Abstract
Background
This study was designed to investigate the effect of metformin and flutamide alone or in combination with anthropometric indices and laboratory tests of obese/overweight PCOS women under hypocaloric diet.
Methods
This single blind clinical trial was performed on 120 PCOS women. At the beginning, hypocaloric diet was recommended for the patients. After one month while they were on the diet, the patients were randomly divided in 4 groups; metformin (500 mg, 3/day), flutamide (250 mg, 2/day), combined, metformin (500 mg, 3/day) with flutamide (250 mg, 2/day) and finally placebo group. The patients were treated for 6 months. Anthropometric indices and laboratory tests (fasting and glucose-stimulated insulin levels, lipid profile and androgens) were measured. A one-way ANOVA (Post Hoc) and paired t-test were performed to analyze data. A p ≤ 0.05 was considered statistically significant.
Results
After treatment, reduction in weight, BMI, hip circumference was significantly greater in the metformin group in comparison to other groups (p<0.05). In addition, the fasting insulin was significantly greater in metformin group and flutamide group in comparison to metformin+flutamide and placebo groups after treatment (p<0.05). Within groups, insulin level showed significant changes (before and after treatment) in metformin+flutamide group and LDL reduction was significant in flutamide group before and after treatment. Post hoc tukey and two-tailed with p≤0.05 were used to define statistical significance.
Conclusion
Using combination of metformin and flutamide improves anthropometric indices and laboratory tests in obese/overweight PCOS women under hypocaloric diet.
Keywords: Body weight, Flutamide, Metformin, Polycystic ovary syndrome
Introduction
Polycystic ovarian morphology is displayed on ultrasound imaging in approximately 11.9±2.4 of women (1). The most common features of PCOS are hyperandrogenism, unovulation, infertility and weight gain. More than 50% of PCOS patients are obese (2). These women are at the risk of diabetes, atherosclerosis, and cardiovascular diseases (1, 3). Since obesity is the most common cause of insulin resistance and hyper-androgenism, weight reduction might be the first recommendation for PCOS women (4, 5). Many researchers introduced hypocaloric diet as an operant treatment for weight loss and improvement of fertility in the overweight/obese PCOS women. However, only 5-10% weight loss has a useful effect on insulin resistance and cardiovascular hemodynamic function (4–6).
Weight loss up to 5-7% during 6 month can restore ovulation and fertility in more than 75% of women (5). Weight loss induced by hypocaloric diet was intensified by treatment with metformin (7, 8). Hyperinsulinemia has a role in anovulation. In addition, several studies have shown weight loss reduces the severity of insulin resistance (9, 10). Treatment with insulin sensitizer agents may also alter endocrine imbalance, improves ovulation and fertility (11). Even some researchers recommended 3 months of pretreatment with metformin for obese women (12, 13). Pasquali et al. showed hirsutism and irregular menses in PCOS patients who received metformin were significantly improved in comparison to placebo group and also hypocaloric diet reduced BMI and waist circumference (WC) in PCOS patients as well as the controls. In both groups, treatment with metformin reduced weight and BMI significantly (14). Treatment with metformin reduces LH and androgens level and restores regular menses and ovulation (1, 2). Also, treatment with the antiandrogen drugs such as flutamide reduced hirsutism and abdominal fat (15). In Sahin et al.'s study, treatment with metformin improved insulin resistance and reduced androgen levels. However, flutamide only reduces androgen levels without improvement of insulin resistance in non-obese patients (16). Gambineri et al. studied 40 obese PCOS patients during 6 months and showed that hypocaloric diet with metformin or flutamide alone or in combination had a significant effect on body lipid distribution, androgen levels, hirsutism and menses (17). Gambineri in another study evaluated 80 women within 12 months and showed the benefits of long term treatment with metformin and flutamide and their compounds in obese PCOS patients under hypocaloric diet. In his study, flutamide reduced visceral fat, androgen levels and lipid profile after 12 months and improved glucose tolerance value in comparison to placebo and metformin though there was no synergistic effect in patients who simultaneously received the combination of the two drugs (18). However, the other study showed that hypocaloric diet with metformin or flutamide alone or in combination had a better therapeutic effect (14).
