Abstract
Background & Aims
Endoscopic intervention or pharmacologic inhibition of cyclooxygenase might be used to prevent progression of Barrett's esophagus (BE) to esophageal adenocarcinoma (EAC). We investigated whether patients with BE prefer endoscopic therapy or chemoprevention of EAC.
Methods
Eighty-one subjects with nondysplastic BE were given a survey that described 2 scenarios. The survey explained that treatment A (ablation), endoscopy, reduced lifetime risk of EAC by 50%, with a 5% risk for esophageal stricture, whereas treatment B (aspirin) reduced lifetime risk of EAC by 50% and the risk of heart attack by 30%, yet increased the risk for ulcer by 75%. Subjects indicated their willingness to undergo either treatment A and/or treatment B if endoscopic surveillance was required every 3–5 years, every 10 years, or was not required. Visual aids were included to represent risk and benefit percentages.
Results
When surveillance was required every 3–5 years, more subjects were willing to undergo treatment A than treatment B (78% [63/81] vs 53% [43/81], P<.01). There were no differences in age, sex, education level, or history of cancer, heart disease, or ulcer between patients willing to undergo treatment A and those willing to undergo treatment B. Altering the frequency of surveillance did not affect patients’ willingness to undergo either treatment.
Conclusion
In a simulated scenario, patients with BE preferred endoscopic intervention over chemoprevention for EAC. Further investigation may be warranted of the shared decision making process regarding preventive strategies for patients with BE.
Keywords: COX inhibitor, surgery, patient choice, esophageal cancer
Introduction
Barrett's esophagus (BE) develops as a consequence of chronic gastroesophageal reflux and is the major identified risk factor for esophageal adenocarcinoma (EAC). Endoscopic screening for BE among patients with gastroesophageal reflux disease (GERD) and endoscopic surveillance among patients with established BE have been justified on the basis of cost-effectiveness analyses as early cancer detection strategies [1-4] and endorsed by societal guidelines. Unfortunately, these practices have failed to impact the rapidly increasing incidence of EAC in the Western world over the past several decades [5].
While a proven EAC prevention strategy does not exist, there is epidemiologic evidence to suggest that there may be a protective association between aspirin use and EAC risk [6], presumably due to effects on cyclooxygenase inhibition. A recent prospective study of patients with BE randomized to esomeprazole plus placebo versus esomeprazole plus aspirin 325 mg daily demonstrated significantly reduced prostaglandin E(2) concentrations in esophageal tissue among those receiving aspirin, prompting renewed enthusiasm for investigation of high dose aspirin as a component of an EAC prevention strategy [7].
A theoretical alternative to chemoprevention is endoscopic eradication therapy, which has demonstrated efficacy in reducing progression from BE with high-grade dysplasia (HGD) to EAC. In the randomized controlled trial with the longest follow-up period, patients who received photodynamic therapy (PDT) with porfimer sodium were found to have a progression rate from HGD to EAC at 5 years of 15% among subjects treated with PDT plus omeprazole versus 29% among subjects treated with omeprazole alone [8]. In the more recent AIM-Dysplasia trial, progression from HGD to EAC at 12 month follow-up was 2.4% among subjects treated with radiofrequency ablation (RFA) compared to 19% among subjects treated with a sham intervention [9]. Whereas the phototoxicity and stricture rate of PDT largely restricted use to patients at high risk for cancer with HGD, the relatively lower adverse effect profile of RFA has enabled consideration of endotherapy as a cancer prevention strategy for an expanded potential treatment pool of BE patients with less advanced pathology than HGD such as low-grade dysplasia (LGD) or no dysplasia [10-12].
Prior work by authors of this study investigating patient preferences for chemoprevention of EAC using standard risk communication techniques demonstrated that, given the option of aspirin versus celecoxib, patients with BE will select a preferred treatment option based on benefits, risks, and tradeoffs [13]. Information regarding patient attitudes towards the benefits and risks of endoscopic intervention would be similarly useful, and may inform the shared decision making process in BE management and in development of future EAC prevention strategies.
The primary aim of this study was to determine whether patients with nondysplastic BE enrolled in a surveillance program prefer endoscopic therapy or chemoprevention for prevention of EAC. The secondary aim was to assess whether altering the frequency of endoscopic surveillance influenced patient selection of endoscopic intervention and/or chemoprevention.
