Abstract
Intracellular zinc levels are tightly regulated since zinc is an essential cofactor for numerous enzymes, yet can be toxic when present in excess. The majority of intracellular zinc is tightly associated with proteins and is incorporated during synthesis from a poorly defined pool of kinetically labile zinc. In Bacillus subtilis, this labile pool is sensed by equilibration with the metalloregulator Zur, as an indication of zinc sufficiency, and by CzrA, as an indication of zinc excess. Here, we demonstrate that the low molecular weight thiol bacillithiol (BSH) serves as a major buffer of the labile zinc pool. Upon shift to conditions of zinc excess, cells transiently accumulate zinc in a low molecular weight pool, and this accumulation is largely dependent on BSH. Cells lacking BSH are more sensitive to zinc stress, and they induce zinc efflux at lower external zinc concentrations. Thiol reactive agents such as diamide and cadmium induce zinc efflux by interfering with the Zn-buffering function of BSH. Our data provide new insights into intracellular zinc buffering and may have broad relevance given the presence of BSH in pathogens and the proposed role of zinc sequestration in innate immunity.
Introduction
Metal ions are essential for life and are required cofactors for ~30% of proteins. Zinc is one of the few metal ions required for all life. Zn(II) functions both as a Lewis acid catalyst and a structural cofactor for numerous enzymes. Due to its ubiquity, overall zinc quotas for many cells are in the range of ~0.1–0.5 mM when averaged over the cell volume (Eide, 2006), which corresponds to ~106 atoms per cell for a bacterium of the size of Bacillus subtilis. As expected from its position in the Irving-Williams series (which describes general trends in the avidity with which metals interact with ligands; Irving & Williams, 1953), Zn(II) often binds to enzymes with very high affinity (measured dissociation constants in the femtomolar range) and, correspondingly, may have very slow off rates (Maret & Li, 2009). However, information on the speciation and exchange kinetics of Zn(II) in vivo is still very limited.
Conceptually, intracellular Zn(II) can be considered either sequestered (non-exchangeable) or kinetically labile. However, this simple nomenclature belies the likely complexity of this pool; the actual kinetics of exchange will vary widely depending on the chemical properties and relative abundance of the various potential zinc ligands. For some proteins, such as the B. subtilis Fur family regulator PerR, Zn(II) is stably bound in a Cys4 structural site and is only released upon protein denaturation (Lee & Helmann, 2006). In such cases, the Zn(II) may remain bound for the lifetime of the protein, and the sequestered Zn(II) is not available for exchange with other proteins. For other proteins, Zn(II) binding and dissociation may be more rapid, perhaps facilitated by ligand exchange reactions (Maret & Li, 2009, Colvin et al., 2010). In these cases, the bound Zn(II) is considered exchangeable and may contribute to the labile Zn(II) pool. Examples include metallothionein, some Zn(II) enzymes, and Zn(II)-sensing metalloregulators (Maret & Li, 2009, Colvin et al., 2010). An intermediate example is provided by those Escherichia coli mononuclear enzymes that are inactivated by mismetallation with Zn(II) under oxidative stress conditions. In this case, reactivation (which is limited by the rate of Zn removal) occurs on a timescale of many minutes in a reaction that may be facilitated by cysteine-dependent ligand exchange reactions (Gu & Imlay, 2013). In addition to its interactions with proteins, the intracellular labile zinc pool is buffered by other molecular constituents of the cell (Colvin et al., 2010).
Zn metalloregulatory proteins sense the labile zinc pool by reversible binding (Helmann et al., 2007, Guerra & Giedroc, 2012, Waldron et al., 2009). Like many bacteria, B. subtilis senses zinc sufficiency by binding of Zn(II) to Zur which activates the repressor to bind DNA (Gaballa & Helmann, 1998, Ma et al., 2011, Ma & Helmann, 2013). When Zn(II) levels are sub-optimal, the Zur regulon is derepressed leading to expression of a high affinity zinc uptake system (ZnuABC, formerly YcdHI-YceA) and its associated accessory protein (ZinT), a putative metallochaperone (YciC), a Zn-independent isozyme for folate biosynthesis (YciA), and alternative (non-Zn-requiring) paralogs of three ribosomal proteins (S14, L31, and L33) (Gaballa et al., 2002, Gabriel & Helmann, 2009, Gabriel et al., 2008, Ma & Helmann, 2013). The Zn-independent ribosomal proteins function both in a strategy of Zn-mobilization (by displacement of Zn(II)-containing proteins from the large subunit of the ribosome; Nanamiya et al., 2004, Akanuma et al., 2006) and to enable the continued synthesis of ribosomes despite severe zinc limitation (in the case of the S14 paralog; Natori et al., 2007).
Just as Zur equilibrates with the labile zinc pool to monitor zinc sufficiency, the CzrA repressor binds Zn(II) under conditions of excess (Moore et al., 2005, Osman & Cavet, 2010). Binding of Zn(II) to CzrA triggers derepression of the CadA efflux ATPase and the CzcD (cobalt-zinc-cadmium) resistance protein. As their names suggest, the CadA and CzcD resistance systems were originally described as contributing to cadmium resistance, in addition to their role in resistance to excess zinc (Gaballa & Helmann, 2003, Solovieva & Entian, 2002, Guffanti et al., 2002, Moore et al., 2005). Together, Zur and CzrA sense changes in the labile Zn(II) pool and effect changes in gene expression that mediate zinc homeostasis (Ma et al., 2011, Ma & Helmann, 2013).
Labile zinc pools have been investigated in a variety of eukaryotic cell types where buffering by metallothionein often plays a key role (Colvin et al., 2010). In bacteria lacking metallothionein, glutathione (GSH) has been implicated as one buffering agent (Helbig et al., 2008). In other cases, including the yeast mitochondrial matrix (Atkinson et al., 2010), the relevant low molecular weight (LMW) ligands have yet to be identified. Operationally, the labile Zn pool has been defined as that portion of cellular zinc accessible to chemical chelators and this is presumed to be comparable to that fraction of the Zn pool that can be sensed by metalloregulatory proteins (Thambiayya et al., 2012).
Although the detailed speciation of zinc within the labile pool is still uncertain, insights into the effective (buffered) concentration of free Zn(II) have been derived from measurements of the biochemical affinity of zinc-sensing metalloregulators for their metal ligands. In E. coli, for example, Zur binds Zn(II) with an affinity (Kd) in the femtomolar (fM) range, whereas the regulator of zinc efflux ZntR binds Zn(II) with a slightly lower affinity (Outten & O'Halloran, 2001). These findings led to the conclusion that there is no free (fully hydrated) Zn(II) in the cell at equilibrium (since 1 atom per cell corresponds to a concentration of ~1 nM). Similarly, studies in B. subtilis have determined that Zur is activated to bind DNA when free Zn(II) levels approach 1 fM (Ma et al., 2011). Although these observations reveal the effective concentration of free cytosolic Zn(II) at equilibrium, the magnitude and composition of the labile zinc pool has remained mysterious.
Here, we explore the contribution of bacillithiol (BSH), the dominant LMW thiol in B. subtilis (Gaballa et al., 2010, Newton et al., 2009), to zinc homeostasis. Zn(II) binds with high affinity to BSH and is coordinated by both its thiolate and carboxylate moieties. Genetic and physiological studies demonstrate that this BSH zinc complex comprises a physiologically relevant pool of chelated Zn(II). This pool of Zn is mobilized under conditions that deplete cellular thiol pools, including treatment with diamide or the thiophilic heavy metal cadmium, and the resulting increase in Zn(II) is sensed by the CzrA metalloregulatory protein.
Results
BSH binds Zn(II) with high affinity
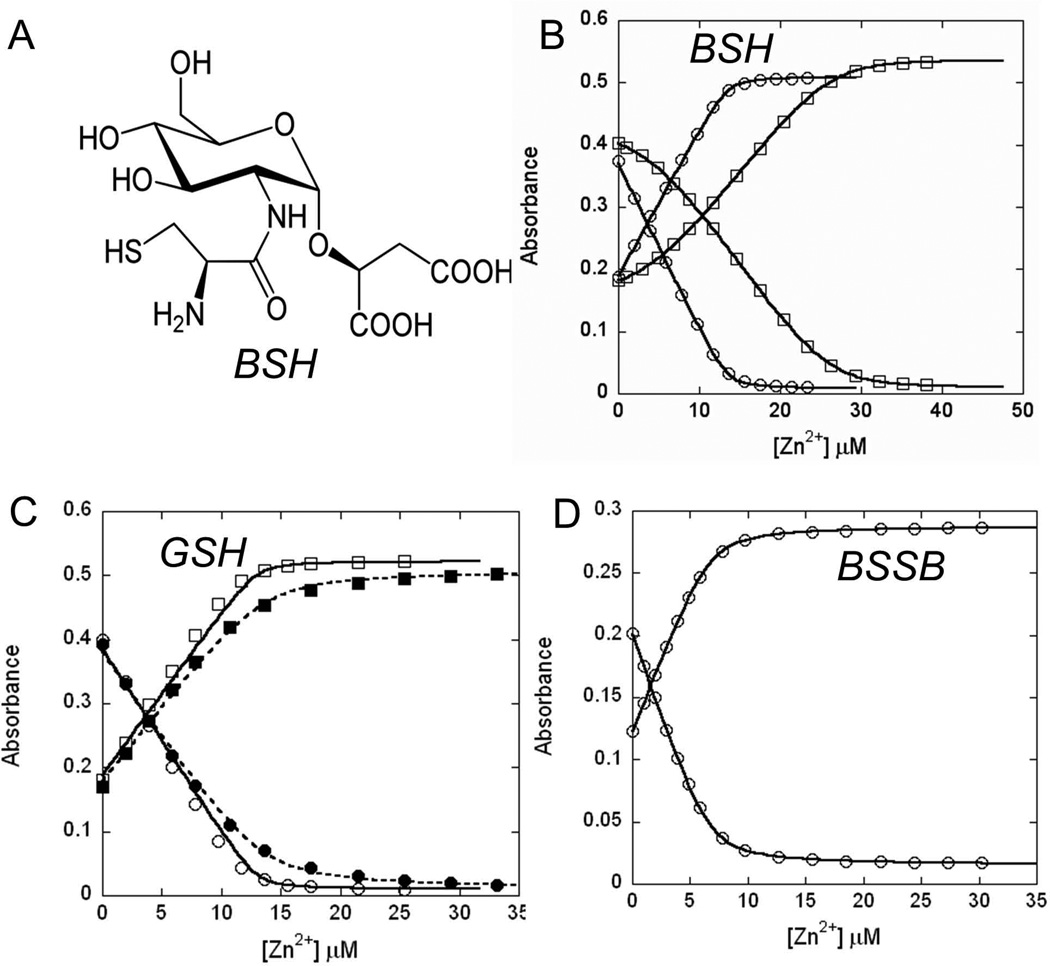
BSH, the α-anomeric glycoside of L-cysteinyl-D-glucosaminyl-L-malate (Fig. 1A) (Newton et al., 2009), is the dominant LMW thiol in B. subtilis with cytosolic concentrations in the millimolar range (Sharma et al., 2013, Gaballa et al., 2010, Helmann, 2011). The thiol group of BSH is slightly more acidic than that in GSH, with ~22% ionization to the thiolate at pH 7.7 (Sharma et al., 2013). We used a spectroscopy-based competition assay to determine the affinity of BSH for Zn(II). These assays were carried out at pH 7.7, the measured intracellular pH for B. subtilis (Kitko et al., 2009). Under our assay conditions, the competitor magfura-2 (MF2) binds one Zn(II) with an affinity of 1.7(±0.1) × 107 M−1 (Fig. 1B), a value similar to the reported affinity of 5.0 × 107 M−1 (VanZile et al., 2000, Simons, 1993). When Zn(II) was titrated into a mixture of MF2 and BSH, significant competition occurs as indicated by the non-linear change of absorbance (Fig. 1B). These data best fit a model in which BSH can form either a 1:1 or 2:1 complex with Zn(II). Initially, BSH is present in excess and addition of Zn(II) leads to the formation of a BSH2:Zn(II) complex. As BSH is consumed, a BSH:Zn(II) complex forms toward the end of the titration. The data are best fit by two step-wise binding constants of K1=7.5 × 106 M−1 and K2=2.5 × 105 M−1, for a cumulative binding constant β2 of 1.9 × 1012 M−2. This allows the physiological BSH pool to buffer Zn in the nM-pM range (see Discussion). Notably, BSH binds Zn(II) with a much higher affinity than the other common biological LMW thiol, GSH. Competition experiments with GSH and MF2 allow estimation of the GSH:Zn(II) binding affinity as ~3 × 104 M−1 (Fig. 1C).
