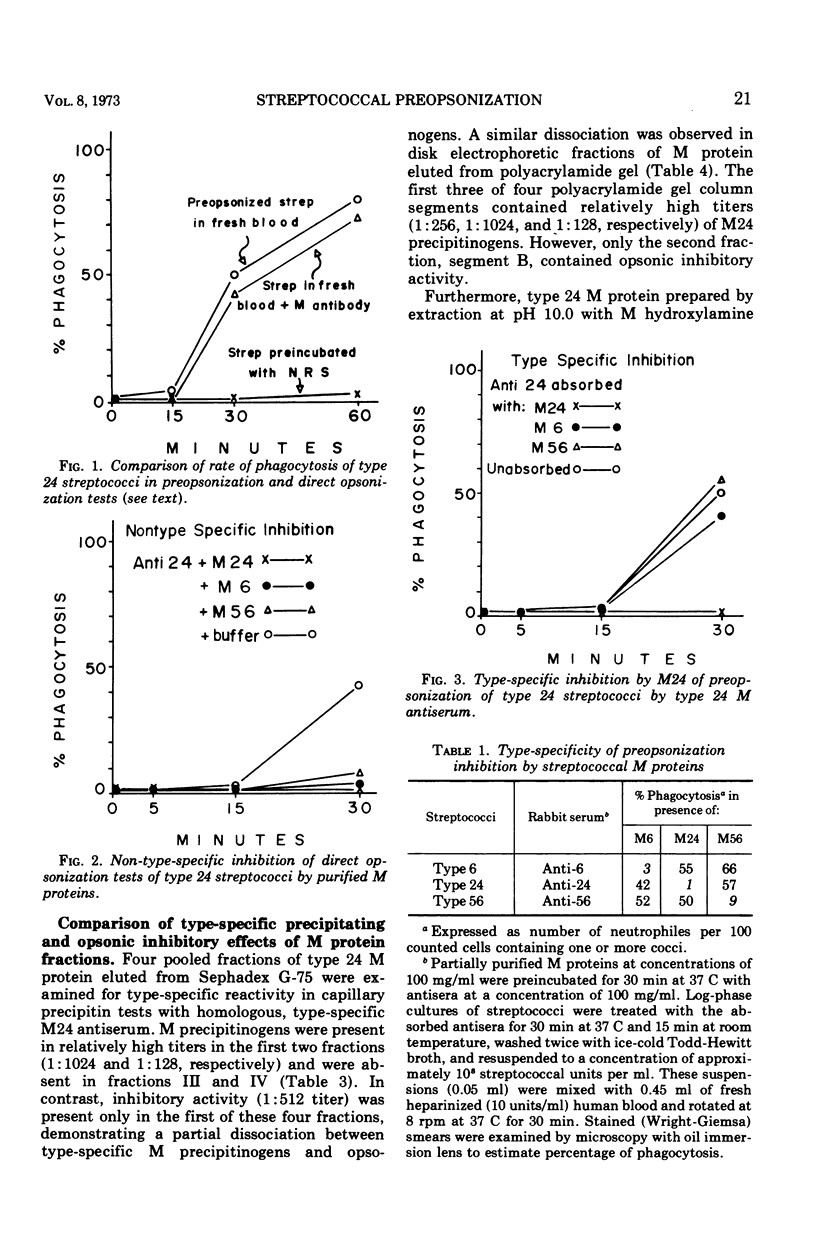
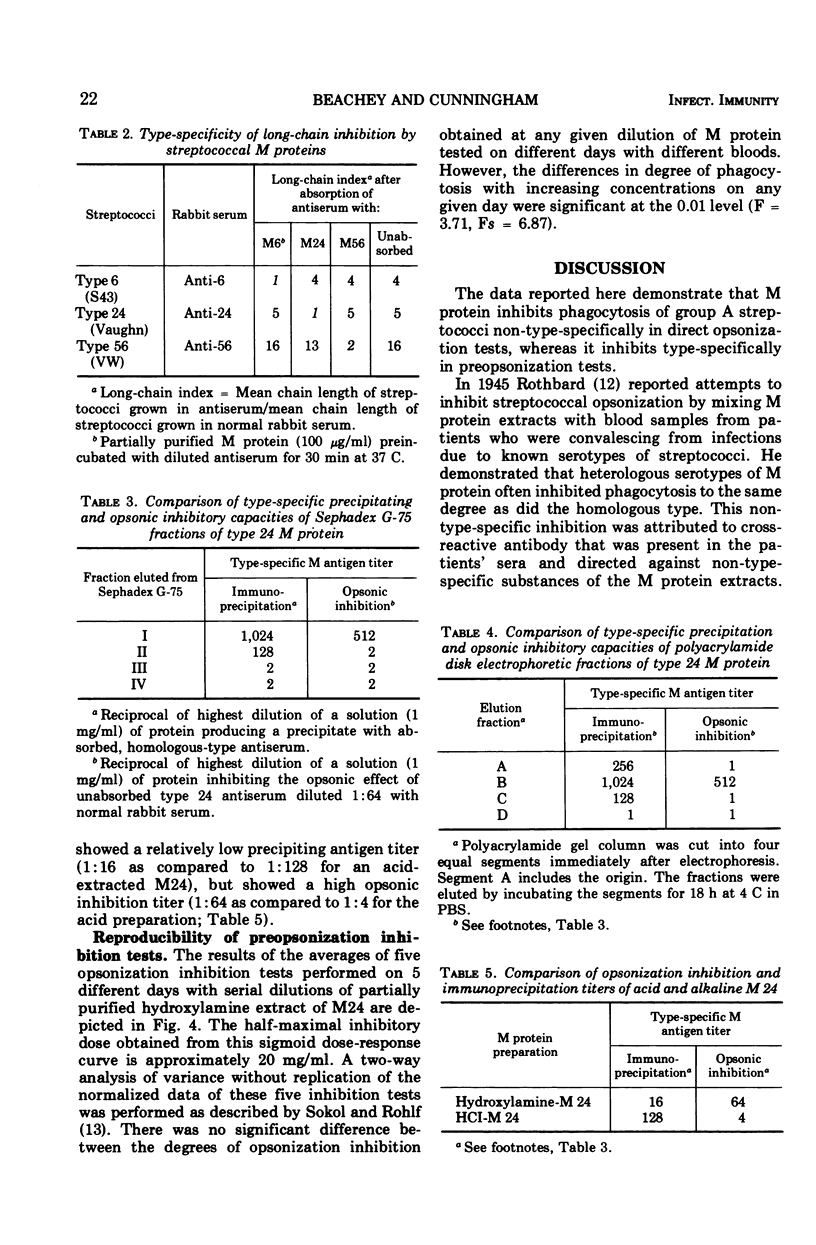
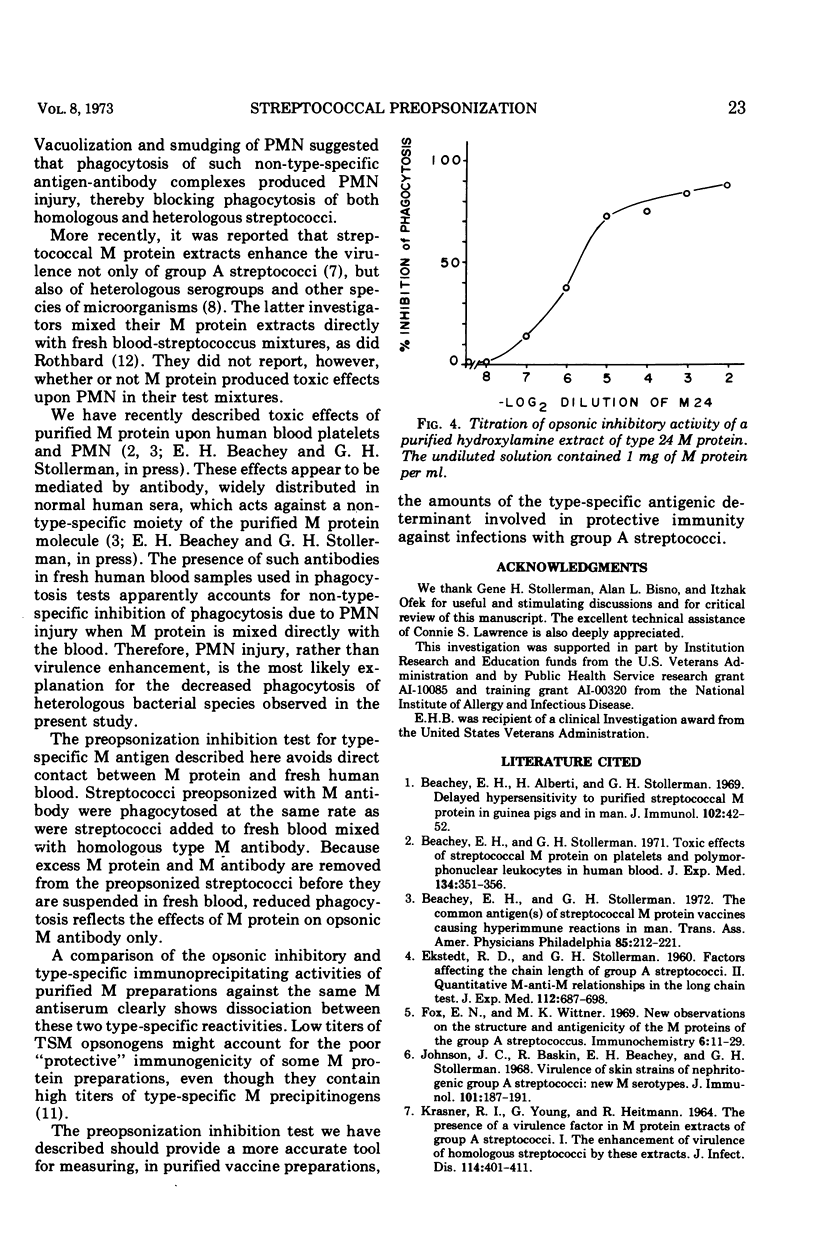
Abstract
Purified M protein of group A streptococci inhibits in vitro phagocytosis of bacteria nonspecifically when it is mixed directly with the test blood. We devised a method of preopsonization inhibition that avoids direct contact between the test blood and the opsonic inhibitory agent. Each of the M proteins tested by this method inhibited opsonization and phagocytosis of only homologous-type streptococci. Titration of the M antigen in various purified preparations demonstrated a clear dissociation between titers of type-specific precipitating M antigen and type-specific opsonic inhibitory M antigen. Since opsonization reflects protective antibody, assays of M protein based on reaction with opsonic antibody should reflect more accurately the presence of the type-specific M determinant involved in protective immunity against streptococcal infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Alberti H., Stollerman G. H. Delayed hypersensitivity to purified streptococcal m protein in guinea pigs and in man. J Immunol. 