Summary
Background
In bright light, mammals use a distinct photopigment (melanopsin) to measure irradiance for centrally mediated responses such as circadian entrainment. We aimed to determine whether the information generated by melanopsin is also used by the visual system as a signal for light adaptation. To this end, we compared retinal and thalamic responses to a range of artificial and natural visual stimuli presented using spectral compositions that either approximate the mouse’s experience of natural daylight (“daylight”) or are selectively depleted of wavelengths to which melanopsin is most sensitive (“mel-low”).
Results
We found reproducible and reversible changes in the flash electroretinogram between daylight and mel-low. Simultaneous recording in the dorsal lateral geniculate nucleus (dLGN) revealed that these reflect changes in feature selectivity of visual circuits in both temporal and spatial dimensions. A substantial fraction of units preferred finer spatial patterns in the daylight condition, while the population of direction-sensitive units became tuned to faster motion. The dLGN contained a richer, more reliable encoding of natural scenes in the daylight condition. These effects were absent in mice lacking melanopsin.
Conclusions
The feature selectivity of many neurons in the mouse dLGN is adjusted according to a melanopsin-dependent measure of environmental brightness. These changes originate, at least in part, within the retina. Melanopsin performs a role analogous to a photographer’s light meter, providing an independent measure of irradiance that determines optimal setting for visual circuits.
Highlights
-
•
Adjustments in visual performance in daylight conditions are driven by melanopsin
-
•
Melanopsin adjusts visual feature selectivity in spatial and temporal dimensions
-
•
Melanopsin facilitates a richer and more reliable encoding of natural scenes
Allen et al. show that aspects of light adaptation rely on a measure of brightness provided by melanopsin, the retina’s most recently discovered photoreceptor. Recordings from the mouse thalamus show that melanopsin controls the types of visual features that excite single neurons, allowing a richer, more reliable representation of natural scenes.
Introduction
The visual system is charged with encoding visual information across the >109-fold change in background light intensity from starlight to cloudless midday. The switch between rod- and cone-based vision and adjustments in photoreceptor sensitivity are central to meeting this challenge. However, the behavior of circuits in the retina is also critical, with multiple examples of visual signals being shifted between parallel pathways with different computational characteristics, and of the behavior of individual elements within these pathways changing as a function of irradiance [1, 2]. Such network changes do not merely adjust sensitivity and avoid saturation, but optimize circuit behavior to ensure efficient extraction of visual information (see [3, 4]).
Effective adaptation relies upon an accurate measure of light intensity. One might expect that adaptation state would be defined by the most accurate available measure of irradiance; under many circumstances, this is provided by a particular class of retinal ganglion cell [5, 6, 7, 8]. These intrinsically photosensitive retinal ganglion cells (ipRGCs) have their own melanopsin-dependent phototransduction mechanism [9, 10, 11], and employ this, along with extrinsic signals originating with rods and cones, to encode light levels over many decimal orders [6].
The information generated by ipRGCs is exported to the brain where it entrains circadian clocks and sets physiological and behavioral states [12, 13]. The hypothesis that ipRGCs also provide irradiance information to the retina contradicts a standard assumption of retinal function that information flows via ganglion cells to the brain, but not back into the retina. However, there is a growing body of evidence that ipRGCs do not obey this rule. In 2002, it was shown that a diurnal rhythm in an aspect of the human cone electroretinogram (ERG) may be regulated by a photoreceptor with melanopsin-like spectral sensitivity [14]. This was followed by data that revealed ipRGCs make gap-junction connections with neighboring amacrine cells [15, 16] and send axon collaterals to the retinal inner plexiform layer [17]. Meanwhile, there is also physiological evidence that ipRGCs excite dopaminergic amacrine cells, which themselves are influential modulators of retinal circuitry [18].
Establishing whether aspects of network light adaptation really are driven by ipRGCs and what impact (if any) this has on visual function is technically challenging. Historically, the starting point for assigning functions to ipRGCs has been eliminating rod and cone photoreception using genetic or pharmacological approaches [19, 20]. However, such preparations are ill suited to revealing ipRGC influences on conventional vision. Comparing visual responses of wild-type and melanopsin knockout mice could be more informative, but interpreting such data is complicated by evidence that retinal development and retinal circadian rhythms are disrupted in animals lacking melanopsin [21, 22, 23, 24].