Fig 1. BSH binds to Zn(II) with high affinity.
(A) Structure of BSH. (B) Zn(II) was titrated into a mixture of 13.5 µM Magfura-2 without (circles) and with 19.2 µM BSH (squares). Absorbance at 325 nm (increasing values) and 366 nm (decreasing values) were plotted. In the absence of BSH, magfura-2 (MF2) binds one Zn(II) with an affinity of 1.7(±0.1) × 107 M−1 (see SI Fig. 1a for comparison with theoretical curves at 10-fold higher and lower affinity), a value similar to the reported affinity of 5.0 × 107 M−1 (VanZile et al., 2000, Simons, 1993). The data from the competitive binding experiment with BSH and Magfura-2 are best fit to a model where BSH forms a 2:1 complex with Zn(II) with stepwise affinity constants of K1= 7.5 (±0.0) × 106 M−1, K2= 2.5 (±0.0) × 105 M−1 (or the accumulative binding constant β2= 1.9 × 1012 M−2) (see SI Fig. 1b for comparison with theoretical curves at 10-fold higher and lower values for K1 and K2). (C) Zn(II) was titrated into a mixture of 13.5 µM Magfura-2 and 18.8 µM (open symbols, solid line) or 188 µM GSH (filled symbols, dashed line). The data acquired with 188 µM GSH allows an estimation of the GSH-Zn binding constant of ~3×104 M−1. Conditions: 20 mM HEPES, pH 7.7, 0.15 M NaCl, 0.1 mM TCEP. (D) BSSB binds Zn(II) with low affinity. Zn(II) was titrated into a mixture of 6.8 µM Magfura-2 and 200 µM BSSB. Only minor competition was observed suggesting an affinity of ~104 M−1 (see SI Fig. 1c for comparison with theoretical curves at 10-fold higher and lower affinity) Conditions: 20 mM HEPES, pH 7.7, 0.15 M NaCl.
Zn(II) is a moderately thiophilic metal ion, so we reasoned that the BSH thiol group was likely to be a key contributor to Zn(II) binding. Evidence for a critical role of the thiol group in Zn(II) binding derives from analysis of oxidized BSH (BSSB), in which the thiol groups are linked as a disulfide. Competition assays with MF2 indicate that BSSB bind Zn(II) much weaker than BSH with an estimated Ka of ~104 M−1 (Fig. 1D). Similarly, we failed to observe significant binding with BOH, a BSH derivative in which the thiol (derived from Cys) is replaced by an alcohol (derived from Ser) (data not shown). Direct evidence for the presence of thiolate-metal bonds was obtained using Co(II) as a surrogate for Zn(II). We monitored the binding of Co(II) and BSH spectroscopically by following the ligand to metal charge transfer (LMCT) indicative of thiolate-Co(II) coordination (SI Fig. S2). In proteins, the presence of S→Co(II) LMCT typically leads to absorption in the near UV with an extinction coefficient at ~320 nm indicative of the number of thiolate ligands (ε320 ~900 to 1200 M−1 cm−1 per S→Co(II) bond; VanZile et al., 2000). Titration of Co(II) into BSH leads to substantial absorbance in the near UV, consistent with thiolate coordination to Co(II). To assess the contribution of the malate carboxylates to Zn(II) binding, we used a previously described BSH derivative (MeO-GlcN-Cys) in which the malate portion is substituted with the uncharged methyl aglycone (Sharma et al., 2013). Unlike BSH, MeO-GlcN-Cys formed a 3:1 complex with Zn with an overall association constant β3 of 3.0 × 1016 M−3 (SI Fig. S3). The coordination complex is distinct from BSH, suggesting that the carboxylate groups are normally involved in metal coordination.
In summary, BSH uses both the thiolate and carboxylate groups to form a complex with Zn(II) with physiologically relevant affinity. Although we do not have a structure of the complex, we suggest that the dominant BSH2:Zn complex likely has two sulfur (thiolate) and two oxygen ligands, presumably from the malate carboxylates, whereas the 3:1 complex with the aglycone likely has three thiolate ligands to Zn(II).
BSH functions as a Zn(II) buffer
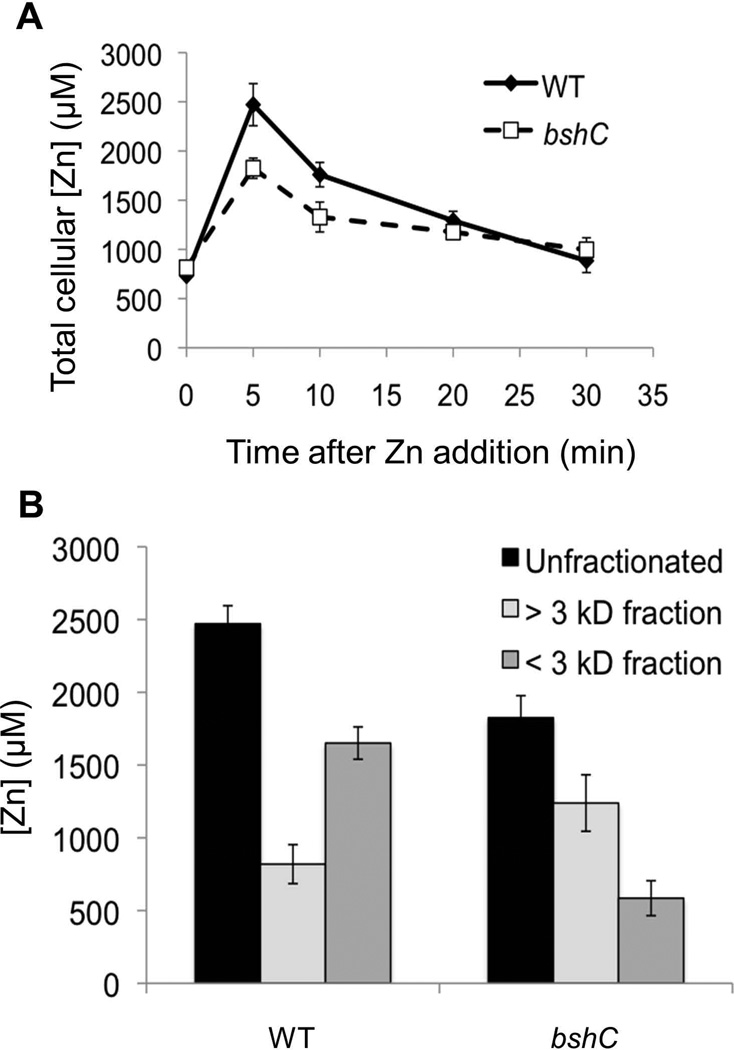
If BSH binds a significant fraction of the total cellular Zn(II), we hypothesized that cells lacking BSH would contain less total Zn(II) than wild-type (WT) cells. To test this notion, we used ICP-MS to monitor the total Zn(II) quota. However, both the WT and the isogenic bshC null mutant strain, which lacks the cysteine-adding enzyme that functions in the last step of BSH synthesis (Gaballa et al., 2010), had equivalent Zn(II) quotas (~800 µM) when growing in LB medium as measured either in logarithmic growth (Fig. 2A; time zero) or stationary phase (data not shown). Next, we determined the Zn(II) quota in cells at various times after challenge with 200 µM Zn(II). Five minutes after imposition of Zn(II) stress, the total Zn(II) quota had increased dramatically, but bshC mutant cells contained ~25% less Zn(II) compared to wild-type (Fig. 2A). Zn(II) levels begin to decrease after 10 minutes, most likely due to the induction of the CadA and CzcD Zn(II) efflux systems. Finally, after about 30 minutes, Zn(II) levels in both wild-type and bshC cells reach a steady state level nearly equivalent to that in the absence of Zn(II) stress, suggesting re-establishment of homeostasis.
Fig 2. BSH function to buffer Zn(II) under zinc stress conditions.
(A) Change of total cellular Zn(II) content in WT (CU1065) and bshC (HB11079) mutant cells following treatment of 250 µM Zn(II) for different time periods. (B) Fraction of total Zn(II) content of WT and bshC cell lysate fractionated through an Amicon ultracentrifugation filter (3 kD molecular weight cutoff) after exposure to 200 µM Zn(II) for 5 min. The data shown represent the mean and standard deviation of three biological replicates.
To identify the fraction of the Zn(II) quota associated with the LMW pool, we quantified the Zn(II) present in cell lysate from Zn(II)-challenged cells after passage through a 3000 Da cut-off ultrafiltration column. Since the molecular weight of BSH is 398 Da (or 861 Da for a BSH2:Zn(II) complex), metals associated with BSH are expected to be recovered in the filtrate. Prior to Zn(II) shock, both WT and BSH null cells contained ~800 µM total Zn(II), with <10% of this Zn(II) in the LMW pool (SI Fig. S4). In contrast, five minutes after challenge with 200 µM Zn(II) the cellular Zn(II) quota has increased several-fold, with ~2/3 partitioning into the LMW fraction in wild-type cells. Significantly, there was about 3-fold less Zn(II) in this LMW fraction in BSH null cells (Fig. 2B). Thus, BSH allows Zn(II)-stressed cells to accumulate substantially higher amounts of Zn(II), and this Zn(II) is largely present in a LMW pool.