Methods
Overview of study design and recruitment
Patients with an established diagnosis of BE were prospectively enrolled at Vanderbilt University Medical Center, University of Colorado Hospital, and the Veterans Administration Eastern Colorado Health Care System. Institutional review board approval was obtained at all sites of patient enrollment.
Study participation for each subject consisted of completion of a questionnaire with accompanying visual aids. Adult subjects (18 years of age and older) with a diagnosis of BE without dysplasia confirmed by tissue histology were candidates for enrollment. Eligible subjects were required to read and understand information in written English. Subjects were recruited by one of two mechanisms. First, potential subjects with a diagnosis of BE scheduled for a clinic or endoscopy visit were identified using an electronic scheduling system and approached by a study investigator at the time of visit. Second, subjects with a known diagnosis of BE were sent a letter by mail with an invitation to participate in the study. Target enrollment was 100 subjects. Enrollment began in May 2011 and was completed in October 2013.
Survey description
Detailed examples of the survey and questionnaire are available in the Appendix 1. Each subject was asked to provide demographic information including age, gender, ethnicity, highest education level completed, and medical history including proton pump inhibitor use, aspirin use, history of cancer, history of heart condition, or history of ulcer.
Next, each subject was provided with a textual description of BE and EAC, and was asked to read about two treatments to prevent EAC, each with risks and benefits. The descriptions were concise in an attempt to simplify the relevant data into a form that would be comprehendible by an individual without formal medical education and to limit cognitive burden [14]. Treatment A and Treatment B were presented anonymously. The order was not randomized; Treatment A was always presented before Treatment B. Benefits and harms were presented as absolute lifetime percentages, standard for risk communication methodology [15].
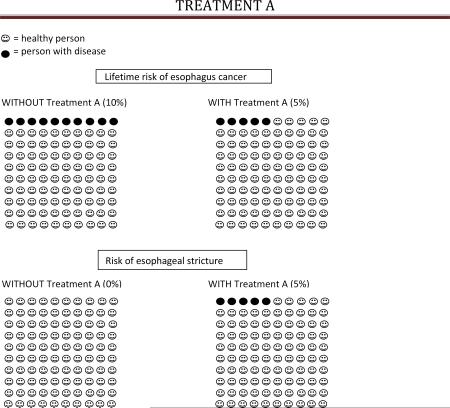
Treatment A (ablation) was described as a treatment performed during upper endoscopy which would remove Barrett's tissue if successful. The benefit of Treatment A was described as a 50% reduction in lifetime EAC risk, from 10% to 5%; the harm of Treatment A was described as a 5% risk of scar tissue in the esophagus which may result in difficulty swallowing (esophageal stricture). Benefits and risks were described in text and summarized in a brief table following the text. In addition, each subject was provided with a visual representation of the percentage risks and benefits of Treatment A (Appendix 2 and 3).
After the scenario was presented, subjects were asked to indicate whether or not they would be willing to undergo Treatment A if 1) an endoscopy were required for surveillance every 3-5 years after Treatment A; 2) an endoscopy were required for surveillance every 10 years after Treatment A; 3) if an endoscopy were never again required for surveillance after Treatment A.
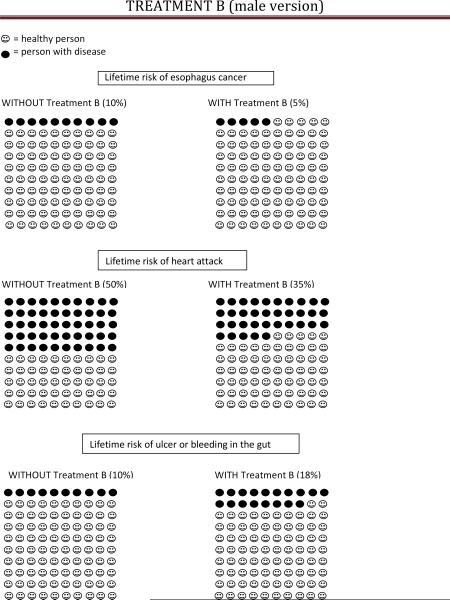
Treatment B (aspirin) was described as a pill taken once daily and which could be purchased over the counter without a prescription. The benefits of Treatment B were described as a 50% reduction in lifetime EAC risk, from 10% to 5%, as well as a 30% reduction in the lifetime risk of heart attack or stroke (from 50 in 100 to 35 in 100 for men, and from 30 in 100 to 20 in 100 for women); the harm of Treatment B was described as a 75% increase in lifetime risk of ulcer or bleeding from stomach/intestines (from 10 in 100 to 18 in 100).
