Abstract
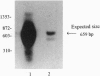
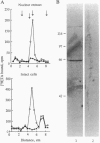
The primary biological effect of the estrogen estradiol-17 beta (17 beta E2) on bone is to decrease bone resorption. However, whether 17 beta E2 affects osteoclast differentiation or function directly or through its action on osteoblasts is unclear. To investigate this question we examined the human preosteoclastic cell line FLG 29.1 for evidence of functional estrogen receptors (ERs). Southern blotting of reverse transcription-PCR amplification products with a 32P-labeled cDNA probe for the human ER mRNA demonstrated that FLG 29.1 cells express ER mRNA. Binding of [3H]17 beta E2 to nuclear ERs was steroid specific with approximately 400 saturable, high affinity (Kd approximately 1 nM) binding sites per cell nucleus. Nuclear ERs covalently labeled with [3H]tamoxifen aziridine showed an apparent molecular weight of 65,000 by SDS/PAGE and Western blotting with the D75 monoclonal antibody to human ER. Pretreatment of cells with 0.1, 1.0, or 10 nM 17 beta E2 induced a dose- and time-dependent specific binding of progesterone to FGL 29.1 cells, and stimulation of the cells with 10 nM and 100 nM 17 beta E2 significantly (P < 0.05) reduced cell proliferation. Transcriptional activity of the ER gene was detected by transient transfection of cells with the pERE-BLCAT plasmid containing the estrogen response element for the vitellogenin A2 gene and the bacterial chloramphenicol acetyltransferase reporter gene. Treatment of FLG 29.1 cells with 10 nM 17 beta E2 increased chloroamphenicol acetyltransferase expression from 5- to 29-fold compared to controls. These observations suggest a potential role for estrogen in osteoclastogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzel U. S. Estrogens in the prevention and treatment of postmenopausal osteoporosis: a review. Am J Med. 1988 Dec;85(6):847–850. doi: 10.1016/s0002-9343(88)80033-0. [DOI] [PubMed] [Google Scholar]
- Brandi M. L., Crescioli C., Tanini A., Frediani U., Agnusdei D., Gennari C. Bone endothelial cells as estrogen targets. Calcif Tissue Int. 1993 Nov;53(5):312–317. doi: 10.1007/BF01351835. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curtis S. W., Korach K. S. Uterine estrogen receptor interaction with estrogen-responsive DNA sequences in vitro: effects of ligand binding on receptor-DNA complexes. Mol Endocrinol. 1990 Feb;4(2):276–286. doi: 10.1210/mend-4-2-276. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Ernst M., Parker M. G., Rodan G. A. Functional estrogen receptors in osteoblastic cells demonstrated by transfection with a reporter gene containing an estrogen response element. Mol Endocrinol. 1991 Nov;5(11):1597–1606. doi: 10.1210/mend-5-11-1597. [DOI] [PubMed] [Google Scholar]
- Ernst M., Schmid C., Froesch E. R. Enhanced osteoblast proliferation and collagen gene expression by estradiol. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2307–2310. doi: 10.1073/pnas.85.7.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli G., Zoppi S., Kohen F., Motta M. Synergistic effect of testosterone and of a luteinizing hormone-releasing hormone agonist on androgen receptor content in the ventral prostate of castrated rats. Steroids. 1989 Jan-Feb;53(1-2):195–217. doi: 10.1016/0039-128x(89)90153-0. [DOI] [PubMed] [Google Scholar]
- Fukayama S., Tashjian A. H., Jr Direct modulation by estradiol of the response of human bone cells (SaOS-2) to human parathyroid hormone (PTH) and PTH-related protein. Endocrinology. 1989 Jan;124(1):397–401. doi: 10.1210/endo-124-1-397. [DOI] [PubMed] [Google Scholar]
- Furlow J. D., Murdoch F. E., Gorski J. High affinity binding of the estrogen receptor to a DNA response element does not require homodimer formation or estrogen. J Biol Chem. 1993 Jun 15;268(17):12519–12525. [PubMed] [Google Scholar]
- Gattei V., Bernabei P. A., Pinto A., Bezzini R., Ringressi A., Formigli L., Tanini A., Attadia V., Brandi M. L. Phorbol ester induced osteoclast-like differentiation of a novel human leukemic cell line (FLG 29.1). J Cell Biol. 1992 Jan;116(2):437–447. doi: 10.1083/jcb.116.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Nolan C., Engler J. P., Jensen E. V. Monoclonal antibodies to human estrogen receptor. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5115–5119. doi: 10.1073/pnas.77.9.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen J. A., Carlson K. E., Heiman D. F., Robertson D. W., Wei L. L., Katzenellenbogen B. S. Efficient and highly selective covalent labeling of the estrogen receptor with [3H]tamoxifen aziridine. J Biol Chem. 1983 Mar 25;258(6):3487–3495. [PubMed] [Google Scholar]
- Koehorst S. G., Jacobs H. M., Tilanus M. G., Bouwens A. G., Thijssen J. H., Blankenstein M. A. Aberrant oestrogen receptor species in human meningioma tissue. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):57–61. doi: 10.1016/0960-0760(92)90187-n. [DOI] [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Hort E., Shine J., Baxter J. D., Greene G. L. Construction of cell lines that express high levels of the human estrogen receptor and are killed by estrogens. Mol Endocrinol. 1990 Oct;4(10):1465–1473. doi: 10.1210/mend-4-10-1465. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markaverich B. M., Williams M., Upchurch S., Clark J. H. Heterogeneity of nuclear estrogen-binding sites in the rat uterus: a simple method for the quantitation of type I and type II sites by [3H]estradiol exchange. Endocrinology. 1981 Jul;109(1):62–69. doi: 10.1210/endo-109-1-62. [DOI] [PubMed] [Google Scholar]
- Nawata H., Chong M. T., Bronzert D., Lippman M. E. Estradiol-independent growth of a subline of MCF-7 human breast cancer cells in culture. J Biol Chem. 1981 Jul 10;256(13):6895–6902. [PubMed] [Google Scholar]
- Oursler M. J., Osdoby P., Pyfferoen J., Riggs B. L., Spelsberg T. C. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensler J. M., Radosevich J. A., Higbee R., Langman C. B. Osteoclasts isolated from membranous bone in children exhibit nuclear estrogen and progesterone receptors. J Bone Miner Res. 1990 Aug;5(8):797–802. doi: 10.1002/jbmr.5650050802. [DOI] [PubMed] [Google Scholar]
- Pfeffer U., Fecarotta E., Castagnetta L., Vidali G. Estrogen receptor variant messenger RNA lacking exon 4 in estrogen-responsive human breast cancer cell lines. Cancer Res. 1993 Feb 15;53(4):741–743. [PubMed] [Google Scholar]
- Ponglikitmongkol M., Green S., Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988 Nov;7(11):3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzukerman M., Zhang X. K., Pfahl M. Inhibition of estrogen receptor activity by the tumor promoter 12-O-tetradeconylphorbol-13-acetate: a molecular analysis. Mol Endocrinol. 1991 Dec;5(12):1983–1992. doi: 10.1210/mend-5-12-1983. [DOI] [PubMed] [Google Scholar]