Here, we therefore set out to explore ipRGC influences on visual responses in animals with an intact retina. Our approach adopts the ideas of metamerism and receptor silent substitution from the field of human psychophysics. In brief, we employed a transgenic mouse (Opn1mwR [25]) in which the spectral sensitivity of cone photoreceptors is substantially shifted compared to that of melanopsin. Using a multispectral light source, we were able to produce background lighting conditions that were equivalent for cones but differed substantially in their effective photon flux for melanopsin. We found substantial differences in retinal and thalamic responses to visual stimuli presented under these two conditions. These differences could be explained, at least in part, by changes in visual feature selectivity of individual units and were associated with alterations in the dorsal lateral geniculate nucleus’ (dLGN’s) ability to encode natural scenes.
Results
Mouse Cone Metamers
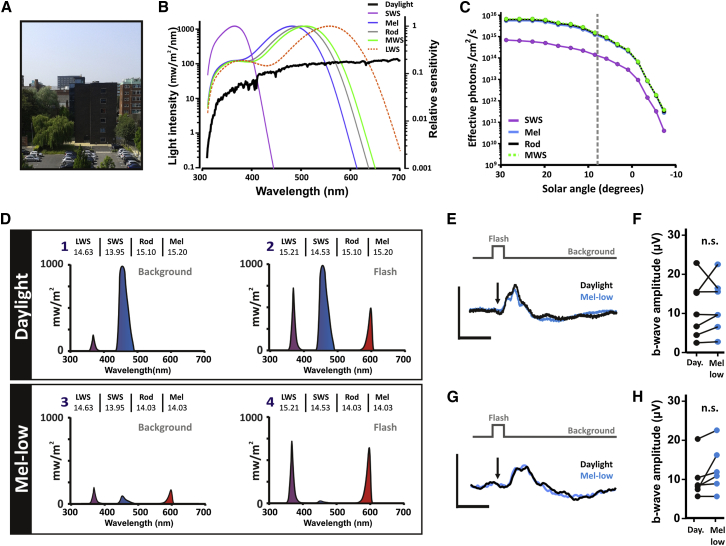
Our strategy for determining whether melanopsin modulates vision was to compare responses to the same visual stimuli presented against backgrounds appearing equivalent to conventional photoreceptors but differing substantially in effective irradiance for melanopsin. Given the similar spectral sensitivity of mouse medium-wavelength-sensitive (MWS) cone opsin and melanopsin (Figure 1B), to achieve this we employed transgenic mice (Opn1mwR) with a fully intact visual system but in which the red-shifted human long-wavelength-sensitive (LWS) cone opsin is knocked into the MWS cone opsin locus [25] (Figure 1B). A bespoke light source in which the output of three spectrally distinct light-emitting diodes (LEDs) could be independently modulated allowed great scope for generating spectra differing in melanopsin effective photon flux but predicted to be isoluminant (“metameric”) for short-wavelength-sensitive (SWS) and LWS cone opsins.
Figure 1.