More recently, literature revealed contradictory findings about adding medicine to the diet and especially there has been little agreement on the beneficial effects of polytherapy; hence, in this study, an attempt was made to address the following question:
Do metformin and flutamide alone or combination of both improve anthropometric indices and laboratory tests in overweight and obese PCOS patients under hypocaloric diet?
Methods
First, 156 overweight and obese infertile PCOS women entered this single blind clinical trial (Clinical trial registration number: IRCT13880 7271760N3, May 21, 2010). These patients were referred to our PCOS clinic (Fatemezahra Infertility and Reproductive Health Research Center) in Babol (north of Iran) in 2009. Out of them, 117 women were recruited and randomized to the treatment after considering exclusion and inclusion criteria.
This study was approved by the ethical committee of Babol University of Medical Science. At the beginning, all participants signed an informed consent and entered the study. Inclusion criteria included all PCOS women of 18-40 years of age, body mass index (BMI) >19 and <35 kg/m2 and waist circumference of at least 88 cm (19). The diagnosis of PCOS was determined by presence of two or more following conditions of Rotterdam criteria; oligo-ovulation or anovulation (manifested as oligomenorrhea or amenorrhea), hyperandrogenemia (elevated levels of circulating androgens), hyperandrogenism (clinical manifestations of androgens excess) and polycystic ovaries detected by ultrasonography (20). Exclusion criteria were any drug consumers (hormonal and metformin) or cases who tried weight loss in 3 recent months, cases with hyperprolactinemia, cushing syndrome, late onset congenital adrenal hyperplasia, thyroid dysfunction, diabetes, cardiovascular, renal and liver diseases, pregnancy and also athletes. They were advised to use barrier methods of contraception throughout the study.
Participants
Before the initiation of the study, the patients underwent the physical examination focused on anthropometric indices (weight, BMI, waist and hip circumferences and hirsutism). The clinical examination focused on the evaluation of anthropometric and parametric values (height, weight and waist circumference) and also hirsutism using the Ferriman-Gallwey score (21). BMI was calculated by weight/height2 (kg/m2). Waist circumference was obtained as the minimum value between the iliac crest and the lateral costal margin, whereas hip circumference was determined as the maximum value over the glottal region to cm. Waist and hip circumferences were measured to the nearest centimeter with a soft tape (cm) according to World Health Organization (WHO) criteria. Before the study, the baseline values of anthropometric parameters were measured. No significant differences were shown in the values.
Hormonal and metabolic determinations included the evaluation of circulating concentrations of free and total testosterone, dehydroepiandroster-one sulfate (DHEA-S), sex hormone-bonding globulin (SHBG), triglyceride (TG), total and high-density lipoprotein (HDL) and Low-density (LDL) cholesterol and triglycerides (TG) in fasting blood samples. Also, fasting glucose test, 2-hour oral glucose tolerance test (OGTT) with 75 gr glucose (Parsazmoon Co, Iran) and fasting Insulin test were administered. All blood tests were performed, regardless of the menstrual cycle, at baseline and after 6 months. A qualified laboratory examined the blood samples (at baseline and the following 6-month reevaluation) through the same methods and materials. First, the baseline values of experiments were analyzed. It should be noted that no significant differences were seen in the anthropometric indices and laboratory tests.