Similarly, benefits and risks were described in text, summarized in a brief table following the text, and via a visual representation of the percentage risks and benefits of Treatment B. And, after the scenario was presented, subjects were asked to indicate whether or not they would be willing to undergo Treatment B if 1) an endoscopy were required for surveillance every 3-5 years after Treatment B; 2) an endoscopy were required for surveillance every 10 years after Treatment B; 3) if an endoscopy were never again required for surveillance after Treatment B.
Estimates for lifetime EAC risk, magnitude of reduction in lifetime EAC risk, reduction in lifetime myocardial infarction risk, and increase in lifetime ulcer/gastrointestinal bleeding risk with aspirin use were established as previously described and employed in a prior study of patient preferences for chemoprevention in BE [13].
Because the lifetime risk of myocardial infarction is different for men and women, separate questionnaires and visual aids were presented to male and female subjects, which differed only in the lifetime risk of heart attack with and without Treatment B, as described above. The questionnaires and visual aids were otherwise identical.
Statistics
The primary endpoint consisted of comparison of the proportion of patients who would be willing to undergo ablation (Treatment A) versus aspirin therapy (Treatment B). Fisher's exact test was used for this comparison. Mcnemar's test was used in determining whether altering the frequency of endoscopic surveillance influenced willingness to undergo treatment. Additional univariate analysis was performed with tests of significance consisting of Student's t-test or ANOVA for continuous variables, and either Chi square or Fisher's exact test for categorical variables. Two-sided P values of <0.05 were considered statistically significant. The statistical plan called for creation of a multivariable logistic regression model if candidate predictors of significance were identified on univariate analysis. Statistical analysis was performed using SAS Version 9.3 software (SAS Institute, Cary, NC USA).
Power calculation was based on the expected proportion of subjects willing to undergo Treatment A and/or Treatment B. Assuming 60% of subjects would be willing to undergo Treatment A and 40% of subjects would be willing to undergo Treatment B, enrollment of 100 subjects would provide 80% power to detect a difference of this magnitude at an alpha level of 0.05.
Results
Participant characteristics
110 subjects were enrolled and completed the survey. 12 subjects were excluded following enrollment due to a diagnosis of BE with dysplasia. An additional 17 subjects were excluded due to incomplete survey responses with respect to willingness to either undergo or not undergo Treatment A and/or Treatment B. Three subjects with incomplete demographic/clinical data were included. The final cohort for analysis therefore consisted of 81 subjects. 65% of subjects were male, 96% were Caucasian, and mean age was 60.2 years. 93% of subjects had completed at least high school, with 68% having completed college or a postgraduate degree. 80% of subjects reported proton pump inhibitor use and 41% reported aspirin use at the time of survey completion. A personal history of cancer, heart condition, or peptic ulcer was reported by 23%, 17%, and 19% of subjects, respectively. Full demographic and clinical data for respondents are summarized in Table 1.
Table 1.
