Introduction
Uterine carcinosarcoma (CS), also known as malignant mixed Mullerian tumors, are very aggressive tumors accounting for <4 % of uterine cancers. These uterine cancers have poor survival rates, even when diagnosed at an early stage [1]. Though surgery is the mainstay of treatment, the role of adjuvant chemotherapy and radiotherapy is being extensively studied as the postoperative recurrence rates and metastases are very high [2]. Hence, various single and combinations of chemotherapeutic regimens and radiotherapeutic approaches are being evaluated [3, 4, 5] Here, we present a rare case of CS with incisional site recurrence which was managed effectively by paclitaxel and cisplatin-based chemotherapy.
Case History
A 52-year-old postmenopausal lady, P5L5, was referred for complain of per vaginal bleeding and discharge for the past 9 month. In general, the examination was within normal limits. Speculum examination revealed the cervix and vagina to be otherwise normal. The uterus was small and retroverted, and both fornices were normal. Rectal mucosa was normal, and a bulky mass was felt in the pouch of Douglas.
The patient’s hematologic and biologic workup was within normal limits, except hypoproteinemia. CT abdomen and pelvis revealed a bulky, retroverted uterus with heterogeneously enhancing thickened endometrium (max thickness 6.5 cm) compressing the urinary bladder anteriorly; however, a fat plane was maintained. The mass was compressing the bilateral lower ureter resulting in bilateral hydroureter and hydronephrosis (R > L). Few enlarged lymph nodes are seen in the paraaortic, aortocaval, and mesenteric region with the largest, 2 × 1.8 cm, in the aortocaval region. Cervical Biopsy on July 10, 2011, reported endometrial stromal tumor. Surgical management was planned after consulting the urologist for D-J stenting.
On August 11, 2011, the patient was operated for total abdominal hysterectomy, bilateral salpingo-oophorectomy, and surgical staging (including peritoneal washings, suspicious areas or peritoneal surfaces sampled, infracolic omental sampling, pelvic and paraaortic lymph node sampling). Histopathology showed a CS homologous type invading the superficial myometrium and cervix. These cells are positive for EMA and negative for CD10; inhibin, Desmin, pelvic, and paraaortic lymph nodes were negative, peritoneal washing cytology was negative, sampling biopsies from the staging surgery were negative, and FIGO stage was IIA.
The patient was advised for adjuvant postoperative radiotherapy, but defaulted. After 4 months, she came with infra-umbilical incisional abdominal wall recurrence as shown in Fig. 1. Metastatic workup was done, which showed local and abdominal wall recurrence. She received paclitaxel (175 mg/m2) and cisplatin (75 mg/m2) chemotherapy for 6 cycles given at 3 week-intervals with complete resolution of abdominal wall tumor as shown in Fig. 2. The chemotherapy was tolerated well with grade II/III nausea and vomiting. After chemotherapy, the patient received radiotherapy and tolerated it well with grade III skin reactions.
Fig. 1.
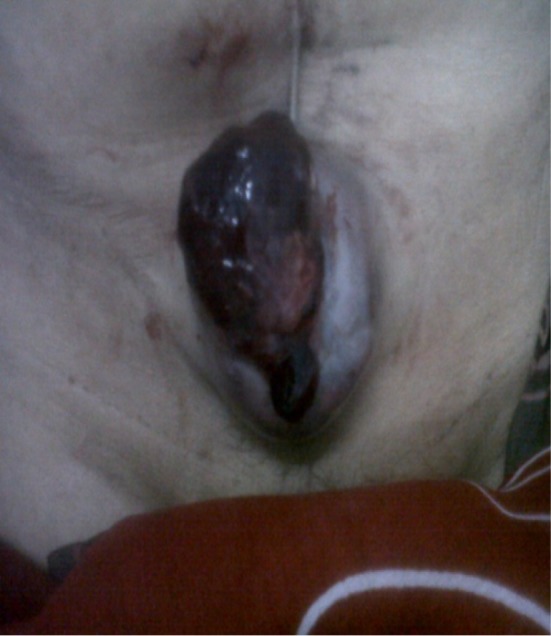
Infra-umbilical incisional recurrence
Fig. 2.