1969 Jan;102(1):42–52. [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H. The common antigen(s) of streptococcal M protein vaccines causing hyperimmune reactions in man. Trans Assoc Am Physicians. 1972;85:212–221. [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H. Toxic effects of streptococcal M protein on platelets and polymorphonuclear leukocytes in human blood. J Exp Med. 1971 Aug 1;134(2):351–365. doi: 10.1084/jem.134.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKSTEDT R. D., STOLLERMAN G. H. Factors affecting the chain length of group A streptococci. II. Quantative M-anti-M relationships in the long chain test. J Exp Med. 1960 Oct 1;112:687–698. doi: 10.1084/jem.112.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. New observations on the structure and antigenicity of the M proteins of the group A streptococcus. Immunochemistry. 1969 Jan;6(1):11–24. doi: 10.1016/0019-2791(69)90174-8. [DOI] [PubMed] [Google Scholar]
- KRASNER R. I., YOUNG G., HEITMANN R. THE PRESENCE OF A VIRULENCE FACTOR IN M PROTEIN EXTRACTS OF GROUP A STREPTOCOCCI. I. THE ENHANCEMENT OF VIRULENCE OF HOMOLOGOUS STREPTOCOCCI BY THESE EXTRACTS. J Infect Dis. 1964 Dec;114:401–411. doi: 10.1093/infdis/114.5.401. [DOI] [PubMed] [Google Scholar]
- Krasner R. I., Heitmann R. The presence of a virulence factor in group A streptococcal acid extracts. 2. The enhancement of virulence of heterologous streptococci and other organisms by these extracts. J Infect Dis. 1968 Feb;118(1):39–46. doi: 10.1093/infdis/118.1.39. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Ofek I., Bergner-Rabinowitz S., Davies A. M. Opsonic capacity of type specific streptococcal antibodies. Isr J Med Sci. 1969 May-Jun;5(3):293–296. [PubMed] [Google Scholar]
- STOLLERMAN G. H., EKSTEDT R. Long chain formation by strains of group A streptococci in the presence of homologous antiserum: a type-specific reaction. J Exp Med. 1957 Sep 1;106(3):345–356. doi: 10.1084/jem.106.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]