Demographic and clinical characteristics—overall and by treatment preference
| Overall | Treatment A only | Treatment B only | Both | Neither | |
|---|---|---|---|---|---|
| N | 81 | 25 | 5 | 38 | 13 |
| Male gender | 65% (51/78) | 71%(17/24) | 80% (4/5) | 61% (22/36) | 62% (8/13) |
| Caucasian ethnicity | 96% (77/80) | 100% (25/25) | 100% (5/5) | 95% (35/37) | 92% (12/13) |
| Mean age | 60.2 yrs | 59.7 yrs | 54.4 yrs | 61.8 yrs | 58.9 yrs |
| Highest education level completed: | |||||
| Postgraduate | 15% (12/81) | 28% (7/25) | 20% (1/5) | 11% (4/38) | 0 |
| College | 53% (43/81) | 52% (13/25) | 80% (4/5) | 45% (17/38) | 69% (9/13) |
| High school | 25% (20/81) | 16% (4/25) | 0 | 32% (12/38) | 31% (4/13) |
| Some high school or less | 7% (6/81) | 4% (1/25) | 0 | 13% (5/38) | 0 |
| Current proton pump inhibitor use | 80% (65/81) | 68% (17/25) | 80% (4/5) | 87% (33/38) | 85% (11/13) |
| Current aspirin use | 41% (33/81) | 32% (8/25) | 60% (3/5) | 45% (17/38) | 38% (5/13) |
| Personal history of cancer | 23% (19/81) | 20% (5/25) | 20% (1/5) | 26% (10/38) | 23% (3/13) |
| Personal history of heart condition | 17% (14/81) | 24% (6/25) | 20% (1/5) | 13% (5/38) | 15% (2/13) |
| Personal history of peptic ulcer | 19% (15/81) | 16% (4/25) | 20% (1/5) | 21% (8/38) | 15 %(2/13) |
Data are presented as reported. Due to incomplete questionnaires, fewer than 81 responses are present for some variables.
Continued surveillance
In base case analysis, with a requirement for ongoing surveillance endoscopy every 3 to 5 years, more subjects were willing to undergo Treatment A (ablation) compared with Treatment B (aspirin) (78% [63/81] vs 53% [43/81], P <0.01) (Table 1). There was no difference in age, sex, education level, or history of cancer, heart disease, or ulcer when comparing subjects willing to undergo Treatment A to those willing to undergo Treatment B. Given the absence of significant univariate predictors, multivariable logistic regression analysis was not performed.
There was no difference in recorded demographic or clinical variables when comparing subjects willing to undergo Treatment A to those unwilling to undergo Treatment A (Table 2), or in comparing subjects willing to undergo Treatment B to those unwilling to undergo Treatment B (Table 3).
Table 2.
Patient preference for Treatment A (ablation), willing vs unwilling subjects: univariate predictors, base case
| Treatment A willing N=63 | Treatment A unwilling N=18 | P= | |
|---|---|---|---|
| Male gender | 65% (39/60) | 67% (12/18) | 0.89 |
| Caucasian ethnicity | 97% (60/62) | 94% (17/18) | 0.54 |
| Age | 61.0 years | 57.6 years | 0.27 |
| Highest education level completed: | 0.25 | ||
| Postgraduate | 17% (11/63) | 6% (1/18) | |
| College | 48% (30/63) | 72% (13/18) | |
| High school | 25% (16/63) | 22% (4/18) | |
| Some high school or less | 10% (6/63) | 0 | |
| Current proton pump inhibitor use | 79% (50/63) | 83% (15/18) | 1.0 |
| Current aspirin use: | 0.92 | ||
| 6 or more doses per week | 29% (18/63) | 33% (6/18) | |
| 5 or fewer doses per week | 11% (7/63) | 11% (2/18) | |
| None | 60% (38/63) | 56% (10/18) | |
| Personal history of cancer | 24% (15/63) | 22% (4/18) | 1.0 |
| Personal history of heart condition | 17% (11/63) | 17% (3/18) | 1.0 |
| Personal history of peptic ulcer | 19% (12/63) | 17% (3/18) | 1.0 |
Table 3.