To eliminate the confounding effect of diet, all patients received a standardized hypocaloric diet containing 1200-1400 kcal/day from a research dietitian. The diet consisted of 50% carbohydrates, 30% proteins and 20% fat prescribed by the same dietician attending our division, who calculated the dietary energy composition by subtracting at least 500 kcal from the usual energy intake of an individual. The data were gathered by means of a questionnaire. The final composition of the diets ranged between 1200 and 1400 kcal/d. It should be noted that all women were visited monthly for compliance with both dietary and pharmacological treatments. Diet was evaluated by the dietician at each monthly visit, according to previously defined criteria providing quantitative information on daily energy intake and composition of the diet consumed during the previous month. There was a strong reason to initiate the hypocaloric diet before treatment with medicine; all patients equally underwent the similar diet as the only impressive element without any medication intervention. Furthermore, patients were checked for their usual physical activity throughout the treatment by the standard physical activity questionnaire (the Persian version with high validity) before, 3 months following and at the end of the study (21, 22).
Randomization and intervention
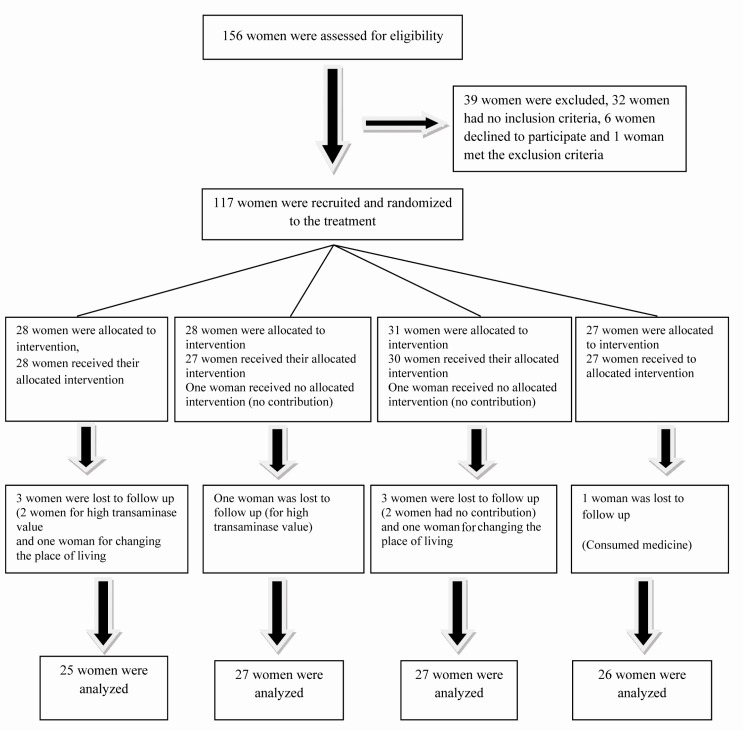
From total of 156 overweight or obese PCOS women, 117 women were recruited and randomized to the treatment (Figure 1). Following one month of diet, the participants were visited again. While continuing dietary treatment, the patients who had anthropometric indices and the recording of all preliminary laboratory tests, were randomly divided in 4 groups including, 1: metformin hydrocloride (Tablet, 500 mg, Tehran-shimi Co., Tehran, Iran), orally, 3/day, 2: flutamide-BIOSYN (Tablet, 250 mg, Sobhan Co., Tehran, Iran), 2/day, 3: metformin 500 mg, 3/day+ flutamide 250 mg, 2/day and 4: placebo group. Patients were randomly assigned to groups to receive the drugs (active or placebo) using a computer generated sequence concealed from the study participants and then allocation sequence was done by a third party. Fatemezahra Investigation Pharmacy transferred metformin, flutamide and placebo capsules with appropriate dose and the same color, then packed and labeled them according to subject number. The patients were blinded to the treatment. All subjects were given a 1-month supply of capsules and they returned for monthly visits. Also, to evaluate compliance with medications and to assess the side effects, patients were visited monthly. To evaluate the liver and renal toxicity at the beginning which were probably the results of medicines, renal and liver functional tests were requested and repeated every 2 months. Moreover, the remaining capsules were counted and a new supply of capsules was given to the patients. The patients were reviewed and recommended to stay on the diet and contraception.