BSH protects from Zn(II) toxicity in cells defective for Zn(II) efflux
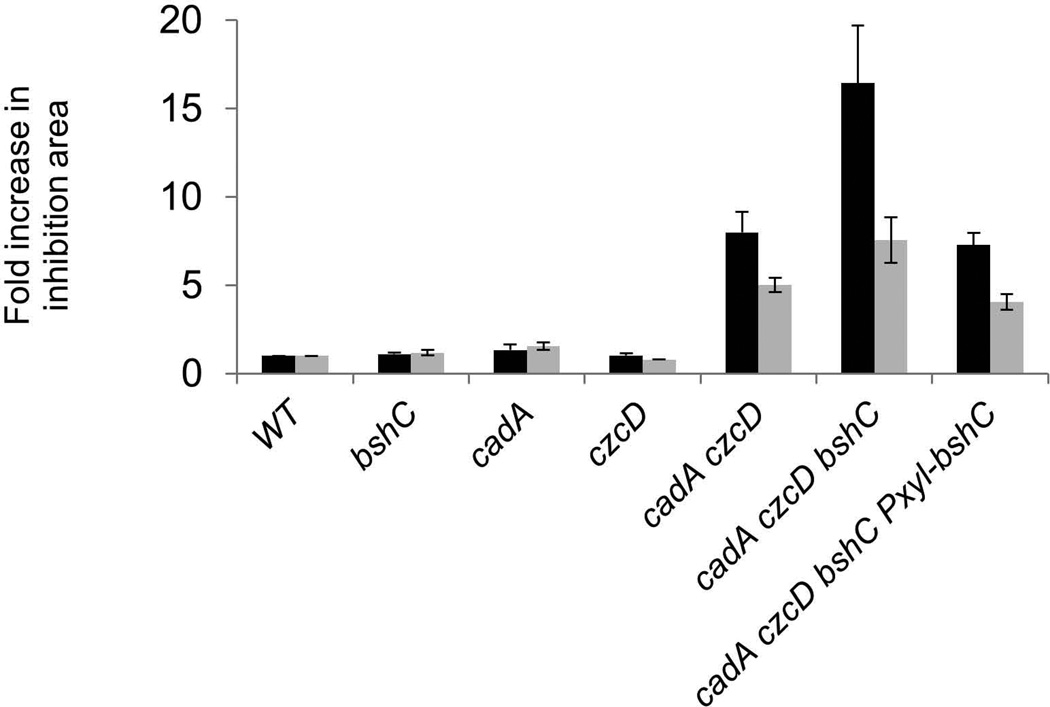
Both CzcD (Guffanti et al., 2002) and CadA (Gaballa & Helmann, 2003) have been previously implicated in Zn(II) and Cd(II) efflux. Using a zone-of-inhibition assay, we here demonstrate that these two transport systems are functionally redundant; single mutants have only a modest effect on metal sensitivity whereas the czcD cadA double mutant is highly sensitive to both Zn(II) and Cd(II) (Fig. 3). Cells lacking BSH (bshC null mutant) were not detectably affected in resistance to Zn(II) or Cd(II) in the wild-type background, but loss of BSH exacerbated the metal sensitivity of the export defective czcD cadA double mutant strain. This phenotype could be fully complemented by expression of a xylose-inducible copy of bshC. These results indicate that BSH protects against the toxic effects of thiophilic metal ions, presumably in part by serving as a buffer, and that this function is most apparent in cells lacking the ability to efflux excess metal. In addition, these results imply that BSH is neither an obligatory co-substrate nor a chaperone for the CadA P1B-type ATPase (Arguello et al., 2011), nor is it required for CzcD, as anticipated from prior biochemical studies that demonstrated an antiport mechanism (Guffanti et al., 2002).
Fig 3. BSH plays a protective role in cells deficient in Zn(II) efflux.
Susceptibility of wild-type and mutant strains (WT, CU1065; bshC, HB11212; cadA, HB11393; czcD, HB11394; cadA czcD, HB11395; cadA czcD bshC, HB11396; cadA czcD bshC Pxyl-bshC, HB11427) to Zn(II) (black bars) or Cd(II) (grey bars) was determined by disk diffusion assay. The data is expressed as the average fold-increase (± standard deviation) of the area of the zone of inhibition relative to wild-type.
Induction of the CadA efflux system by Zn(II) is increased in cells lacking BSH
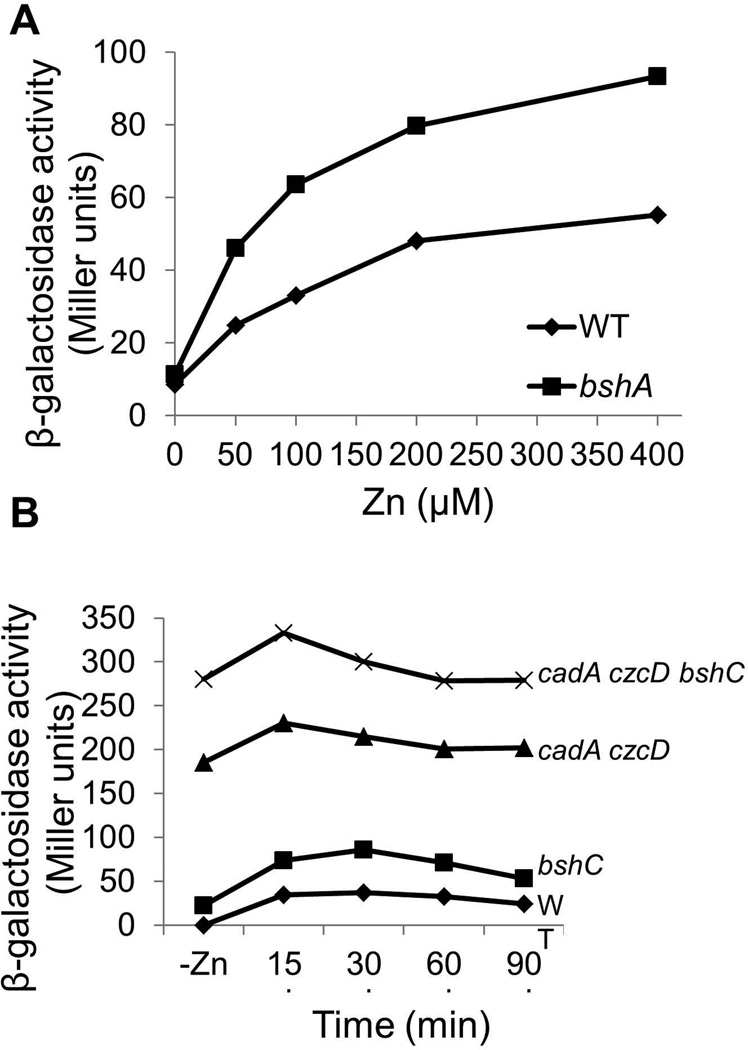
The millimolar levels of BSH in the cytosol represent a substantial pool of potential Zn(II) binding ligands. To determine if BSH affects the ability of Zn(II) to induce the CzrA-regulated Zn(II) efflux systems we monitored the expression of a PcadA-lacZ fusion. Cells lacking BSH displayed an increased induction when compared to wild-type at all tested Zn(II) concentrations, suggesting that CzrA had an increased responsiveness to Zn(II) in the absence of the competing ligand BSH (Fig. 4A). Similar results were seen when induction of a second CzrA-repressed gene, czcD, was monitored as a function of time (Fig. 4B). Even prior to challenge with external Zn(II), the basal expression of the PcadA-lacZ fusion was slightly elevated in the bshC null mutant, and expression was dramatically elevated in the efflux defective cadA czcD double mutant; this derepression was further enhanced in cells additionally lacking BSH (Fig. 4B). These data are consistent with a significant loss of intracellular Zn(II) buffering capacity in the absence of BSH.
Fig 4. Induction of cadA in response to Zn(II) is increased in cells lacking BSH.
(A) Induction of PcadA-cat-lacZ as a function of Zn(II) concentration. Wild-type (HB11058) cells (diamonds) and an isogenic bshA null (HB11061) mutant (squares) carrying a cadA-cat-lacZ reporter fusion were grown to mid-logarithmic phase in LB medium with the indicated concentration of Zn(II) and assayed for β-galactosidase. (B) PcadA-lacZ activity in wild-type and mutant strains was monitored as a function of time after 50 µM Zn(II) addition. Representative results are shown for experiments repeated at least three times.
Induction of the CadA efflux system by diamide is decreased in cells lacking BSH
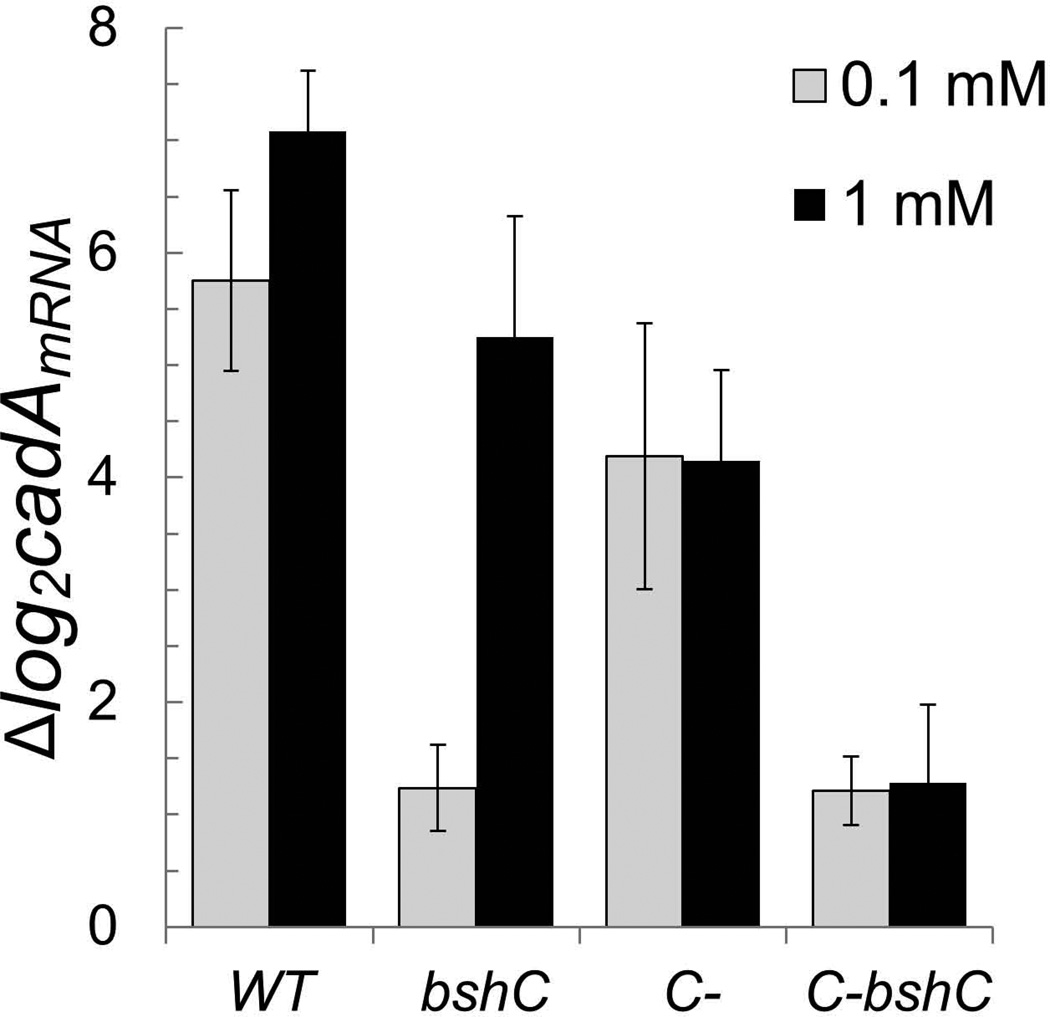
CadA is one of the most highly induced genes upon treatment with the thiol oxidant diamide (Leichert et al., 2003). Derepression of the CzrA regulon by diamide was unexpected since CzrA lacks cysteine residues, and is therefore not an obvious target for inactivation by disulfide stress. Using qRT-PCR, we confirmed that cadA mRNA levels increased >50-fold in wild-type cells upon treatment with either 0.1 mM or 1 mM diamide for 5 minutes (Fig. 5). Remarkably, induction by 0.1 mM diamide was almost completely eliminated (~2-fold residual induction) in cells lacking BSH. However, a full response was still obtained with 1 mM diamide. These results indicate that the major effect of 0.1 mM diamide is to oxidize intracellular BSH pools to BSSB with concomitant release of bound Zn(II), and this released Zn(II) is then sensed by CzrA leading to induction of cadA.
Fig 5. Induction of cadA in response to diamide is reduced in cells lacking mobilizable Zn(II) pools.
Expression of cadA was monitored by qRT-PCR in wild-type (CU1065), bshC (HB11212), C- (rpmGA rpmGB rpmE mutant; HB6916), and C- bshC (HB15912) mutant strains after the treatment with 0.1 or 1 mM diamide for 5 minutes. 23S rRNA was used as an internal control and the fold-change between treated and untreated samples were plotted. Results are the mean and standard deviation of 3 biological replicates.
