
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Amoh Y, Li L, Katsuoka K, Hoffman RM. Chemotherapy targets the hair-follicle vascular network but not the stem cells. J Invest Dermatol. 2007;127:11–5. doi: 10.1038/sj.jid.5700486. [DOI] [PubMed] [Google Scholar]
- Amoh Y, Li L, Yang M, Jiang P, Moossa AR, Katsuoka K, et al. Hair follicle-derived blood vessels vascularize tumors in skin and are inhibited by Doxorubicin. Cancer Res. 2005;65:2337–43. doi: 10.1158/0008-5472.CAN-04-3857. [DOI] [PubMed] [Google Scholar]
- Bodo E, Tobin DJ, Kamenisch Y, Biro T, Berneburg M, Funk W, et al. Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol. 2007;171:1153–67. doi: 10.2353/ajpath.2007.061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo E, van Beek N, Naumann V, Ohnemus U, Brzoska T, Abels C, et al. Modulation of chemotherapy-induced human hair follicle damage by 17-beta estradiol and prednisolone: potential stimulators of normal hair regrowth by “dystrophic catagen” promotion? J Invest Dermatol. 2009;129:506–9. doi: 10.1038/jid.2008.228. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA. Molecular mechanisms of chemotherapy-induced hair loss. J Invest Dermatol Symp Proc. 2003;8:72–5. doi: 10.1046/j.1523-1747.2003.12175.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Komarova EV, Siebenhaar F, Botchkareva NV, Komarov PG, Maurer M, et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res. 2000;60:5002–6. [PubMed] [Google Scholar]
- Botchkarev VA, Peters EM, Botchkareva NV, Maurer M, Paus R. Hair cycle-dependent changes in adrenergic skin innervation, and hair growth modulation by adrenergic drugs. J Invest Dermatol. 1999;113:878–87. doi: 10.1046/j.1523-1747.1999.00791.x. [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Ahluwalia G, Shander D. Apoptosis in the hair follicle. J Invest Dermatol. 2006;126:258–64. doi: 10.1038/sj.jid.5700007. [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Kahn M, Ahluwalia G, Shander D. Survivin in the human hair follicle. J Invest Dermatol. 2007;127:479–82. doi: 10.1038/sj.jid.5700537. [DOI] [PubMed] [Google Scholar]
- Bremer E, van Dam G, Kroesen BJ, de Leij L, Helfrich W. Targeted induction of apoptosis for cancer therapy: current progress and prospects. Trends Mol Med. 2006;12:382–93. doi: 10.1016/j.molmed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Di Benedetto G, Scollo M, Paterniti I, Cuzzocrea S, Bosco P, et al. Neutralization of tumor necrosis factor-related apoptosis-inducing ligand reduces spinal cord injury damage in mice. Neuropsychopharmacology. 2010;35:1302–14. doi: 10.1038/npp.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cece R, Cazzaniga S, Morelli D, Sfondrini L, Bignotto M, Menard S, et al. Apoptosis of hair follicle cells during doxorubicin-induced alopecia in rats. Lab Invest. 1996;75:601–9. [PubMed] [Google Scholar]
- Cheng H, Hong B, Zhou L, Allen JE, Tai G, Humphreys R, et al. Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation. Cell Cycle. 2012;11:3312–23. doi: 10.4161/cc.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Cotter TG. Live or let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signalling. 2003;15:983–92. doi: 10.1016/s0898-6568(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, et al. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995;147:145–54. [PMC free article] [PubMed] [Google Scholar]
- Fessing MY, Atoyan R, Shander B, Mardaryev AN, Botchkarev VV, Jr, Poterlowicz K, et al. BMP signaling induces cell-type-specific changes in gene expression programs of human keratinocytes and fibroblasts. J Invest Dermatol. 2010;130:398–404. doi: 10.1038/jid.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessing MY, Mardaryev AN, Gdula MR, Sharov AA, Sharova TY, Rapisarda V, et al. p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J Cell Biol. 2011;194:825–39. doi: 10.1083/jcb.201101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity: characterising and avoinding the problem. Drugs. 1991;42:781–95. doi: 10.2165/00003495-199142050-00005. [DOI] [PubMed] [Google Scholar]
- Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med. 1996;2:574–7. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- Hendrix S, Handjiski B, Peters EM, Paus R. A guide to assessing damage response pathways of the hair follicle: lessons from cyclophosphamide-induced alopecia in mice. J Invest Dermatol. 2005;125:42–51. doi: 10.1111/j.0022-202X.2005.23787.x. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, et al. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13:31–8. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JJ, Yunis AA. Protection from 1-beta-d-Arabinofuranosylcytosine-induced alopecia by epidermal growth factor and fibroblast growth factor in the rat model. Cancer Res. 1992;52:413–5. [PubMed] [Google Scholar]
- Kavuri SM, Geserick P, Berg D, Dimitrova DP, Feoktistova M, Siegmund D, et al. Cellular FLICE-inhibitory protein (cFLIP) isoforms block CD95- and TRAIL death receptor-induced gene induction irrespective of processing of caspase-8 or cFLIP in the death-inducing signaling complex. J Biol Chem. 2011;286:16631–46. doi: 10.1074/jbc.M110.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribayashi K, Krigsfeld G, Wang W, Xu J, Mayes PA, Dicker DT, et al. TNFSF10 (TRAIL), a p53 target gene that mediates p53-dependent cell death. Cancer Biol Ther. 2008;7:2034–8. doi: 10.4161/cbt.7.12.7460. [DOI] [PubMed] [Google Scholar]