Figure 1.
Patients recruited during 6-month treatment with hypocaloric diet and placebo, metformin, flutamide, or metformin+flutamide
Finally, at the end of the sixth month of treatment, physical examination focusing on anthropometric indices and all preliminary laboratory tests were done again and hirsutism was reevaluated. The same researcher performed all these assessments. All patients underwent anthropometric, hormonal and metabolic evaluation at baseline and 6 months following.
Statistical Analysis
Statistical evaluations were performed by running SPSS software package (SPSS Inc., Chicago, IL, USA) on a personal computer. The sample size was calculated by G*power software and Set α error prob to 0.05, Power to 0.80. The values in ANOVA had normal distribution and the variances of assumed variables were equal. The measures were reported as mean±SD.
Paired t-test was performed to estimate the within-group modification (before and after treatment at each time point). A one-way ANOVA (Post Hoc) was applied to compare values amongst the four groups at each time point (baseline, 6 months following), whereas tukey test was applied to find differences amongst the groups. The p≤0.05 were used to define statistical significance.
Results
Out of 156 PCOS patients who were eligible, 39 women were excluded; 32 women had no inclusion criteria, 6 women declined to participate and one woman met the exclusion criteria. 117 women were randomized in 4 groups; 28 women were allocated to receive metformin, 28 received flu-tamide (1 woman had no contribution) and 31 women received metformin+flutamide (1 woman had no contribution) and 27 women received placebo. Out of the metformin group, 3 women (1 woman for high transaminase level and 2 women for refusing to follow up), from the flutamide group, one woman for wrong use of medicine, were lost to follow up. Also, from the metformin+flutamide group, 4 women (2 women for the high transaminase level, 1 for refusing the follow up and one woman for the wrong use of medicine) and from placebo group, one woman were lost to follow up (for changing the place of living). Finally, 25 women in the metformin group, 27 in the flutamide group, 27 in the metformin+flutamide group and 26 in the placebo group were analyzed. Nobody was excluded from analysis (Figure 1). Their mean age was 25.6±4.02 and mean weight was 75.5±9.82 kg. No significant difference was seen in the anthropometric indices and laboratory tests in baseline values.
Comparing changes within the four groups (before and after treatment)
Table 1 summarizes the anthropometric indices and laboratory test changes (before and after treatment) within the four groups. To determine the within-group changes, paired t-test was applied.
Table 1.
Changes in anthropometric indices and laboratory tests within the 4 groups under study (before and after treatment)
| Variables | Changes within groups | |||
|---|---|---|---|---|
|
| ||||
| Metformin (n=25) | Flutamide (n=27) | Metformin+Flutamide (n=27) | Placebo (n=26) | |
| BMI(Kg/m2)d | 4.2±2 c | 2.8±1.9 c | 3 ±1.9 c | 3.9±1.9 c |
| WC(cm) | 10.9±6.8 c | 6.6±6.6 c | 9.4±5.7 c | 8.4±5.9 c |
| HC(cm)e | 10.4±7 c | 3.2±8.6 | 7.1±7 c | 6.5±7.8 c |
| W/H(cm) | 0.1 c | 0 b | 0.05 b | 0.05 b |
| Hirsutism score 1 | 2.5±3 c | 3.8±2.7 c | 4.3±11.5 c | 4.7±7 b |
| FBS( mg/dl ) | 2.6±12.8 | -0.8±10.5 | 6.9±10 c | -4.8±11.4 a |
| OGTT(2hr)(mg/dl) | -0.28±25.1 | 9.1±25.2 | 10±38.6 | 10.6±42.1 |