Ribosomal proteins represent a second pool of mobilizable Zn(II)
Induction of cadA mRNA was similar in both wild-type and BSH null cells when treated with 1.0 mM diamide. Previous studies have suggested that the L31 and L33 Zn(II) metalloproteins, associated with the surface of the large subunit of the ribosome, each contain a Cys4:Zn site. This Zn(II) that can be mobilized by a protein displacement mechanism in times of Zn(II) deprivation (Akanuma et al., 2006, Gabriel & Helmann, 2009, Nanamiya et al., 2004). To determine whether or not Zn(II)-binding ribosomal proteins contribute to Zn(II) mobilization in response to diamide we used a strain (designated C-) lacking both of the Zn(II)-containing L33 paralogs (RpmGA and RpmGB) and L31 (RpmE). As reported previously, this strain is viable and still encodes the non-Zn(II) binding L31 (YtiA) and L33 (YhzA) paralogs that are regulated by Zur (Akanuma et al., 2006, Gabriel & Helmann, 2009, Nanamiya et al., 2004). Induction of cadA expression after diamide treatment is only modestly lower in the C- mutant than in wild-type (Fig. 5). However, Induction of cadA by diamide was greatly reduced in a C- bshC mutant (lacking both the zinc-storing ribosomal proteins and BSH) even when cells were treated with 1.0 mM diamide (Fig. 5). This suggests that BSH and the surface-exposed, Zn(II)-storing ribosomal proteins are the two major sources of Zn(II) released by diamide under these conditions. Diamide presumably releases Zn(II) from ribosomal proteins by oxidation of the coordinating cysteine residues.
Cd(II) induction of the CzrA regulon is a consequence of Zn(II) mobilization
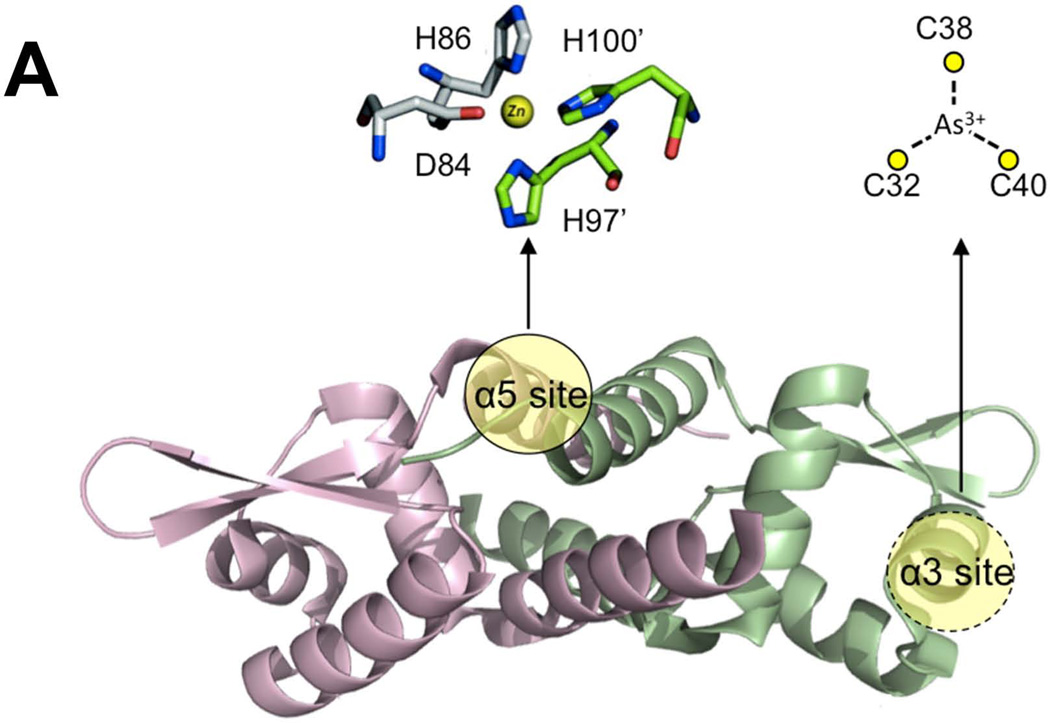
Previous studies demonstrate that the CzrA regulon was broadly induced by a wide variety of metals/metalloids (Gaballa & Helmann, 2003, Moore et al., 2005). A greater than 10-fold induction of the CzrA regulon was observed in transcriptome analyses after treatment with various thiophilic metals including Ag(I), As(V), Cd(II), Ni(II), and Zn(II), with only slightly lower induction by Cu(II). This is curious because B. subtilis CzrA does not contain any cysteine residues in its metal-sensing site in the α5 helix (Fig. 6A). Based on the known metal specificity determinants of the ArsR/SmtB family of proteins, B. subtilis CzrA is thought to be a bona fide Zn(II) sensor, much like the well studied S. aureus ortholog (Busenlehner et al., 2003, Ma et al., 2009b, Osman & Cavet, 2010). We therefore hypothesized that the induction of the CzrA regulon by thiophilic metals is likely indirect, with binding of thiophilic metals to BSH displacing the activating Zn(II) ion.
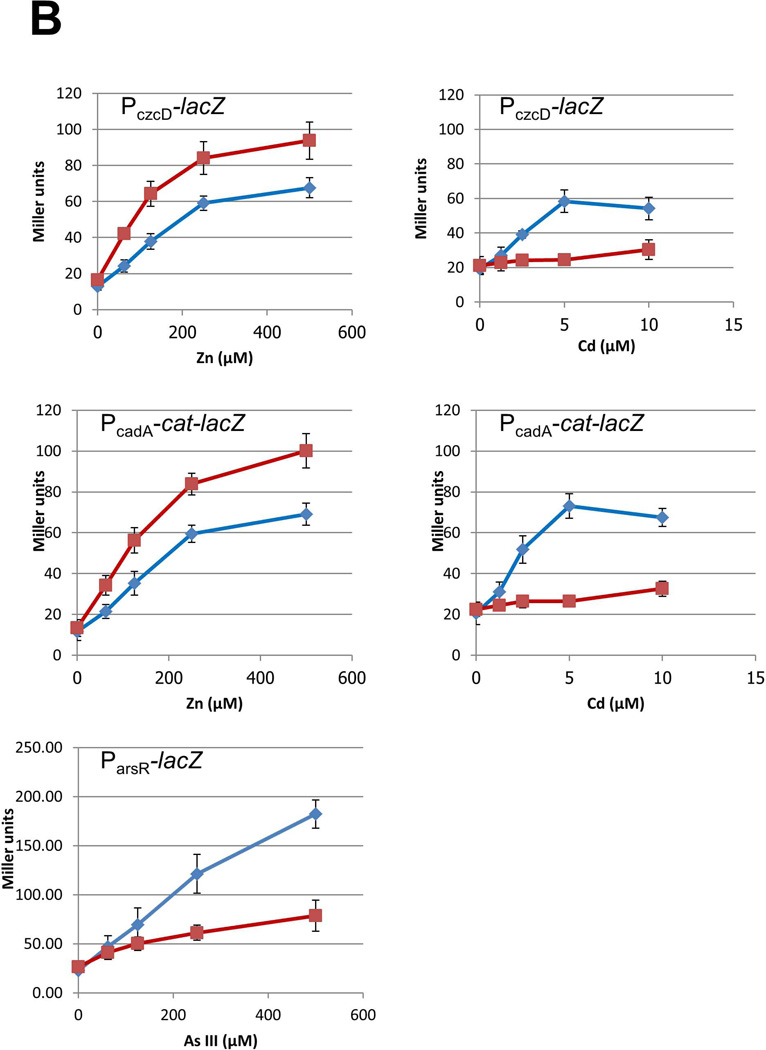
Fig 6. BSH null mutants are affected in metalloregulation.
(A) CzrA and ArsR are both members of the ArsR/SmtB family of repressors, but they differ in the location and composition of their sensing sites. The crystal structure of S. aureus CzrA dimer (PDB: 1r1v; 49% identical to the B. subtilis CzrA ortholog) is shown with the two protomers colored green and pink, respectively (Eicken et al., 2003). In CzrA, Zn is coordinated at the α5 site by D84, H86 along with H97’ and H100’ from the opposite protomer (adapted from Pennella et al., 2006). In ArsR, As(III) is coordinated by three Cys residues in the α3 site (putative location highlighted in yellow) (Numbering based on B. subtilis ArsR by alignment with the E. coli ArsR sensing site; Shi et al., 1996). (B) Wild-type cells (blue diamonds) and an isogenic bshC null mutant (red squares) carrying the indicated reporter fusion (PczcD-lacZ WT, HB11423; bshC, HB11424; PcadA-lacZ WT, HB8125; bshC, HB16680; ParsR-lacZ WT, HB5015; bshC, HB16666) were grown to mid-log phase in LB medium with the indicated concentration of metal ions and assayed for β-galactosidase. Results are shown as the mean and standard deviation of triplicate cultures.
To test this hypothesis, we monitored induction of both CzrA repressed operons, cadA and czcD, by Zn(II) and Cd(II) in wild-type and BSH null cells. Consistent with the results of Fig. 4A, Zn(II) induction was increased for each gene in the absence of BSH. With Cd(II), however, there was essentially no (<2-fold) induction for either cadA or czcD in cells lacking BSH (Fig. 6B). This supports the hypothesis that Cd(II) mobilizes Zn(II) by displacement from BSH. In addition, these results indicate that, unlike high concentrations of diamide, Cd(II) does not significantly displace Zn(II) from the L31 or L33 ribosomal proteins.
BSH broadly affects metalloregulation
In contrast with the results for the CzrA regulon, BSH has a relatively modest effect on metalloregulation mediated by the Cu(I)-sensor CsoR, and the paralogous As(III) sensors, ArsR and AseR (Fig. 6B and SI Fig. S5). The ArsR and AseR regulated arsenate/arsenite resistance systems are induced by As(III), either directly or after reduction of externally added As(V) by arsenate reductase (ArsC) (Gaballa & Helmann, 2003, Moore et al., 2005). The autoregulated arsR operon responds most strongly to As(III), and this response is reduced by ~2-fold in cells lacking BSH (Fig. 6B). In contrast with ArsR, the autoregulated aseR operon is only weakly regulated, as noted previously (Moore et al., 2005, Harvie et al., 2006), and its responsiveness is insensitive to the presence or absence of BSH (SI Fig. S5). There is little difference in the case of added As(V), in which case induction may be limited by the rate of reduction of As(V) to As(III). The mechanism underlying the decreased response of AsrR to As(III) in the absence of BSH is not immediately apparent. It is possible that As(III), like Cd(II), mobilizes Zn(II) from BSH and that released Zn(II) serves as an antagonist of ArsR DNA-binding. Alternatively, in the absence of BSH the mobility of As(III) within the cell may be reduced, due to lack of the facilitating effects of an abundant LMW thiol ligand, and this may reduce the efficacy of As(III) loading into ArsR.
The CsoR protein senses Cu(I), reduced in the cell from externally added Cu(II), by coordination to one His and two Cys residues which triggers the allosteric change required for repressor dissociation (Ma et al., 2009a). Induction of the CsoR-repressed copZA operon was slightly elevated in the BSH null mutant cells (SI Fig. S5). This is analogous to the increased induction of the Zn(II)-sensitive CzrA regulon in the absence of BSH, and may reflect the lack of competition from BSH.
Next, we wished to determine if the effects of BSH on induction of metal/metalloid resistance operons had physiological implications. We used a growth inhibition assay to monitor the effects of Zn(II), Cd(II), Cu(II), As(III) and As(V) added at levels that led to reductions of up to 10-fold in cell growth. There was no discernable effect on metal ion sensitivity for BSH null mutants when tested with Zn(II) (consistent with Fig. 3), Cu(II), or As(V) (SI Fig. S6). In contrast, BSH null mutant cells were modestly more sensitive to both Cd(II) and As(III), with an ~2-fold reduction in the amount of metal/metalloid that led to a reduction in growth yield by 50% (SI Fig. S6). While the origins of these effects are not entirely clear, we note that in the bshC null background Cd(II) is an extremely poor inducer of the CzrA regulon (Fig. 6B), which includes the primary Cd(II) resistance determinant CadA, and As(III) is reduced in its ability to induce the arsR operon, which is the primary resistance determinant for As(III) (Gaballa & Helmann, 2003, Moore et al., 2005). This correlation suggests that BSH plays a role in Cd(II) and As(III) resistance, perhaps by facilitating the efficient induction of the corresponding resistance operons. In contrast, the role of BSH in resistance to Zn(II) is proposed to result directly from its role in Zn(II) buffering, and this effect is only detectable in cells lacking the major Zn(II) efflux systems (Fig. 3).