- Lee J, Hoi CS, Lilja KC, White BS, Lee SE, Shalloway D, et al. Runx1 and p21 synergistically limit the extent of hair follicle stem cell quiescence in vivo. Proc Natl Acad Sci U S A. 2013;110:4634–9. doi: 10.1073/pnas.1213015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverkus M, Neumann M, Mengling T, Rauch CT, Brocker EB, Krammer PH, et al. Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes. Cancer Res. 2000;60:553–9. [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen) Amer J Pathol. 1997;151:1601–17. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yue P, Khuri FR, Sun SY. p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res. 2004;64:5078–83. doi: 10.1158/0008-5472.CAN-04-1195. [DOI] [PubMed] [Google Scholar]
- Matsunaga R, Abe R, Ishii D, Watanabe SI, Kiyoshi M, Nocker B, et al. Bidirectional binding property of high glycine-tyrosine keratin-associated protein contributes to the mechanical strength and shape of hair. J Struct Biol. 2013 doi: 10.1016/j.jsb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Meyn RE, Stephens LC, Hunter NR, Milas L. Apoptosis in murine tumors treated with chemotherapy agents. Anticancer Drugs. 1995a;6:443–50. doi: 10.1097/00001813-199506000-00013. [DOI] [PubMed] [Google Scholar]
- Meyn RE, Stephens LC, Hunter NR, Milas L. Kinetics of cisplatin-induced apoptosis in murine mammary and ovarian adenocarcinomas. Int J Cancer. 1995b;60:725–9. doi: 10.1002/ijc.2910600526. [DOI] [PubMed] [Google Scholar]
- Mitsui S, Ohuchi A, Adachi-Yamada T, Hotta M, Tsuboi R, Ogawa H. Cyclin-dependent kinase inhibitors, p21(waf1/cip1) and p27(kip1), are expressed site- and hair cycle-dependently in rat hair follicles. J Dermatol Sci. 2001;25:164–9. doi: 10.1016/s0923-1811(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Peters EJ, Botchkarev VA, Panteleyev A, Paus R. Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J Histochem Cytochem. 1998;46:1401–10. doi: 10.1177/002215549804601209. [DOI] [PubMed] [Google Scholar]
- Muller M, Strand S, Hug H. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403–13. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Tsuboi M, Hikita K, Kaneda N. Protective effects of neurotrophic factors on tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis of murine adrenal chromaffin cell line tsAM5D. J Biol Chem. 2006;281:22503–16. doi: 10.1074/jbc.M602579200. [DOI] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–8. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Paus R, Handjiski B, Eichmuller S, Czarnetzki BM. Chemotherapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol. 1994;144:719–34. [PMC free article] [PubMed] [Google Scholar]
- Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:e50–9. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(Apo-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Philpott MP, Sanders D, Westgate GE, Kealey T. Human hair growth in vitro: a model for study of hair follcile biology. J Dermatol Sci. 1994;7(Suppl):S55–S72. doi: 10.1016/0923-1811(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Ramot Y, Bíró T, Tiede S, Tóth BI, Langan EA, Sugawara K, Foitzik K, Ingber A, Goffin V, Langbein L, Paus R. Prolactin - a novel neuroendocrine regulator of human keratin expression in situ. FASEB J. 2010;24:1768–79. doi: 10.1096/fj.09-146415. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Langbein L, Praetzel-Wunder S, Winter H, Schweizer J. Human hair keratin-associated proteins (KAPs) Int Rev Cytol. 2006;251:209–63. doi: 10.1016/S0074-7696(06)51006-X. [DOI] [PubMed] [Google Scholar]
- Scheitz CJ, Tumbar T. New insights into the role of Runx1 in epithelial stem cell biology and pathology. J Cell Biochem. 2013;114:985–93. doi: 10.1002/jcb.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleri S, Seltmann H, Gariboldi S, Shirai YF, Balsari A, Zouboulis CC, et al. Doxorubicin-induced alopecia is associated with sebaceous gland degeneration. J Invest Dermatol. 2006;126:711–20. doi: 10.1038/sj.jid.5700175. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Fessing M, Atoyan R, Sharova TY, Haskell-Luevano C, Weiner L, et al. Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proc Natl Acad Sci U S A. 2005;102:93–8. doi: 10.1073/pnas.0408455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Li GZ, Palkina TN, Sharova TY, Gilchrest BA, Botchkarev VA. Fas and c-kit are involved in the control of hair follicle melanocyte apoptosis and migration in chemotherapy-induced hair loss. J Invest Dermatol. 2003;120:27–35. doi: 10.1046/j.1523-1747.2003.12022.x. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, Weiner L, et al. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci U S A. 2006;103:18166–71. doi: 10.1073/pnas.0608899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Siebenhaar F, Sharova TY, Botchkareva NV, Gilchrest BA, Botchkarev VA. Fas signaling is involved in the control of hair follicle response to chemotherapy. Cancer Res. 2004;64:6266–70. doi: 10.1158/0008-5472.CAN-04-1367. [DOI] [PubMed] [Google Scholar]
- Siebenhaar F, Sharov AA, Peters EM, Sharova TY, Syska W, Mardaryev AN, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata) J Invest Dermatol. 2007;127:1489–97. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- Siu WY, Arooz T, Poon RY. Differential responses of proliferating versus quiescent cells to adriamycin. Exp Cell Res. 1999;250:131–41. doi: 10.1006/excr.1999.4551. [DOI] [PubMed] [Google Scholar]
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–70. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- Trueb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28:11–4. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Wu XX, Jin XH, Zeng Y, El Hamed AM, Kakehi Y. Low concentrations of doxorubicin sensitizes human solid cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing TRAIL-R2 expression. Cancer Sci. 2007;98:1969–76. doi: 10.1111/j.1349-7006.2007.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.