| Fasting Insulin(pM/l) | 1.4±11.1 | -1.4±11.3 | 10.0±8.4 c | 0.2±12.4 |
| TG (mM/l)d | 6.4±48 | 3.84±2.7 | 4.1±4.5 | 7±65.7 |
| Total Cholesterol( mM/l ) | 10.1±24.5 a | 21.3±33.7 b | 8.2±57.9 | 19.7±31 a |
| HDL( mM/l ) e | -1.2±12 | -3.8±14.4 | 4.2±11.5 | -3.8±11.3 |
| LDL( mM/l ) | 10.9±29.5 c | 19.9±30.1 b | -1±58.8 | 18.7±27.2 a |
| Total testosterone( nM/l ) | 0.4±0.6 c | 0.2±0.47 a | 0.4±0.6 b | 0.7 |
| Free testosterone( pM/l ) | 0.3±1.1 | -0.3±3.1 | 0.6±1.7 | -0.5±3.2 |
| DHEAS( µM/l ) | 2.7±71.1 | 31.2±126.7 | 42.3±82.4 a | 38.2±93.6 a |
| SHBG( nM/l ) | 1.5±18.2 | -5.5±47.8 | 0.63±14.6 | 2.8±16.3 |
Changes (post treatment value- pre treatment value) was significant at p<0.05
Changes (post treatment value- pre treatment value) was significant at p<0.01
Changes (post treatment value- pre treatment value) was significant at p<0.001
Comparing changes between groups was significant at p<0.05
Comparing changes between groups was significant at p<0.01
Ferriman-Gallway score
As well as placebo, the groups treated with metformin, flutamide, and metformin+flutamide showed significant reduction in all antropometric indices following 6 months in comparison to their baseline (p<0.01) except HC in flutamide group which was not significant.
In the glucose profile, the FBS initially demonstrated significant reduction within metformin+flutamide group and then within placebo group before and after treatment, respectively (p<0.001) (p<0.05). However, the fasting insulin showed no further modification after treatment compared to baseline except within metformin+flutamide group (p<0.001).
In the lipid profile, LDL had significant reduction in all groups except metformin+ flutamide following the treatment compared to baseline (metformin; p<0.001), (flutamide; p<0.01) (placebo; p<0.05).
In sex hormones, all groups demonstrated no significant reduction except DHEA (S) in which significant reduction was seen in metformin+flutamide group and also placebo after treatment compared to the baseline.
The menstrual pattern of our patients showed no significant differences before and after treatment.
Comparing amongst the four groups
The anthropometric indices and laboratory tests of four groups after treatment are summarized in Table 2.
Table 2.
Parametric indices and metabolic parameters of women among the four groups under study
| Criteria | Groups | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| After treatment Met (n=25) | After treatment Flu (n=27) | After treatment Met + flu (n=27) | After treatment placebo (n=26) | ||
| BMI (Kg/m2) | 28.9±5* | 29.57±4* | 29.3±2.6* | 29.2±3.6* | M vs. F vs. M+F vs. P: <0.001 |
| WC (cm) | 89.4±9.5* | 88.19±9* | 91.44±6.1* | 89.16±7.5* | M vs. F vs. M+F vs. P: <0.001 |
| HC ( cm ) | 110.6±10.8* | 110.1±11 | 109.74±6.5* | 110.36±5* | M vs. M+F vs. P: <0.001 |
| W/H ( cm ) | 0.8±0.1 | 0.8 | 0.83±0.04* | 0.8±0.05* | F vs. F+M vs. P: <0.001 |
| Hirsutism score 1 | 7.08±3.8* | 5±2.5* | 7.52±3.8 | 4.8±2.4* | P vs. F vs. M: <0.001 |
| FBS ( mg/dl ) | 81.9±8.1 | 83.7±7.2 | 79.26±8.3* | 85.73±10.2* | M+F vs. P: <0.001 |
| OGTT (2 hr ) ( mg/dl ) | 112.1±30.5 | 102.56±20.1 | 107.22±25.9 | 95.7±31.3 | NS |
| Fasting Insulin ( pM/l ) | 13.7±7.1* | 14.6±6.2 | 11.6±6.2* | 12.01±10.1* | M+F vs. M vs. P: <0.001 |
| TG ( mM/l ) | 122.3±41.1 | 133.6±72* | 140.6±65.9* | 128.6±76.4* | M+F vs. F, M, P: <0.001 |
| Total Cholesterol ( mM/l ) | 171.3±23.2* | 178.5±48* | 180.74±40.8 | 171.3±27.8* | P vs. F vs. M: <0.001 |