CzrA is a Zn(II) selective metalloregulator
Zn(II) homeostasis in B. subtilis is controlled by a pair of metalloregulatory proteins, Zur and CzrA, via the regulation of Zn(II) uptake and efflux gene expression, respectively (Ma & Helmann, 2013, Moore & Helmann, 2005). Previously, Zur was shown to bind two Zn(II) ions per dimer in a high affinity structural site and two additional Zn(II) ions in the sensing site that results in activation of DNA-binding. The two sensing sites (site 2) in each dimer (one per monomer) bind Zn(II) with negative cooperativity with Ka of 1.8 × 1013 M−1 for the first bound Zn(II) and 8.3 × 1011 M−1 for the second (Ma et al., 2011). Since Zn(II) binding activates DNA-binding, the converse is also true. When the sensitivity of Zur to added Zn(II) was measured using a DNA-binding assay it was noted that levels of free Zn(II) <10−15 M activate binding (Ma et al., 2011). These results suggest that Zn(II) import is repressed when free Zn(II) levels rise much above the fM level.
CzrA is the major regulator of Zn(II) efflux, so we presume that the affinity of CzrA for Zn(II) represents the concentration judged to represent Zn(II) excess. To determine the affinity of CzrA for Zn(II) we measured binding by using FluoZin-3 and Quin-2 as competitors. CzrA binds Zn(II) with an affinity of 1.7(±0.2) × 1013 M−1 (SI Fig. S7a and b). This defines a lower limit, in the pM range, for the concentration of free Zn(II) that might trigger derepression of Zn(II) efflux due to binding of Zn(II) to apo-CzrA in solution together with rapid equilibration of solution and DNA-bound CzrA. However, since CzrA is a DNA-binding repressor and derepression occurs only upon Zn(II) binding, the relevant Zn(II) binding affinity for CzrA may be that of the CzrA-DNA complex. Since Zn(II) binding negatively regulates DNA binding affinity ≥1000-fold (SI Fig. S8), it is expected that Zn(II) will bind to the CzrA-DNA complex with an affinity ≥1000-fold lower than for free CzrA (Guerra & Giedroc, 2012). If binding to the repression complex is the relevant parameter, accumulation of nM levels of free Zn(II) may be needed to trigger derepression. Indeed, transient increases in free Zn(II) levels to the nM level have been reported in Zn(II) shocked E. coli (Wang et al., 2012).
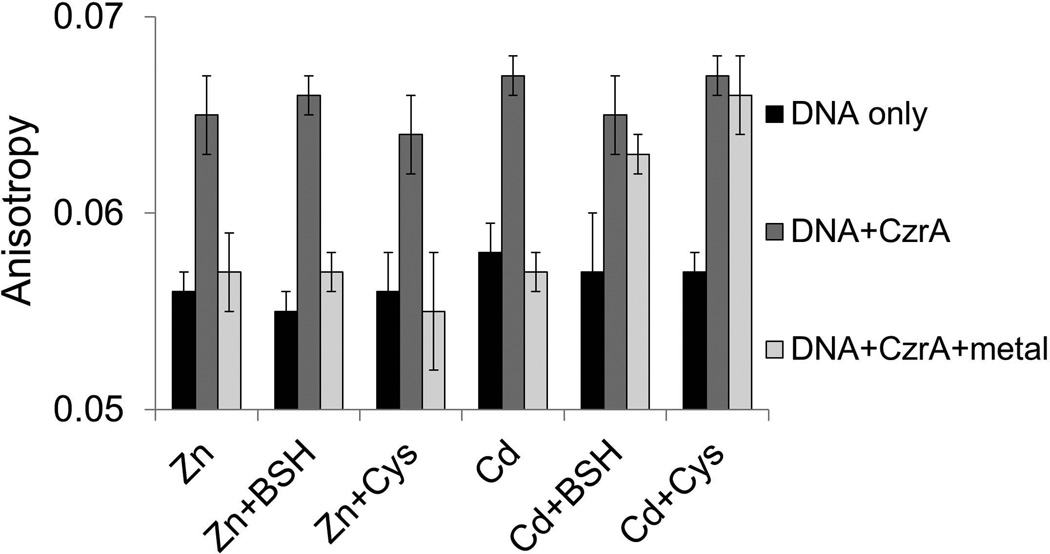
Previous studies have demonstrated that the metal-responsiveness of CzrA can be monitored in vitro using a fluorescence anisotropy-based DNA-binding assay (Harvie et al., 2006). Using a similar approach, we monitored the ability of various metals to release CzrA from DNA. In initial studies, we noted that both Zn(II) and Cd(II) eliminated DNA-binding activity in vitro, and DNA-binding was restored by addition of an excess EDTA, consistent with prior studies of both B. subtilis CzrA (Harvie et al., 2006) and the S. aureus ortholog (Pennella et al., 2003). The ability of Cd(II) to directly inactivate the CzrA repressor in vitro contrasts with the apparent BSH-dependence of the in vivo response (Fig. 6B). We reasoned that a trivial explanation for this discrepancy might be the lack of competing thiols in our in vitro reactions. Indeed, inclusion of either Cys or BSH in the DNA-binding assays eliminated the response to Cd(II), but not to Zn(II) (Fig. 7). A similar effect was noted previously in studies of the mammalian metal-regulatory transcription factor MTF1; in this case the ability of Cd(II) to activate transcription in vitro was dependent on the release of Zn from Zn-metallothionein (Zhang et al., 2003). These results highlight the importance of including physiologically relevant competing ligands in efforts to reconstitute the selective responsiveness of metalloregulatory proteins.
Fig 7. CzrA responds directly to Zn(II), but not to Cd(II), in the presence of physiological levels of thiols.
The association and dissociation of CzrA-DNA complexes was monitored by fluorescence anisotropy. Anisotropy was determined with 10 nM DNA only (white bars) and after addition of 250 nM CzrA monomer (light grey bars), 1 µM indicated metal ions (deep grey bars). 300 µM thiols were added as indicated. Conditions: 20 mM Tris, pH 8.0, 0.4 M NaCl. Anisotropy values are mean and standard deviations of >4 technical replicates and are representative of experiments repeated at least 3 times with similar magnitude changes, but different absolute anisotropy values.
BSH facilitates Zn(II) dissociation from CzrA
The above results highlight several mechanisms by which BSH might affect metalloregulation. The effects on Zn(II) sensing by CzrA are most simply explained by simple competition; in the presence of millimolar BSH the cell must accumulate higher Zn(II) levels in order to have sufficient free Zn(II) to bind CzrA and trigger derepression. In the case of Cd(II), cellular pools of BSH2:Zn(II) can be destabilized in the presence of the thiophilic Cd(II) ion leading to a release of Zn(II) into the cytosol which is then sensed by CzrA. Finally, we suggest that the decreased efficacy of As(III)-mediated induction of the arsR operon may result from the ability of BSH to help mobilize As(III) within the cell thereby facilitating binding to ArsR. Testing this latter proposal is a challenge, however, since metalloregulation by the B. subtilis ArsR protein has not been successfully reconstituted in vitro. Therefore, we chose to test the idea that BSH may facilitate metal association and dissociation from regulatory proteins using CzrA as a model system.
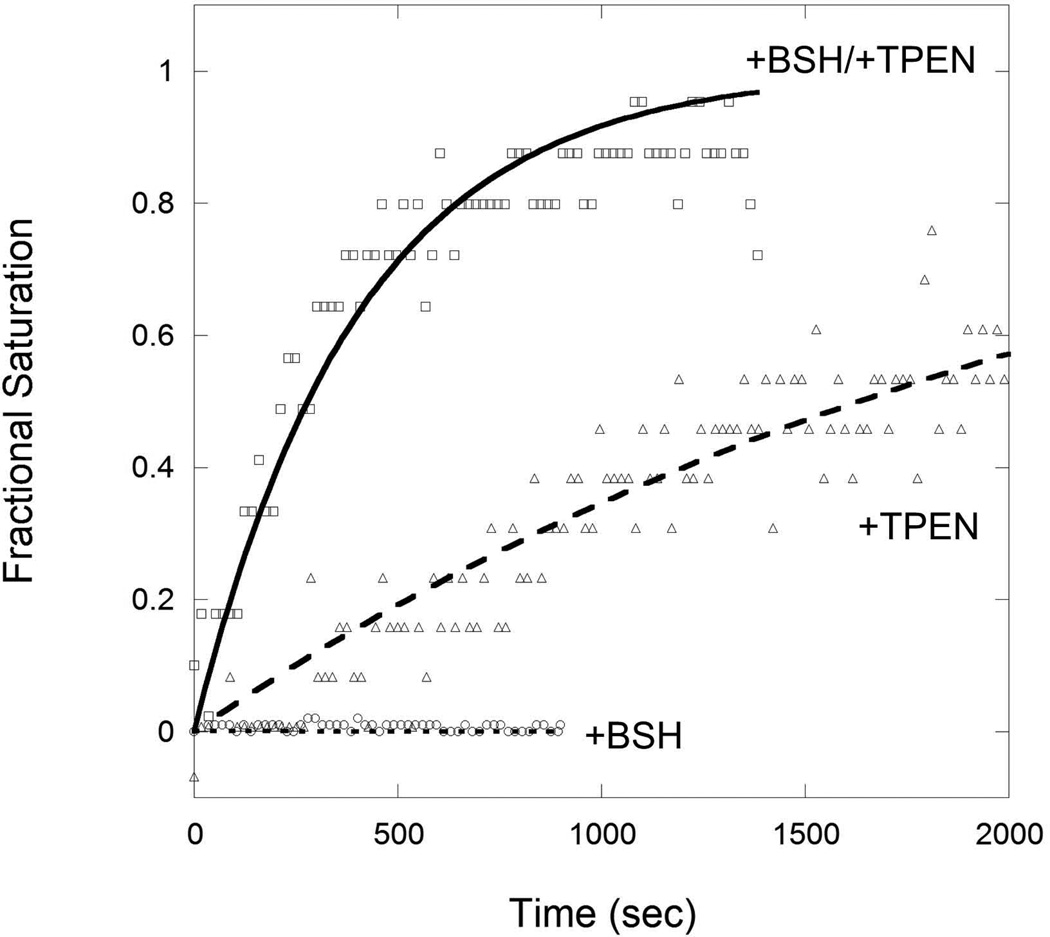
We monitored the DNA-binding activity of CzrA using fluorescence anisotropy as a readout of the ability of BSH to remove Zn(II) from CzrA. Zn(II)-loaded CzrA was mixed with FAM-labeled cadA promoter DNA. The metal chelator, TPEN, and/or BSH were then added and CzrA binding was monitored (Fig. 8). Although TPEN has a high affinity for Zn(II) (~1015 M−1), the Zn(II) bound by CzrA is only slowly transferred to TPEN, as indicated by the slow association of the resultant apo-CzrA with DNA (t1/2 ~1.6 × 103 sec). Even this relatively slow reaction may be facilitated by ligand exchange reactions since TPEN has four flexibly linked pyridine ligands. When 300 µM BSH was present, the dissociation of Zn(II) from CzrA and transfer to TPEN occurred approximately six times faster as monitored by the rate of DNA-binding (t1/2 ~280 sec). This suggests that BSH facilitates the removal of Zn(II) from CzrA, and its ultimate transfer to the high affinity TPEN ligand, through relatively facile ligand exchange reactions. In contrast, 300 µM GSH was relatively inefficient in facilitating Zn(II) removal with only a slight decrease in the CzrA:Zn half-time (t1/2 ~1.1 × 103 sec) relative to TPEN alone (data not shown). Together, these data suggest that, as cells transition from Zn(II) excess to normal conditions, BSH may accelerate the repression of CzrA regulated Zn(II) efflux genes, cadA and czcD. Conversely, BSH may facilitate Zn(II)-loading into DNA-bound apo-CzrA and thereby increase the kinetics of derepression. Further analyses of these proposed facilitating mechanisms are underway.