Patient preference for Treatment B (aspirin), willing vs unwilling subjects: univariate predictors, base case
| Treatment B willing N=43 | Treatment B unwilling N= 38 | P= | |
|---|---|---|---|
| Male gender | 63% (26/41) | 68% (25/37) | 0.7 |
| Caucasian ethnicity | 95% (40/42) | 97% (37/38) | 1.0 |
| Age | 60.9 years | 59.4 years | 0.56 |
| Highest education level completed: | 0.36 | ||
| Postgraduate | 12% (5/43) | 18% (7/38) | |
| College | 49% (21/43) | 58% (22/38) | |
| High school | 28% (12/43) | 21% (8/38) | |
| Some high school or less | 12% (5/43) | 3% (1/38) | |
| Current proton pump inhibitor use | 86% (37/43) | 74% (28/38) | 0.16 |
| Current aspirin use: | 0.52 | ||
| 6 or more doses per week | 35% (15/43) | 24% (9/38) | |
| 5 or fewer doses per week | 12% (5/43) | 1% (4/38) | |
| None | 53% (23/43) | 66% (25/38) | |
| Personal history of cancer | 26% (11/43) | 21% (8/38) | 0.63 |
| Personal history of heart condition | 14% (6/43) | 21% (8/38) | 0.56 |
| Personal history of peptic ulcer | 21% (9/43) | 16% (6/38) | 0.55 |
Reduced or eliminated surveillance
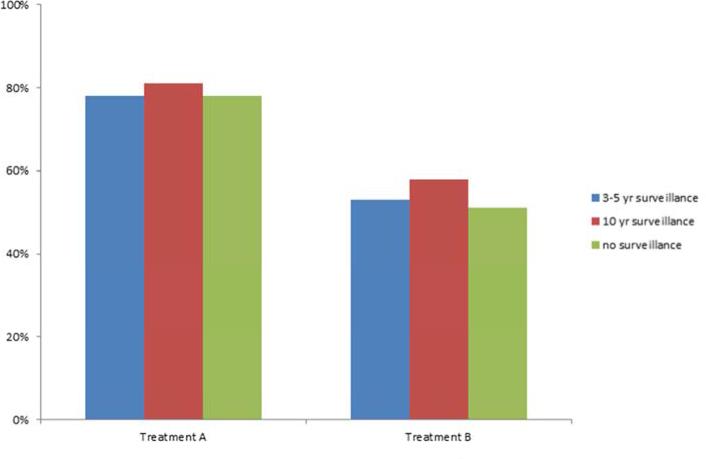
Altering the frequency of endoscopic surveillance did not influence willingness to undergo either Treatment A or Treatment B. While 78% (63/81) of respondents were willing to undergo Treatment A with endoscopic surveillance every 3-5 years, 81% (66/81) were willing to undergo Treatment A if surveillance endoscopy were required every 10 years (P=0.55 for comparison), and 78% (63/81) were willing to undergo Treatment A if surveillance endoscopy were never required again (P=1.0 for comparison) (Figure 1).
Figure 1.
Patient willingness to undergo treatment while altering frequency of surveillance
53% (43/81) of respondents were willing to undergo Treatment B with endoscopic surveillance every 3-5 years, 58% (47/81) were willing to undergo Treatment B if surveillance endoscopy were required every 10 years (P=0.22 for comparison), and 51% (41/81) were willing to undergo Treatment B if surveillance endoscopy were never required again (P=0.75 for comparison) (Figure 1).
Discussion
Benefits of preventive interventions do not clearly outweigh the risks for all patients. The factors affecting a decision to pursue a preventive intervention may not be straightforward, and in many scenarios, may be subject to patient preferences. The decision to take aspirin or selective COX-inhibitors as a chemopreventive strategy by patients with BE, for instance, may be a preference-sensitive decision [13]. The availability of current generation endoscopic therapy has expanded management options for BE, and has increased the complexity of medical decision-making faced by patients with BE. Current American Gastroenterological Association practice guidelines suggest that RFA should be an option for patients with low-grade dysplasia, and select individuals with nondsyplastic BE judged to be at risk for progression—while endorsing the concept of shared decision making between patient and physician [16].
This study analyzed patient preferences for two EAC prevention strategies. The cohort demographics were representative of typical individuals with BE with respect to age, sex, and gender. When informed about the benefits and harms of an ablation option and chemoprevention option for EAC prevention, patients with BE preferred the endoscopic ablation strategy—with the majority (78%) willing to undergo ablation, and approximately half (53%) willing to select chemoprevention.
The risk estimates presented to subjects, derived from our prior study of patient preferences [13], deserve critical inspection. Prior estimates of annual EAC risk for patients with BE, 0.5% per year, were based largely on a study to assess the presence of publication bias in the reporting of EAC risk [17]. Subsequent studies have led to substantially lower estimates of EAC risk. A large-scale Danish study reported an annual incidence rate of 0.29% -- 0.12% if cases of prevalent EAC were excluded [18]. A multicenter United States registry estimated an annual progression from nondysplastic BE to EAC of 0.3% [19]. Assuming that this rate is constant over a lifetime, and after hazarding using DEALE transformation method [20], over a 30 year time horizon the estimated progression rate to EAC is approximately 9%--similar to the 10% risk lifetime risk described to study subjects. A reduction in lifetime risk from 10% to 5% with aspirin use was based largely on a meta-analysis reporting a protective effect of aspirin use (OR 0.5 [95% CI 0.38-0.66]) on esophageal cancer development [6].