| HDL ( mM/l ) | 41.3±11.3 | 43±12.2 | 37.85±6 | 46.73±9.1 | NS |
| LDL ( mM/l ) | 100.74±19.7* | 105.8±32* | 121.04±81.2 | 99.12±23.7* | P vs. M vs. F vs. P: <0.001 |
| Total testosterone ( nM/l ) | 0.7±0.4* | 0.55±0.2 | 0.6±0.06* | 0.95±0.9* | P vs. M+F vs. M: <0.01 |
| Free testosterone ( pM/l ) | 2.32±1.3 | 2.18±1.9 | 2.6±1.3 | 2.9±2.7 | M+F: <0.05 |
| DHEAS (µM/l) | 222.5±129.1 | 145.46±81* | 156.08±73.6* | 161.52±68.07* | P vs. F, M+F, P: <0.05 |
| SHBG (nM/l) | 26.9±18.9 | 41.08±39 | 22.64±9.7 | 24.14±11.3 | NS |
Results are expressed as Mean±SD. The values were evaluated with the Post Hoc test; No significant differences among groups in baseline values; Difference by group p-≤0.05; NS=Non significant >0.05; Met=Metformin; Flu=Flutamide; P=Placebo
The difference between groups (in a row) is significant
Ferriman-Gallway score
There were no significant differences in the baseline values in our study. In anthropometric indices, significantly greater reductions were observed in BMI, WC and HC in the metformin group after treatment in comparison to other groups (p< 0.001). In sugar profile, the post treatment fasting blood sugar and fasting insulin were diminished significantly in metformin+flutamide group compared to other groups after treatment (p<0.001), while in the lipid profile, metformin+flutamide revealed reduction in TG and placebo reduced LDL significantly after treatment compared to other groups (p<0.001). In sex hormone profiles, the flutamide group was superior to others in reduction of total testosterone (p<0.01) and in the placebo, the reduction of DHEA(S) was observed after treatment (p<0.05) (Table 2).
It should be mentioned that, throughout the study, markers of liver dysfunction such as transaminases and lactate dehydrogenase were checked monthly and they remained unaltered except among 3 women.
Discussion
The result of our study indicates that the use of metformin and flutamide improves anthropometric indices and laboratory tests in obese/over-weight PCOS women under hypocaloric diet.
Metformin alone was successful to reduce BMI in PCOS patients. Generally, in obese women with anovulatory infertility, weight loss makes spontaneous ovulation facile and improves the chance of spontaneous conception (24). Those patients who ovulate and conceive while remaining obese will encounter predictable risks during pregnancy and post-pregnancy. Since pre-pregnancy weight loss reduces the incidence of gestational diabetes in these women (25), most therapists encourage PCOS women to reduce weight before pregnancy.
The findings of the current study are consistent with that of Morin-Papunen et al. who found metformin is able to decrease glucose disposal in skeletal muscle (26). Also, a number of studies have found the reduction in weight and BMI by metformin (7, 27, 28), however, there are many conflicting ideas about the effect of metformin on weight and BMI. Tang et al. concluded that metformin is less effective in very obese anovulatory women and a higher dose instead of routinely prescribed doses and a longer duration of therapy are necessary for these patients (29). In addition, Nieuwenhuis-Ruifrok in his systematic review concluded that addition of metformin treatment does not lead to greater weight loss in patients who are on a diet or who follow a lifestyle program (8). A possible explanation for those might be that regarding our patients’ mean BMI (32 k/m2), no further doses were needed for them.