Fig 8. Bacillithiol (BSH) facilitates Zn-dissociation from CzrA.
10 nM FAM-labeled cadA promoter DNA was mixed with 300 nM CzrA and 1 µM Zn in 20 mM Tris, pH 8.0. 0.4 M NaCl. 100 µM TPEN was then added with 300 µM BSH. DNA binding was monitored by fluorescence anisotropy with normalized fractional saturation plotted on the y-axis. x-axis represents the time after TPEN addition. All data were fit to a simple first-order decay model with the half-life (t1/2) of CzrA-Zn complex under these conditions being 1633 s (triangles; TPEN only) and 278 s (squares; TPEN and BSH).
Discussion
Zn(II) plays an essential role as a structural and catalytic cofactor in numerous cell processes. Here, we have identified the LMW thiol, BSH, as a key buffer for the labile Zn(II) pool in B. subtilis. We propose that BSH helps maintain free Zn(II) concentrations at levels high enough for proper metallation of Zn(II) requiring proteins, and low enough to prevent rampant mismetallation of other proteins. The high affinity of BSH for Zn(II) is largely dependent on the cysteine-derived thiol group, although the malate carboxylates also contribute (Fig. 1). The dominant complex at low levels of saturation, which is likely the case in vivo, is a BSH2:Zn(II) complex.
The intracellular concentration of BSH is ~1 mM for mid-logarithmic phase cells, and can increase to ~5 mM during stationary phase (Sharma et al., 2013). Thus, there are likely 6 × 105 molecules or more of BSH per µm3 of cell volume. At equilibrium, it is likely that BSH2:Zn(II) complexes represent a small fraction of total BSH. Based on our measured affinity of Zn(II) for formation of BSH2:Zn(II) complexes, if 1% of BSH (at 1 mM) is associated with Zn(II) (corresponding to ~5 µM BSH2:Zn(II)) the free Zn(II) concentration would be buffered to ~2.5 pM, the Zur protein would be metallated, and further transcription of the znuABC operon, encoding the major high affinity Zn(II) uptake system, would be inhibited. Indeed, our biochemical measurements imply that free CzrA (although not necessarily DNA-bound CzrA) would also be inactivated at this level of free Zn(II), possibly leading to expression of efflux. However, the actual sensitivity of zinc efflux systems in vivo, and whether they are under thermodynamic or kinetic control, remains unresolved (Wang et al., 2012). Moreover, the effective rate of efflux may be limited by the affinity of the efflux systems for Zn(II) rather than their expression level.
The level of zinc bound to BSH likely rises much higher in Zn(II) shocked cells. The maximum Zn(II) binding capacity of a 1 mM pool of BSH is between ~0.5 mM (as BSH2:Zn(II)) and ~1 mM (as BSH:Zn(II)). It is interesting to note that 15 min. after a Zn(II) shock, the difference in total cell-associated Zn(II) between WT and BSH null cells is ~0.6 mM, and most of the difference corresponds to a LMW pool of Zn(II) (Fig. 2B and SI Fig. S4). One interpretation of this result is that the BSH pool becomes nearly saturated with Zn(II), at least transiently. Under these conditions, the calculated level of free Zn(II) will rise to the nM level (80% saturation of a 1 mM BSH pool buffers free Zn(II) to ~ 5 nM), more than sufficient to inactivate the CzrA repressor and derepress the corresponding Zn(II) efflux pumps which are presumably quite active at these high concentration of Zn(II). In summary, these calculations suggest that BSH can serve as a Zn(II) buffer in the pM to nM range, which is within the likely intracellular free Zn(II) concentration range determined based on the Zn(II) binding affinities of the regulators of Zn(II) homeostasis, Zur (Ma et al., 2011) and CzrA (SI Fig. S7). These results are consistent with recent studies using ratiometric fluorescent reporters that suggest that pM to nM levels of free Zn(II) are common in several systems (Qin et al., 2011, Vinkenborg et al., 2009). To date, most of these studies are in eukaryotic systems, but studies of E. coli have suggested free Zn values in the 10–200 pM range (Wang et al., 2011, Haase et al., 2013), rising to >10 nM after a Zn shock (Wang et al., 2012).
Several observations support the notion that BSH is a physiologically significant buffer for intracellular Zn(II). First, cells lacking the Zn(II) efflux systems encoded by the czcD and cadA operons are sensitive to elevated Zn(II), and this sensitivity is exacerbated in cells lacking BSH (Fig. 3). Second, the CzrA regulon is more sensitive to induction by Zn(II) in cells lacking BSH (Fig. 4 and 6B), consistent with the loss of significant intracellular zinc buffering capacity. Third, the presence of a significant pool of BSH2:Zn(II), and the metal binding potential of BSH itself, can explain many other previously puzzling observations. For example, the thiol oxidant diamide strongly induces the cadA and czcD operons repressed by CzrA (Leichert et al., 2003), a repressor protein that itself lacks any Cys residues (Harvie et al., 2006, Moore et al., 2005). We here demonstrate that induction with 0.1 mM diamide is largely dependent on BSH, presumably because the BSH2:Zn(II) pool is much more sensitive to this thiol oxidant than most protein-bound Zn(II). However, 1 mM diamide additionally mobilizes Zn(II) from L31 and L33 (Fig. 5), consistent with the proposal that these two proteins represent a significant and mobilizable store of Zn(II) (Akanuma et al., 2006, Gabriel & Helmann, 2009, Nanamiya et al., 2004). Since these proteins are normally synthesized as Zn(II) metalloproteins in Zn(II) sufficient cells, they represent a mobilizable store of Zn(II), but do not buffer the cell against Zn(II) shock.
Release of Zn(II) from cellular BSH2:Zn(II) pools may also contribute to several other previously noted stress responses. For example, the CzrA regulon is induced by numerous thiophilic metal ions (Moore et al., 2005), by acid stress (Wilks et al., 2009), and by reactive electrophiles such as methylglyoxal and formaldehyde (Nguyen et al., 2009). One plausible model posits that in each case induction results from release of Zn(II) from BSH2:Zn(II) pools. Indeed, methylglyoxal has been shown to rapidly deplete the BSH pool in the cell (Chandrangsu et al., 2014), and reduction of intracellular pH may release bound Zn(II) by protonation of the BSH thiolate (Sharma et al., 2013).
As the dominant LMW thiol in the cell, BSH has numerous functions. We here document its dominant role in buffering Zn(II), consistent with prior suggestions that GSH may play a similar role in other cells (Helbig et al., 2008), despite its significantly lower affinity for Zn(II) (Fig. 1C). In addition, BSH likely buffers the impact of other thiophilic metals/metalloids, as here shown for Cd(II). It has been suggested that GSH, for example, serves as a buffer for the labile Fe(II) pool (Hider & Kong, 2013, Hider & Kong, 2011). BSH also functions, by virtue of its ability to form mixed disulfides with proteins (S-bacillithiolation), to protect Cys residues against overoxidation in response to oxidative stress (Chi et al., 2011, Chi et al., 2012, Gaballa et al., 2014). Finally, BSH is a cofactor for BSH-S-transferases such as the fosfomycin detoxifying enzyme FosB (Roberts et al., 2012, Thompson et al., 2013), DinB family enzymes (Perera et al., 2014), and BSH-dependent glyoxalases (Chandrangsu et al., 2014). In addition, it is likely that BSH plays a key facilitating role in the ligand transfer reactions that allow metals/metalloids to equilibrate rapidly with ligands within the cell. Indeed, as shown here, addition of BSH increases the rate of Zn(II) dissociation from CzrA as measured by the rate of apo-CzrA DNA association (Fig. 8). Similarly, mobility of As(III) in biological systems is facilitated by LMW thiols and loading of As(III) into the ArsD metallochaperone is stimulated by GSH (Yang et al., 2010). We speculate that a similar kinetic role for BSH in loading of As(III) into ArsR could account for the decreased efficacy of induction of the arsR operon noted in the BSH null cells (Fig. 6B).
BSH and other LMW thiols could also play a crucial role in the host-pathogen competition for zinc. Zinc in the second most abundant transition metal in the host and is important for proper immune function (Kehl-Fie & Skaar, 2010). In response to infection by Staphylococcus aureus, neutrophils release calprotectin, a protein which sequesters zinc, thereby limiting its availability to the pathogen and increasing sensitivity to superoxide stress (Kehl-Fie et al., 2011). To circumvent nutritional limitation by the host, bacteria express high affinity metal uptake systems, such as the Znu-like systems (Liu et al., 2012). Once Zn(II) is brought in to the cell, BSH may contribute by providing an effective means for bacteria to store Zn(II) in an easily accessible form.
The nature of the labile Zn(II) pool in bacteria has been elusive. Our work suggests that BSH forms high affinity interactions with Zn(II) and plays a dominant role in Zn(II) buffering. Moreover, our suggest results that the Zn(II) associated with ribosomal proteins can be mobilized in response to diamide stress, consistent with the prior suggestion that zinc-containing ribosomal proteins serve as a storage form of Zn(II) (Gabriel and Helmann, 2009). Since BSH is widely distributed among the Firmicutes, whose members include important human pathogens such as Bacillus anthracis and S. aureus, our findings may also have implications for understanding the intense competition for metal ions during host-pathogen interactions.
Experimental Procedures
Bacterial strains, plasmids, and growth conditions
Strains and plasmids used in this study are listed in Table 1. Bacteria were grown in the media described in the following sections. When necessary, antibiotics were used at the following concentrations: chloramphenicol (10 µg ml−1), kanamycin (15 µg ml−1), spectinomycin (100 µg ml−1), and tetracycline (5 µg ml−1). Gene deletions were constructed using long flanking homology PCR as previously described (Mascher et al., 2003). Chromosomal DNA transformation was performed as described (Harwood & Cutting, 1990). To generate promoter–lacZ fusions, a DNA fragment containing czcD promoter was PCR-amplified using czcD-F (5'CGCGAATTCTTTCAGTTACAAGTAAATCC3') and czcD-R (5'CGCGGATCCTTCATTATGATTGTGACCCA3') and cloned into vector pDG1661 (Guerout-Fleury et al., 1996).