Development of safe and effective endoscopic treatment modalities for eradication of BE has allowed consideration of whether endoscopic treatment, previously reserved for patients with HGD or more advanced pathology, may be appropriate for an expanded at risk pool of patients with less advanced pathology including nondysplastic BE. The ablation option presented as Treatment A, though not explicitly specified in the scenario, has a risk profile (5% stricture rate) most similar to RFA. With respect to reported endoscopic outcomes for endoscopic therapy of BE, the magnitude of risk reduction presented in Treatment A is most similar to a 50% reduction in risk of progression to EAC at 5-year follow-up after treatment of BE with HGD with porfimer sodium PDT [8]. While these data may not be directly applicable to endoscopic management of patients with nondysplastic BE, compelling data regarding the estimated magnitude of reduction in lifetime EAC risk following RFA of nondysplastic BE do not exist at present.
Decision models suggest that the cost-utility of ablation for BE would be influenced by the frequency of endoscopic surveillance [21]. A hypothesis of this study was that frequency of surveillance would influence patient willingness to undergo treatment—specifically, that either endoscopic ablation or chemoprevention might become an increasingly attractive option with decreasing frequency of required surveillance. Contrary to this hypothesis, however, altering the frequency of surveillance did not influence willingness of subjects to undergo ablation or chemoprevention in this study. The reasons for this—for instance, whether endoscopic surveillance provides some measure of reassurance in a cohort recruited largely from individuals enrolled in and accustomed to endoscopic surveillance programs—are unclear. Moreover, the fact that subjects were recruited from a surveillance cohort is a potential source of bias in this study. There are data to suggest that endoscopic surveillance may be overutilized among patients with BE, with endoscopy performed more frequently than recommended by practice guidelines [22]. Such utilization may be driven by both physician factors (such as medical-legal fears and financial incentives) and patient factors (such as access to care and medical copayments), which may be either promoters of or barriers to use [23]. Future investigation could include assessment of preferences for endoscopic intervention versus chemoprevention among patients naïve to endoscopic screening and surveillance.
The proportion of patients willing to select aspirin as a chemoprevention strategy was considerably lower than that observed in a prior patient preference study (76%) [13], despite an identical risk-benefit scenario, suggesting that the appeal of chemoprevention may be diminished by the appeal of a competing endoscopic ablation prevention strategy. Part of this effect may be due to “framing” bias, in which the order of questions could influence subjects’ perceptions of risk and response to treatment options; in this case, the order of the scenarios was not randomized and the option of Treatment A (ablation) was always presented before the option of Treatment B (aspirin). However, the relatively low enthusiasm for aspirin as a chemopreventive strategy in this study may have implications for future EAC prevention strategies—particularly if these evolve in complexity to include polypharmacy such as aspirin plus PPI, or conceivably plus statin therapy [24], rather than aspirin monotherapy alone.
An alternative, valid interpretation of the study findings could be that some patients prefer endoscopy, some prefer chemoprevention, some would choose both, and still others would choose neither. A values clarification exercise accompanying a formal patient decision aid may have helped further describe the effects of treatment options in this case [25], and such preference elicitation might further assist in understanding how a shared decision making process arrives at a patient-centered decision – which, by necessity and definition, may differ among patients. In particular, it is possible that personalized data with respect to risk for peptic ulcer or cardiovascular disease might influence treatment choice. Post hoc subgroup analysis detected no evidence of a preference for either endoscopic intervention or chemoprevention among subjects on aspirin therapy (N=33), subjects with prior history of peptic ulcer disease (N=15), or subjects with prior history of coronary disease (N=14), although testing for statistical significance was limited by sample size.