Our unanticipated finding shows that the combination of the two drugs as a part of polytherapy provides beneficial effects on glucose reduction. Wadden concluded that more weight loss is achieved by combination of a lifestyle modification program with drug therapy in comparison to a lifestyle program alone (30). However, in Gambineri et al.'s research hypocaloric diet alone has a main role in improving insulin resistance and hyperinsulinaemia. Gambineri also conducted another study on PCOS patients during 12 months and concluded metformin alone decreased glucose-stimulated insulin but the combination of both drugs had no additive effect. He explained that some therapeutic effects would not be present during the first six months of treatment (18) though, in this paper this incidence took place within 6 month.
Our interesting finding was that metformin+ flutamide reduced TG more than other medicines except hypocaloric diet. This finding is not in agreement with Gambineri et al.‘s findings which showed none of the active treatment groups differed in changes of lipid concentrations after 6 months of treatment, whereas they observed a greater reduction in LDL cholesterol after 12 months in the metformin+flutamide group. Maybe the initial results of LDL reduction in our study relates to the lesser weights of our patients in comparison to the patients in their study (18).
One of the limitations of our study was small sample size of the obese women which overlapped with overweight women, so the effect of medicine on them versus overweight women could not be evaluated.
In our study, the menstrual pattern of our patients showed no significant differences with taking medicines or diet before and after treatment, whereas metformin further increased frequency of menstruation. Paradisi et al. reported that flutamide therapy showed a significant enhancement in the percentage of ovulation cycles and regulated the menstrual profile in their PCOS patients. Also, Gambineri reported that metformin further increased frequency of menstruation compared with placebo after 6 months (18, 32). This difference is probably related to higher BMI of their patients. Maybe, by dividing our patients’ menstruation pattern to regular and irregular, further beneficial findings could be offered. Well designed clinical trials on the current topic are therefore recommended, especially use of flutamide and metformin and also hypocaloric diet and determining their effect on clinical and biochemical characteristics of PCOS versus non PCOS patients can be an important area for investigation.
Lifestyle modification is strongly suggested for the treatment of overweight-obese PCOS women. It causes improvement in their general health and prevents long term consequences. Also, different beneficial treatment options according to our desired outcomes in the diet of overweight/obese PCOS women would help the experts to plan and treat patients according to each of their profile disturbance (any of the 4 groups). Considering the beneficial effects of hypochaleric diet compared to the medicine, life style and nutrition modification in PCOS patients was also effective in our study. Of course, considering findings about metformin and flutamide versus diet alone, it is supposed that our conclusions should be considered with caution and judiciously and they require more attention before clinical recommendations.
Conclusion
The most obvious finding of this study is that the use of metformin and flutamide improves and accelerates normalizing the anthropometric indices and laboratory tests in obese/overweight PCOS women under hypocaloric diet.