Table.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| CU1065 | WT | Laboratory stock |
| HB11002 | CU1065 bshA::kan | Gaballa et al. (2010) |
| HB11079 | CU1065 bshC::kan | Gaballa et al. (2010) |
| HB11212 | CU1065 bshC::mls | Chandrangsu et al. (2014) |
| HB11675 | CU1065 bshC::spec | This work |
| HB11393 | CU1065 cadA::kan | This work |
| HB11394 | CU1065 czcD::tet | This work |
| HB11395 | CU1065 cadA::kan czcD::tet | This work |
| HB11396 | CU1065 cadA::kan czcD::tet bshC::mls | This work |
| HB11427 | CU1065 cadA::kan czcD::tet bshC::mls amyE::Pxyl-bshC | This work |
| HB11423 | CU1065 PczcD-lacZ (pDG1661) cat | This work |
| HB11424 | HB11423 bshC::mls | This work |
| HB11436 | HB11423 cadA::kan czcD::tet | This work |
| HB11437 | HB11423 cadA::kan czcD::tet bshC::mls | This work |
| HB11058 | CU1065 SPβ(cadA’-cat-lacZ) (mls, neo) | This work |
| HB11061 | HB11058 bshA::kan | This work |
| HB5015 | CU1065 ParsR-lacZ (pDG1661) cat | Moore et al. (2005) |
| HB16666 | HB5015 bshC::mls | This work |
| HB8125 | CU1065 PcadA-lacZ (pDG1661) cat | Moore et al. (2005) |
| HB16680 | HB8125 bshC::spec | This work |
| HB6916 | CU1065 rpmGA::tet rpmGB::cm rpmGE::spc (C-) | Gabriel and Helmann (2009) |
| HB15192 | HB6916 bshC::mls | This work |
Disk diffusion assays
Strains were grown in LB at 37 °C with vigorous shaking to an OD600~0.4. A 100 µl aliquot of these cultures was added to 4 ml of LB soft agar (0.7% agar) and poured on to prewarmed LB agar plates. The plates were then allowed to solidify for 10 minutes at room temperature in a laminar flow hood. Filter disks (6.5 mm) were placed on top of the agar and 10 µl of Zn(II) or Cd(II) (50 mM) was added to the disks and allowed to absorb for 10 minutes. The plates were then incubated at 37 °C for 16–18 hours. The diameter of the zone of inhibition was measured. The data shown represent the average and standard deviation of three biological replicates.
Growth inhibition assays
Strains were grown in LB at 37 °C with vigorous shaking overnight. An aliquot of the overnight culture was used to inoculate LB containing Zn(II), Cd(II), Cu(II), or As(III) at the indicated concentrations to a starting OD600 of 0.02. The cultures were allowed to grow overnight, at which time the final OD600 was measured. The data shown represent the average and standard deviation of three biological replicates.
β-Galactosidase assays
Cells containing cadA-lacZ or czcD-lacZ were grown in LB with different concentrations of Zn(II) or Cd(II) to mid-exponential phase. Cells containing arsR-lacZ were grown in LB with different concentrations of As(III) or As(V) to mid-exponential phase. β-galactosidase assays were done as described previously (Chen et al., 1993, Miller, 1972). For monitoring the time-course of induction, cells containing czcD-lacZ were grown in LB until mid-exponential phase and 50 µM Zn was added. Samples were taken before and after Zn(II) addition at the indicated time.
Quantification of total Zn(II) quota by ICP-MS
Cells for quantification of the total Zn(II) quota were grown in 5 ml LB medium in the presence or absence of 200 µM ZnCl2 to mid-log phase. For fractionation experiments, 25 ml of cells were grown in presence or absence of 200 µM Zn(II). Samples were prepared and analyzed by ICP-MS using the same protocol as previously described (Faulkner et al., 2012). Cells were collected and washed with PBS buffer containing 1mM nitrotriacetic acid followed by two PBS only washes. Cells were then resuspended in PBS and a fraction was used for an OD600 measurement. The remaining cells were lysed by incubation at 37C for 20 minutes in the presence of 10 ug of lysozyme. For fractionation experiments, the cell lysate was passed through a 3000 dalton cutoff ultrafiltration column (Ambion). Nitric acid (final concentration of 3%) and 0.1% (vol/vol) Triton-X100 was then added and the samples were boiled at 95C for 30 minutes. Samples were then centrifuged and subjected to ICP-MS (Perkin Elmer ELAN DRC II using ammonia as the reaction gas and gallium as an internal standard). Zn(II) content was averaged over the cell volume and cell number assuming 5 × 108 cells OD600−1 and 1.0 × 10−14 liters cell−1. The data shown represent the average and standard deviation of three biological replicates.
qRT-PCR
qRT-PCR was performed as previously described (Guariglia-Oropeza & Helmann, 2011). Briefly, cells were grown at 37 °C in LB medium with rigorous shaking till OD600 ~0.4. 1 ml aliquots were treated with freshly prepared diamide for 5 min. Total RNA from both treated and untreated samples were extracted RNeasy Mini Kit following the manufacturer's instructions (Qiagen Sciences, Germantown, MD). RNA samples were then treated with Turbo-DNA free DNase (Ambion) and precipitated with ethanol overnight. RNA samples were re-dissolved in RNase-free water and quantified by NanoDrop spectrophotometer. 2 µg total RNA from each sample was used for cDNA synthesis with TaqMan reverse transcription reagents (Applied Biosystems). qPCR was then carried out using iQ SYBR green supermix in an Applied Biosystems 7300 Real Time PCR System. 23S rRNA was used as an internal control and fold-changes between treated and untreated samples were plotted.
Metal binding experiments with BSH
All metal binding experiments involving GSH, BSH and its derivatives were carried out in 20 mM HEPES, pH 7.7, 0.15 NaCl. 0.1 mM TCEP as reductant was added to prevent oxidation. This concentration of TCEP was shown not to interfere with metal binding. Concentrations of BSH and GSH were determined using DTNB assay. For Co(II) titration, Co(II) was titrated into a solution containing BSH and the UV-vis absorption spectrum was recorded after each addition. For Zn(II) titration, the competitor magfura-2 was included in the solution. After each addition, 10 min equilibration time was allowed and then the UV-vis absorption spectrum was recorded. Absorption at 325 and 366 nm were plotted for analysis. Data were fitted using Dynafit with competition model as described in the text. Experiments with BSH derivatives and GSH were performed in a similar manner.
Overexpression and purification of CzrA
B. subtilis CzrA was overexpressed in E. coli BL21 pLysS strain carrying a pET-16b plasmid containing CzrA coding sequence. A single colony was inoculated and grown in LB medium with 100 µg ml−1 ampicillin at 37 °C overnight. The overnight culture was then diluted 1:100 with fresh LB medium containing 100 µg ml−1 ampicillin. Cells were grown at 37°C with rigorous shaking. 1 mM IPTG was added at mid-log phase (OD600~0.4) and cells were harvest by centrifugation after 2 hours of induction. Cell pellets were resuspended in 15 ml buffer A (20 mM Tris, pH 8.0, 0.1 M NaCl) and lysed by sonication. CzrA remained in the lysis pellet but can be recovered by stirring in 50 ml of the same buffer overnight. After centrifugation, the supernatant was loaded onto a MonoQ anion exchange column and eluted with Buffer A with salt gradient from 0.1 to 1 M. Fractions containing CzrA were pooled and found to be ~90% pure as judged by coomassie blue stained SDS-PAGE gel. CzrA was finally dialyzed against Buffer B (20 mM Tris, pH 8.0, 0.4 M NaCl) and stored in −80 °C. Relatively high salt condition was used to increase the solubility of CzrA. These buffer conditions were also used in subsequent metal and DNA binding experiments with CzrA. Due to the low calculated extinction coefficient, the concentration of CzrA was determined by two independent titration methods: i) Zn(II) titration monitored by tyrosine fluorescence, which assumes CzrA binds 1.0 Zn(II) per monomer; ii) DNA binding monitored by fluorescence anisotropy, which assumes 1:1 binding of CzrA dimer to a 28 bp operator DNA. Those assumptions are based on the known properties of the closely related CzrA from S. aureus (46.5% sequence identity) (Arunkumar et al., 2009). Both methods provide similar results on the concentration of CzrA.
Zn(II) binding by CzrA
Binding of Zn(II) to CzrA was monitored by competition assay using FluoZin3 (Fz3) or Quin-2 as competitor, which binds one Zn(II) with affinities of 5.0 × 107 M−1 and 2.7 × 1011 M−1, respectively. All experiments were carried out in Buffer B as previously described (Ma et al., 2011). Data were analyzed using Dynafit.
DNA binding by CzrA
DNA binding by CzrA was monitored by fluorescence anisotropy. A 28 bp 6-FAM-labeled DNA derived from the cadA promoter was used (5’-6-FAM-TTATATATGA GTATATGCTCATATATAT-3’ and its complement). Fluorescence anisotropy was measured with λex = 495 nm and λem = 520 nm in Buffer B. The averaged anisotropy value from at least five measurements was reported. For experiments measuring DNA binding of CzrA in the presence of BSH, 10 nM FAM-labeled was mixed with 300 nM CzrA and 1 µM Zn (20 mM Tris, pH 8.0. 0.4 M NaCl). 100 µM TPEN was then added with 300 µM BSH.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (GM059323 to JDH), NSF (MCB1020481 to JDH), and an NIH postdoctoral fellowship to PC (1F32GM106729). A.R. was supported by the Royal Golden Jubilee Ph.D. Program (PHD/0294/2550) with joint funding from the Thailand Research Fund and Mahidol University. The authors thank Dr. Chris J. Hamilton (Univ. East Anglia) for the gift of BSH and BSH analogs and Alexandra Roberts for assistance with the BSSB Zn(II) affinity measurements.