Additional limitations include the fact that the survey, although previously utilized for research purposes [13], is not a validated instrument or formal decision aid, and did not include all items or criteria suggested for decision aides, including a values clarification exercise, an effort to tailor outcome probabilities to personalized risk factors, and description of others’ experience [25, 26]. Attempts to present treatment options in a succinct scenario aim to provide accurate information while not unduly influencing cognitive burden [14]. Risk estimates were presented to patients by percentiles and pictographs, but not by ratios with realistic denominators. Furthermore, in an effort to maintain a survey which could be completed by patients in a time-sensitive manner, additional survey items to allow further sensitivity analyses (thresholds of benefit or risk which might alter a given subject's willingness to undergo treatment), most important factor analysis, and decision quality measures (such as decisional uncertainty, decisional satisfaction, involvement in decision process) were not included.
In summary, when presented with the option of endoscopic or pharmacologic therapy of equivalent efficacy in reducing lifetime EAC risk and known adverse effect profiles, in this simulated scenario patients with BE preferred endoscopic intervention over chemoprevention for EAC prevention. Modifying the frequency of surveillance did not alter patient treatment choice. Further investigation of the shared decision making process regarding preventive interventions in patients with BE may prove valuable in the design of EAC prevention strategies.
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health: grant R01CA140574 to Dr. Hur. Dr. Wani is supported by the AGA Takeda Research Scholar Award in GERD and Barrett's esophagus. Research Electronic Data Capture (REDCap) supported by grant UL1 TR000445 from NCATS/NIH.
Appendix 1—Treatment scenarios (male version)
We will ask you about your health and esophagus cancer prevention and then ask you to make some choices.
Scenario A:
As a patient with Barrett's esophagus, you are at higher risk for getting cancer of your esophagus. There are treatments available that may help prevent esophagus cancer. Each treatment has risks and side effects. Please read the following description of one of these treatments, Treatment A. Once you have read the description, please answer the questions at the bottom of the page.
Treatment A:
Is a treatment performed during an upper endoscopy.
Will remove Barrett's tissue if successful.
Lowers your risk of getting esophagus cancer by 50%. As a person with Barrett's, your lifetime risk of getting esophagus cancer is about 10% (or 10 in 100), so this treatment reduces your risk to 5% (or 5 in 100) (see pictures).
Harms and side effects
- Causes esophageal stricture in 5% of patients (or 5 in 100) who undergo treatment A (see pictures). This is scar tissue in the esophagus which may result in di fficulty swallowing. Stretching/dilation of the esophagus can correct this side effect in most patients. At least one additional upper endoscopy is required to perform the stretching.
Treatment A summary: Benefits Harms • Lowers your lifetime risk of esophagus cancer from 10% to 5% • Increases risk of esophageal stricture from 0% to 5% Without treatment A, you are expected to have endoscopies every 3-5 years for surveillance. 1) Would you be willing to undergo Treatment A if you required endoscopy for surveillance every 3-5 years after the procedure? Yes No 2) Would you be willing to undergo Treatment A if you required endoscopy for surveillance every 10 years after the procedure? Yes No 3) Would you be willing to undergo Treatment A if you never needed another endoscopy again? Yes No
Scenario B:
Now please think about a DIFFERENT treatment that helps prevent esophagus cancer, Treatment B.
Treatment B:
Is a pill that you take once a day.
Can be purchased over the counter (you don’t need a prescription).
Lowers your risk of getting esophagus cancer by 50%. As a person with Barrett's, your lifetime risk of getting esophagus cancer is about 10% (or 10 in 100), so this medicine reduces your risk to 5% (or 5 in 100) (see pictures).
Lowers your risk of a heart attack by 30%. The average man's lifetime risk of a heart attack is 50% (or 50 in 100), so Treatment B lowers your risk of a heart attack to 35% (or 35 in 100).
Harms and side effects
- Increases the risk of problems in your gut (stomach or intestines) by 75%. This may include getting an ulcer or having bleeding in your stomach or intestines. Over a lifetime, 10% of people (or 10 in 100) will have an ulcer or bleeding in their gut. Treatment B increases your risk of having these problems to 18% (or 18 in 100).