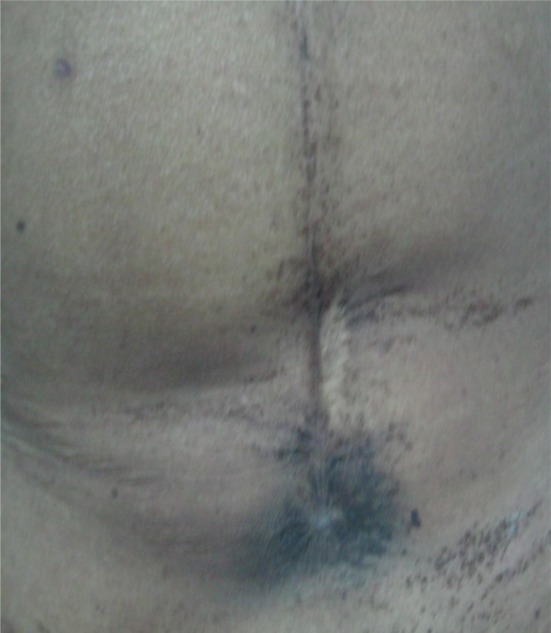
Post-chemotherapy response
Discussion
Uterine sarcomas account for up to 3 % of uterine malignancies, of which CS constitutes almost half with an overall survival rate of 30–40 % [2]. According to the revised FIGO 2009 classification, CS is separated from uterine sarcomas and has been classified with endometrial carcinomas. It has also been termed malignant mixed Mullerian tumor of the uterus. Most of the cases reported are of the postmenopausal age group and are usually present with postmenopausal bleeding or pelvic mass. Surgery (total abdominal hysterectomy with bilateral salpingo-oophorectomy and peritoneal washings with or without pelvic and paraaortic lymphadenectomy) remains the standard management, but the high rate of recurrence and metastasis necessitate adjuvant therapy [1].
In over half of the patients, recurrences in uterine CSs are seen after primary surgical and adjuvant therapy, mostly within 1 year. Recurrences are also associated with distant metastasis. Lung, peritoneum, pelvic or paraaortic lymph nodes, adrenal gland or bone, heart or pericardium, and/or brain are the common sites of metastasis.
This patient was diagnosed with CS homologous type, stage IIA, invading the superficial myometrium and cervix, and underwent surgery. Postoperatively, the patient was advised radiotherapy, which the patient defaulted on, then presented with rare recurrence at the incisional site on the infra-umbilical abdominal wall region after 4 months of surgery. Recurrence at the abdominal wall incisional site is a rare incidence, but similar recurrences have been documented in a case of adenocarcinoma of the endometrium.
Both the adjuvant radiotherapy and chemotherapy alone have not shown any overall survival benefit, though radiotherapy improves local control regardless of the lymph node involvement. Chemotherapy studies have shown differing response rates with various chemotherapeutic combinations. Phase II trials evaluating the combination of paclitaxel with carboplatin in advanced CS have shown an ORR between 54 and 62 % [5, 6].
Paclitaxel and cisplatin have demonstrated moderate activity in patients with advanced carcinoma of the endometrium, and the combination of these two agents has shown good ORR in a variety of solid tumors, but its activity in CS is yet to be studied. This patient has received paclitaxel and cisplatin chemotherapy for 6 cycles where the tumor responded completely. The patient also tolerated the therapy well. Thus, the regimen of cisplatin-paclitaxel is another combination therapy that could be evaluated further.
References
- 1.Sartori E, Bazzurini L, Gadducci A, et al. Carcinosarcoma of the uterus: a clinicopathological multicenter CTF study. Gynecol Oncol. 1997;67:70–75. doi: 10.1006/gyno.1997.4827. [DOI] [PubMed] [Google Scholar]
- 2.Callister M, Ramondetta LM, Jhingran A, et al. Malignant mixed mullerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004;58(3):786–796. doi: 10.1016/S0360-3016(03)01561-X. [DOI] [PubMed] [Google Scholar]
- 3.Thigpen JT, Blessing JA, Orr JW, Jr, et al. Phase II trial of cisplatin in the treatment of patients with advanced or recurrent mixed mesodermal sarcomas of the uterus: a gynecologic oncology group study. Cancer Treat Rep. 1986;70:271–274. [PubMed] [Google Scholar]
- 4.Sutton GP, Blessing JA, Rosenshein N, et al. Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a gynecologic oncology group study) Am J Obstet Gynecol. 1989;161:309–312. doi: 10.1016/0002-9378(89)90507-3. [DOI] [PubMed] [Google Scholar]
- 5.Powell MA, Filiaci VL, Rose PG, et al. Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a gynecologic oncology group study. J Clin Oncol. 2010;28:2727–2731. doi: 10.1200/JCO.2009.26.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacour RA, Euscher E, Atkinson EN, et al. A phase II trial of paclitaxel and carboplatin in women with advanced or recurrent uterine carcinosarcoma. Int J Gynecol Cancer. 2011;21:517–522. doi: 10.1097/IGC.0b013e31820da9e2. [DOI] [PubMed] [Google Scholar]