References
- Akanuma G, Nanamiya H, Natori Y, Nomura N, Kawamura F. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product, YtiA, in Bacillus subtilis. J Bacteriol. 2006;188:2715–2720. doi: 10.1128/JB.188.7.2715-2720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello JM, Gonzalez-Guerrero M, Raimunda D. Bacterial transition metal P(1B)-ATPases: transport mechanism and roles in virulence. Biochemistry. 2011;50:9940–9949. doi: 10.1021/bi201418k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunkumar AI, Campanello GC, Giedroc DP. Solution structure of a paradigm ArsR family zinc sensor in the DNA-bound state. Proc Natl Acad Sci U S A. 2009;106:18177–18182. doi: 10.1073/pnas.0905558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson A, Khalimonchuk O, Smith P, Sabic H, Eide D, Winge DR. Mzm1 influences a labile pool of mitochondrial zinc important for respiratory function. J Biol Chem. 2010;285:19450–19459. doi: 10.1074/jbc.M110.109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busenlehner LS, Pennella MA, Giedroc DP. The SmtB/ArsR family of metalloregulatory transcriptional repressors: Structural insights into prokaryotic metal resistance. FEMS microbiology reviews. 2003;27:131–143. doi: 10.1016/S0168-6445(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Chandrangsu P, Dusi R, Hamilton CJ, Helmann JD. Methylglyoxal resistance in Bacillus subtilis: Contributions of bacillithiol-dependent and independent pathways. Molecular Microbiology. 2014;91:706–715. doi: 10.1111/mmi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, James LP, Helmann JD. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BK, Gronau K, Mader U, Hessling B, Becher D, Antelmann H. S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Molecular & cellular proteomics : MCP. 2011;10 doi: 10.1074/mcp.M111.009506. M111 009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BK, Roberts AA, Huyen TT, Basell K, Becher D, Albrecht D, Hamilton CJ, Antelmann H. S-Bacillithiolation Protects Conserved and Essential Proteins Against Hypochlorite Stress in Firmicutes Bacteria. Antioxidants & redox signaling. 2012;8:1273–1295. doi: 10.1089/ars.2012.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Holmes WR, Fontaine CP, Maret W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics : integrated biometal science. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- Eicken C, Pennella MA, Chen X, Koshlap KM, VanZile ML, Sacchettini JC, Giedroc DP. A metal-ligand-mediated intersubunit allosteric switch in related SmtB/ArsR zinc sensor proteins. Journal of molecular biology. 2003;333:683–695. doi: 10.1016/j.jmb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochimica et biophysica acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Faulkner MJ, Ma Z, Fuangthong M, Helmann JD. Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. J Bacteriol. 2012;194:1226–1235. doi: 10.1128/JB.06566-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa A, Chi BK, Roberts AA, Becher D, Hamilton CJ, Antelmann H, Helmann JD. Redox regulation in Bacillus subtilis: the bacilliredoxins BrxA(YphP) and BrxB(YqiW) function in de-bacillithiolation of S-bacillithiolated OhrR and MetE. Antioxidants & redox signaling. 2014;21:357–367. doi: 10.1089/ars.2013.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa A, Helmann JD. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa A, Helmann JD. Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems. Biometals. 2003;16:497–505. doi: 10.1023/a:1023425321617. [DOI] [PubMed] [Google Scholar]
- Gaballa A, Newton GL, Antelmann H, Parsonage D, Upton H, Rawat M, Claiborne A, Fahey RC, Helmann JD. Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli. Proc Natl Acad Sci U S A. 2010;107:6482–6486. doi: 10.1073/pnas.1000928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa A, Wang T, Ye RW, Helmann JD. Functional analysis of the Bacillus subtilis Zur regulon. J Bacteriol. 2002;184:6508–6514. doi: 10.1128/JB.184.23.6508-6514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Helmann JD. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol. 2009;191:6116–6122. doi: 10.1128/JB.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Miyagi F, Gaballa A, Helmann JD. Regulation of the Bacillus subtilis yciC gene and insights into the DNA-binding specificity of the zinc-sensing metalloregulator Zur. J Bacteriol. 2008;190:3482–3488. doi: 10.1128/JB.01978-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Imlay JA. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol Microbiol. 2013;89:123–134. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariglia-Oropeza V, Helmann JD. Bacillus subtilis sigma(V) confers lysozyme resistance by activation of two cell wall modification pathways, peptidoglycan O-acetylation and D-alanylation of teichoic acids. J Bacteriol. 2011;193:6223–6232. doi: 10.1128/JB.06023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerout-Fleury AM, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- Guerra AJ, Giedroc DP. Metal site occupancy and allosteric switching in bacterial metal sensor proteins. Archives of biochemistry and biophysics. 2012;519:210–222. doi: 10.1016/j.abb.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti AA, Wei Y, Rood SV, Krulwich TA. An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+ Mol Microbiol. 2002;45:145–153. doi: 10.1046/j.1365-2958.2002.02998.x. [DOI] [PubMed] [Google Scholar]
- Haase H, Hebel S, Engelhardt G, Rink L. Application of Zinpyr-1 for the investigation of zinc signals in. Escherichia coli Biometals. 2013;26:167–177. doi: 10.1007/s10534-012-9604-0. [DOI] [PubMed] [Google Scholar]
- Harvie DR, Andreini C, Cavallaro G, Meng W, Connolly BA, Yoshida K, Fujita Y, Harwood CR, Radford DS, Tottey S, Cavet JS, Robinson NJ. Predicting metals sensed by ArsR-SmtB repressors: allosteric interference by a non-effector metal. Mol Microbiol. 2006;59:1341–1356. doi: 10.1111/j.1365-2958.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- Harwood CR, Cutting SM. Molecular Biological Methods for Bacillus. Chichester: John Wiley and Sons, Ltd.; 1990. [Google Scholar]
- Helbig K, Bleuel C, Krauss GJ, Nies DH. Glutathione and transition-metal homeostasis in Escherichia coli. J Bacteriol. 2008;190:5431–5438. doi: 10.1128/JB.00271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann JD. Bacillithiol, a new player in bacterial redox homeostasis. Antioxidants & redox signaling. 2011;15:123–133. doi: 10.1089/ars.2010.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann JD, Soonsanga S, Gabriel S. Metalloregulators: Arbiters of Metal Sufficiency. In: Nies DH, Silver S, editors. Molecular Microbiology of Heavy Metals. Berlin: Springer-Verlag; 2007. pp. 37–71. [Google Scholar]
- Hider RC, Kong X. Iron speciation in the cytosol: an overview. Dalton Trans. 2013;42:3220–3229. doi: 10.1039/c2dt32149a. [DOI] [PubMed] [Google Scholar]
- Hider RC, Kong XL. Glutathione: a key component of the cytoplasmic labile iron pool. Biometals. 2011;24:1179–1187. doi: 10.1007/s10534-011-9476-8. [DOI] [PubMed] [Google Scholar]
- Irving H, Williams R. 637. The stability of transition-metal complexes. Journal of the Chemical Society (Resumed) 1953:3192–3210. [Google Scholar]
- Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell host & microbe. 2011;10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Current opinion in chemical biology. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitko RD, Cleeton RL, Armentrout EI, Lee GE, Noguchi K, Berkmen MB, Jones BD, Slonczewski JL. Cytoplasmic acidification and the benzoate transcriptome in Bacillus subtilis. PloS one. 2009;4:e8255. doi: 10.1371/journal.pone.0008255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Helmann JD. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J Biol Chem. 2006;281:23567–23578. doi: 10.1074/jbc.M603968200. [DOI] [PubMed] [Google Scholar]
- Leichert LI, Scharf C, Hecker M. Global characterization of disulfide stress in Bacillus subtilis. J Bacteriol. 2003;185:1967–1975. doi: 10.1128/JB.185.6.1967-1975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, Pasquali P, Lane TE, Chazin WJ, Vogl T, Roth J, Skaar EP, Raffatellu M. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell host & microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Cowart DM, Scott RA, Giedroc DP. Molecular insights into the metal selectivity of the copper(I)-sensing repressor CsoR from Bacillus subtilis. Biochemistry. 2009a;48:3325–3334. doi: 10.1021/bi900115w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Gabriel SE, Helmann JD. Sequential binding and sensing of Zn(II) by Bacillus subtilis Zur. Nucleic acids research. 2011;39:9130–9138. doi: 10.1093/nar/gkr625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Helmann JD. Metal homeostasis and oxidative stress in Bacillus subtilis. In: Scott RA, editor. Encyclopedia of Inorganic and Bioinorganic Chemistry. Chichester, UK: John Wiley & Sons, Ltd; 2013. pp. eibc2129. [Google Scholar]
- Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chemical reviews. 2009b;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W, Li Y. Coordination dynamics of zinc in proteins. Chemical reviews. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- Moore CM, Gaballa A, Hui M, Ye RW, Helmann JD. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol Microbiol. 2005;57:27–40. doi: 10.1111/j.1365-2958.2005.04642.x. [DOI] [PubMed] [Google Scholar]
- Moore CM, Helmann JD. Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol. 2005;8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Nanamiya H, Akanuma G, Natori Y, Murayama R, Kosono S, Kudo T, Kobayashi K, Ogasawara N, Park SM, Ochi K, Kawamura F. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol Microbiol. 2004;52:273–283. doi: 10.1111/j.1365-2958.2003.03972.x. [DOI] [PubMed] [Google Scholar]
- Natori Y, Nanamiya H, Akanuma G, Kosono S, Kudo T, Ochi K, Kawamura F. A fail-safe system for the ribosome under zinc-limiting conditions in Bacillus subtilis. Mol Microbiol. 2007;63:294–307. doi: 10.1111/j.1365-2958.2006.05513.x. [DOI] [PubMed] [Google Scholar]
- Newton GL, Rawat M, La Clair JJ, Jothivasan VK, Budiarto T, Hamilton CJ, Claiborne A, Helmann JD, Fahey RC. Bacillithiol is an antioxidant thiol produced in Bacilli. Nat Chem Biol. 2009;5:625–627. doi: 10.1038/nchembio.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Eiamphungporn W, Mader U, Liebeke M, Lalk M, Hecker M, Helmann JD, Antelmann H. Genome-wide responses to carbonyl electrophiles in Bacillus subtilis: control of the thiol-dependent formaldehyde dehydrogenase AdhA and cysteine proteinase YraA by the MerR-family regulator YraB (AdhR) Mol Microbiol. 2009;71:876–894. doi: 10.1111/j.1365-2958.2008.06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman D, Cavet JS. Bacterial metal-sensing proteins exemplified by ArsR-SmtB family repressors. Natural product reports. 2010;27:668–680. doi: 10.1039/b906682a. [DOI] [PubMed] [Google Scholar]
- Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Pennella MA, Arunkumar AI, Giedroc DP. Individual metal ligands play distinct functional roles in the zinc sensor Staphylococcus aureus CzrA. Journal of molecular biology. 2006;356:1124–1136. doi: 10.1016/j.jmb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Pennella MA, Shokes JE, Cosper NJ, Scott RA, Giedroc DP. Structural elements of metal selectivity in metal sensor proteins. Proc Natl Acad Sci U S A. 2003;100:3713–3718. doi: 10.1073/pnas.0636943100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera VR, Newton GL, Parnell JM, Komives EA, Pogliano K. Purification and characterization of the Staphylococcus aureus bacillithiol transferase BstA. Biochimica et biophysica acta. 2014;1840:2851–2861. doi: 10.1016/j.bbagen.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci U S A. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AA, Sharma SV, Strankman AW, Duran SR, Rawat M, Hamilton CJ. Mechanistic studies of FosB: a divalent metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus. The Biochemical journal. 2012;451:69–79. doi: 10.1042/BJ20121541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Arbach M, Roberts AA, Macdonald CJ, Groom M, Hamilton CJ. Biophysical features of bacillithiol, the glutathione surrogate of Bacillus subtilis and other firmicutes. Chembiochem: a European journal of chemical biology. 2013;14:2160–2168. doi: 10.1002/cbic.201300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Dong J, Scott RA, Ksenzenko MY, Rosen BP. The role of arsenic-thiol interactions in metalloregulation of the ars operon. J Biol Chem. 1996;271:9291–9297. doi: 10.1074/jbc.271.16.9291. [DOI] [PubMed] [Google Scholar]
- Simons TJ. Measurement of free Zn2+ ion concentration with the fluorescent probe mag-fura-2 (furaptra) Journal of biochemical and biophysical methods. 1993;27:25–37. doi: 10.1016/0165-022x(93)90065-v. [DOI] [PubMed] [Google Scholar]
- Solovieva IM, Entian KD. Investigation of the yvgW Bacillus subtilis chromosomal gene involved in Cd(2+) ion resistance. FEMS microbiology letters. 2002;208:105–109. doi: 10.1111/j.1574-6968.2002.tb11068.x. [DOI] [PubMed] [Google Scholar]
- Thambiayya K, Kaynar AM, St Croix CM, Pitt BR. Functional role of intracellular labile zinc in pulmonary endothelium. Pulmonary circulation. 2012;2:443–451. doi: 10.4103/2045-8932.105032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MK, Keithly ME, Harp J, Cook PD, Jagessar KL, Sulikowski GA, Armstrong RN. Structural and chemical aspects of resistance to the antibiotic fosfomycin conferred by FosB from Bacillus cereus. Biochemistry. 2013;52:7350–7362. doi: 10.1021/bi4009648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanZile ML, Cosper NJ, Scott RA, Giedroc DP. The zinc metalloregulatory protein Synechococcus PCC7942 SmtB binds a single zinc ion per monomer with high affinity in a tetrahedral coordination geometry. Biochemistry. 2000;39:11818–11829. doi: 10.1021/bi001140o. [DOI] [PubMed] [Google Scholar]
- Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nature methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- Wang D, Hosteen O, Fierke CA. ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. Journal of inorganic biochemistry. 2012;111:173–181. doi: 10.1016/j.jinorgbio.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hurst TK, Thompson RB, Fierke CA. Genetically encoded ratiometric biosensors to measure intracellular exchangeable zinc in Escherichia coli. Journal of biomedical optics. 2011;16 doi: 10.1117/1.3613926. 087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks JC, Kitko RD, Cleeton SH, Lee GE, Ugwu CS, Jones BD, BonDurant SS, Slonczewski JL. Acid and base stress and transcriptomic responses in Bacillus subtilis. Applied and environmental microbiology. 2009;75:981–990. doi: 10.1128/AEM.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Rawat S, Stemmler TL, Rosen BP. Arsenic binding and transfer by the ArsD As(III) metallochaperone. Biochemistry. 2010;49:3658–3666. doi: 10.1021/bi100026a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Georgiev O, Hagmann M, Gunes C, Cramer M, Faller P, Vasak M, Schaffner W. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Molecular and cellular biology. 2003;23:8471–8485. doi: 10.1128/MCB.23.23.8471-8485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.