To cite this article: Amiri M, Golsorkhtabaramiri M, Esmaeilzadeh S, Ghofrani F, Bijani A, Ghorbani L, et al. Effect of Metformin and Flutamide on Anthropometric Indices and Laboratory Tests in Obese/Overweight PCOS Women under Hypocaloric Diet. J Reprod Infertil. 2014;15(4):205-213.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Glueck CJ, Phillips H, Cameron D, Sieve-Smith L, Wang P. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reducefirst-trimester spontaneous abortion: a pilot study. Fertil Steril. 2001;75(1):46–52. doi: 10.1016/s0015-0282(00)01666-6. [DOI] [PubMed] [Google Scholar]
- 4.Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81(3):630–7. doi: 10.1016/j.fertnstert.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82(2):421–9. doi: 10.1016/j.fertnstert.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 6.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60(2):241–9. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 7.Berek JS, Novak E. 14th ed. Philadelphia: Lippincott William & Wilkins; 2007. Berek & Novak's gynecology. [Google Scholar]
- 8.Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: systematic review and meta-analysis. Hum Reprod Update. 2009;15(1):57–68. doi: 10.1093/humupd/dmn043. [DOI] [PubMed] [Google Scholar]
- 9.Glueck CJ, Aregawi D, Agloria M, Winiarska M, Sieve L, Wang P. Sustainability of 8% weight loss, reduction of insulin resistance, and amelioration of atherogenic-metabolic risk factors over 4 years by metformin-diet in women with polycystic ovary syndrome. Metabolism. 2006;55(12):1582–9. doi: 10.1016/j.metabol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev. 2003;(3):CD003053. doi: 10.1002/14651858.CD003053. [DOI] [PubMed] [Google Scholar]
- 11.Ghazeeri GS, Nassar AH, Younes Z, Awwad JT. Pregnancy outcomes and the effect of metformin treatment in women with polycystic ovary syndrome: anoverview. Acta Obstet Gynecol Scand. 2012;91(6):658–78. doi: 10.1111/j.1600-0412.2012.01385.x. [DOI] [PubMed] [Google Scholar]
- 12.Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippelainen M, Perheentupa A, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97(5):1492–500. doi: 10.1210/jc.2011-3061. [DOI] [PubMed] [Google Scholar]
- 13.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012;5:CD003053. doi: 10.1002/14651858.CD003053.pub5. [DOI] [PubMed] [Google Scholar]
- 14.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–52. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85(8):2767–74. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 16.Sahin I, Serter R, Karakurt F, Demirbas B, Culha C, Taskapan C, et al. Metformin versus flutamide in the treatment of metabolic consequences of non-obese young women with polycystic ovary syndrome: a randomized prospective study. Gynecol Endocrinol. 2004;19(3):115–24. doi: 10.1080/09513590400004736. [DOI] [PubMed] [Google Scholar]
- 17.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60(2):241–9. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 18.Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab. 2006;91(10):3970–80. doi: 10.1210/jc.2005-2250. [DOI] [PubMed] [Google Scholar]
- 19.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i-xii):1–253. [PubMed] [Google Scholar]
- 20.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 21.Cook H, Brennan K, Azziz R. Reanalyzing the modified Ferriman-Gallwey score: is there a simpler method for assessing the extent of hirsutism? Fertil Steril. 2011;96(5):1266–70.e1. doi: 10.1016/j.fertnstert.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidlines for data processing and analaysis of the international physical activity questionnaire (IPQA) short and long forms. Med Sci Sports Exerc. 2005;37(11 Suppl):531–43. [Google Scholar]
- 23.Kelishadi R, Rabiei K, Khosravi A, Ardalan G, Gheiratmand R, Delavari A, et al. Assessment of physical activity in adolescents of Isfahan. J Shahrekord Univ Med Sci. 2001;3(2):55–66. [Google Scholar]
- 24.Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10(10):2705–12. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 25.Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733–7. doi: 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- 26.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: a randomized study. J Clin Endocrinol Metab. 2000;85(9):3161–8. doi: 10.1210/jcem.85.9.6792. [DOI] [PubMed] [Google Scholar]
- 27.Harborne L, Fleming R, Lyall H, Norman J, Sattar N. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361(9372):1894–901. doi: 10.1016/S0140-6736(03)13493-9. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo controlled, double-blind multicentre study. Hum Reprod. 2006;21(1):80–9. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 31.Li XJ, Yu YX, Liu CQ, Zhang W, Zhang HJ, Yan B, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf) 2011;74(3):332–9. doi: 10.1111/j.1365-2265.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 32.Paradisi R, Fabbri R, Battaglia C, Venturoli S. Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol Endocrinol. 2013;29(4):391–5. doi: 10.3109/09513590.2012.754876. [DOI] [PubMed] [Google Scholar]