Treatment B summary: Benefits Harms • Lowers your lifetime risk of esophagus cancer from 10% to 5%
• Lowers your lifetime risk of heart attack from 50% to 35%• Increases the lifetime risk of having an ulcer or bleeding in the gut from 10% to 18% Without treatment B, you are expected to have endoscopies every 3-5 years for surveillance. 4) Would you be willing to undergo Treatment B if you required endoscopy for surveillance every 3-5 years after the procedure? Yes No 5) Would you be willing to undergo Treatment B if you required endoscopy for surveillance every 10 years after the procedure? Yes No 6) Would you be willing to undergo Treatment B if you never needed another endoscopy again? Yes No
Appendix
Appendix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study design: PY, EO, CH; data acquisition: TG, EH, TH, AP, KB, LH, PMK; data interpretation: PY, SW, CH; statistical analysis: KP; drafting of manuscript: PY; revision of manuscript: PY, SW, CH.
Disclosure: None of the authors has any financial disclosures or potential conflicts of interest to report relevant to the content of this manuscript:
References
- 1.Provenzale D, Kemp JA, Arora S, Wong JB. A guide for surveillance of patients with Barrett's esophagus. Am J Gastroenterol. 1994;89:670–680. [PubMed] [Google Scholar]
- 2.Provenzale D, Schmitt C, Wong JB. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 3.Soni A, Sampliner RE, Sonnenberg A. Screening for high-grade dysplasia in gastroesophageal reflux disease: is it cost-effective? Am J Gastroenterol. 2000;95:2086–93. doi: 10.1111/j.1572-0241.2000.02173.x. [DOI] [PubMed] [Google Scholar]
- 4.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 5.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 6.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:147–156. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 7.Falk GW, Buttar NS, Foster NR, et al. A combination of esomeprazole and aspirin reduces tissue concentration of prostaglandin E(2) in patients with Barrett's esophagus. Gastroenterology. 2012;143:917–926. doi: 10.1053/j.gastro.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 10.Fleischer DE, Odze R, Overholt BF, et al. The case for endoscopic treatment of non-dysplastic and low-grade dysplastic Barrett's esophagus. Dig Dis Sci. 2010;55:1918–1931. doi: 10.1007/s10620-010-1218-1. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Graham DY. Routine polypectomy for colorectal polyps and ablation for Barrett's esophagus are intellectually the same. Gastroenterology. 2011;140:386–388. doi: 10.1053/j.gastro.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Wani S, Puli SR, Shaheen NJ, et al. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastro. 2009;104:502–513. doi: 10.1038/ajg.2008.31. [DOI] [PubMed] [Google Scholar]
- 13.Hur C, Broughton DE, Ozanne E, et al. Patient preferences for the chemoprevention of esophageal adenocarcinoma in Barrett's esophagus. AM J Gastroenterol. 2008;103:2432–2442. doi: 10.1111/j.1572-0241.2008.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlong W FD, Torrance GW, Barr R, Horsman J. Guide to Design and Development of Health-State Utility Instrumentation. Centre for Health Economics and Policy Analysis Working Paper Series #90-9. McMaster University; Hamilton, Ontario, Canada: 1990. [Google Scholar]
- 15.Malenka DJ, Baron JA, Johansen S, et al. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;8:543–548. doi: 10.1007/BF02599636. [DOI] [PubMed] [Google Scholar]
- 16.American Gastroenterological Association. Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett's esophagus. Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 18.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 19.Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett's esophagus have low risk for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2011;9:220–227. doi: 10.1016/j.cgh.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (The “DEALE”). II. Use in medical decision-making. Am J Med. 1982;73:889–897. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 21.Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett's esophagus. Gastroenterology. 2012;143:567–575. doi: 10.1053/j.gastro.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crockett SD, Lipkus IM, Bright SD, et al. Overutilization of surveillance endoscopy in nondysplastic Barrett's esophagus: a multicenter study. Gastrointest Endosc. 2012;75:23–31. doi: 10.1016/j.gie.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naik AD, Hinojosa-Lindsey M, Arney J, et al. Choosing wisely and the perceived drivers of endoscopy use. Clin Gastroenterol Hepatol. 2013;1:753–755. doi: 10.1016/j.cgh.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandre L, Clark AB, Bhutta HY, et al. Statin use is associated with reduced risk of histologic subtypes of esophageal cancer: a nested case-control analysis. Gastroenterology. 2013 Dec 5; doi: 10.1053/j.gastro.2013.11.046. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Elwyn G, O'Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor AM, Bennett C, Stacey D, et al. Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis. Med Decis Making. 2007;27:554–574. doi: 10.1177/0272989X07307319. [DOI] [PubMed] [Google Scholar]