Polyspectral Stimuli Allowing Independent Control of Irradiance as Experienced by Cones versus Melanopsin
(A–H) To ensure that some of our stimuli approximated the mouse’s experience of natural light, we recorded spectral irradiance in an urban scene (A) in Manchester over a dusk transition. The resultant spectral power distributions (B, black line shows data for solar angle 11.2°) were multiplied by spectral efficiencies of mouse SWS opsin, melanopsin, rod opsin, and MWS opsin (purple, blue, gray, and green lines, respectively) to calculate effective corneal irradiance in photons/cm2/s for each photopigment over a range of solar angles (C). To allow independent modulation of cone opsins versus melanopsin, these experiments employed Opn1mwR mice in which mouse MWS opsin is replaced by red-shifted human LWS cone opsin (red dotted line in B). A three primary LED light source (peak emission at 365, 460, and 600 nm) produced four spectrally distinct stimuli shown in (D) with log10 effective photon fluxes for each photopigment in inset. Spectra 1 and 2 approximated the mouse’s experience of natural light at solar angle +8°, while spectra 3 and 4 were selectively denuded of those wavelengths to which melanopsin is most sensitive. The individual elements of these daylight and mel-low stimulus pairs were designed to be rod and melanopsin isoluminant but to differ in effective irradiance for SWS opsin and LWS opsin. By contrast, spectra 1 and 3 and spectra 2 and 4 were designed to be cone isoluminant but to differ substantially in effective photon flux for melanopsin and rods. As a result, switching from either spectrum 1 to 2 or spectrum 3 to 4 produced a 58% Michelson contrast step to cones presented against backgrounds differing substantially in effective photon flux for rods and melanopsin. This was validated by measuring ERG responses to 200 repeats of 1 Hz, 50 ms transitions from either spectrum 1 to 2 and back again (daylight) or spectrum 3 to 4 and back (mel-low). Two control ERG measurements were made in response to daylight and mel-low stimuli: (1) in Opn1mwR mice at a moderate intensity (100-fold dimmer than maximum and hence with reduced melanopsin excitation; E and F) and (2) in Opn4−/−;Opn1mwR mice at the maximum intensity (G and H). (E) and (G) show representative traces in daylight (black traces) and mel-low (blue traces); arrow indicates time of flash. Scale bars, 100 ms (x); 40 μV (y). Population response amplitudes are plotted in (F) and (H). Data were compared with paired t tests. In each control condition, responses to daylight and mel-low had equivalent amplitudes (p > 0.05).
From spectra matching this requirement, we chose two pairs (Figure 1D) that could be used to generate a 50 ms “flash” (stimuli 2 and 4) presented against backgrounds differing in melanopsin photon flux (stimuli 1 and 3). These combinations had the following characteristics. (1) The flash should be visible to cones but not rods or melanopsin. We were interested in modulatory rather than direct contributions of melanopsin to flash responses and thus aimed to make the elements of each background and flash combination melanopsin isoluminant. By working at high irradiances, we hoped to minimize rod influences on our recordings. Nevertheless, as a further precaution, we set background and flash elements to be rod isoluminant. (2) The cone experience of the flash stimulus should be equivalent for the two pairs. Setting both backgrounds and both flashes isoluminant for both SWS and LWS cone opsins across the pairs ensured that the two stimulus conditions were equivalent for each individual cone irrespective of its relative expression of the two pigments [26]. (3) One of the conditions approximates the mouse’s experience of natural daylight. We recorded spectral irradiance profiles in horizontal view over a dusk transition in an urban setting (solar angles from −9° to 30° under clear skies but outside of direct sunlight; Figures 1A and 1B) and modeled the mouse’s experience of these conditions by calculating the effective photon flux for each of the mouse photopigments (Figure 1C). At all positive solar elevations, the effective photon flux (melanopsin, rod opsin, and MWS opsin) was roughly equivalent and ∼10 times greater than that for SWS opsin. Our first stimulus pair (spectra 1 and 3) maintained these activity ratios and recreated the mouse’s experience of a solar angle of +8° on our representative day. We therefore refer to this condition as “daylight.” In the other stimulus pair (spectra 2 and 4; termed “mel-low”), the effective photon flux for melanopsin was selectively reduced by 10 times.
As these experiments rely upon the daylight and mel-low conditions appearing equivalent to mouse cones (at least within the resolution of our methods of assaying visual response), we first undertook control experiments to confirm that this was true (see also Figure S1 available online). Initially, we based these upon electroretinography (although see also Figure 3). As melanopsin is increasingly active at brighter backgrounds, our first control was to show that ERG responses to daylight and mel-low conditions (50 ms transition from “background” to “flash” spectra at 1 Hz) were indistinguishable at a moderate irradiance (Figures 1E and 1F). We next showed that responses to these stimuli were identical in mice lacking melanopsin (Opn4−/−;Opn1mwR) at both moderate (not shown) and high background irradiances (Figures 1G and 1H).
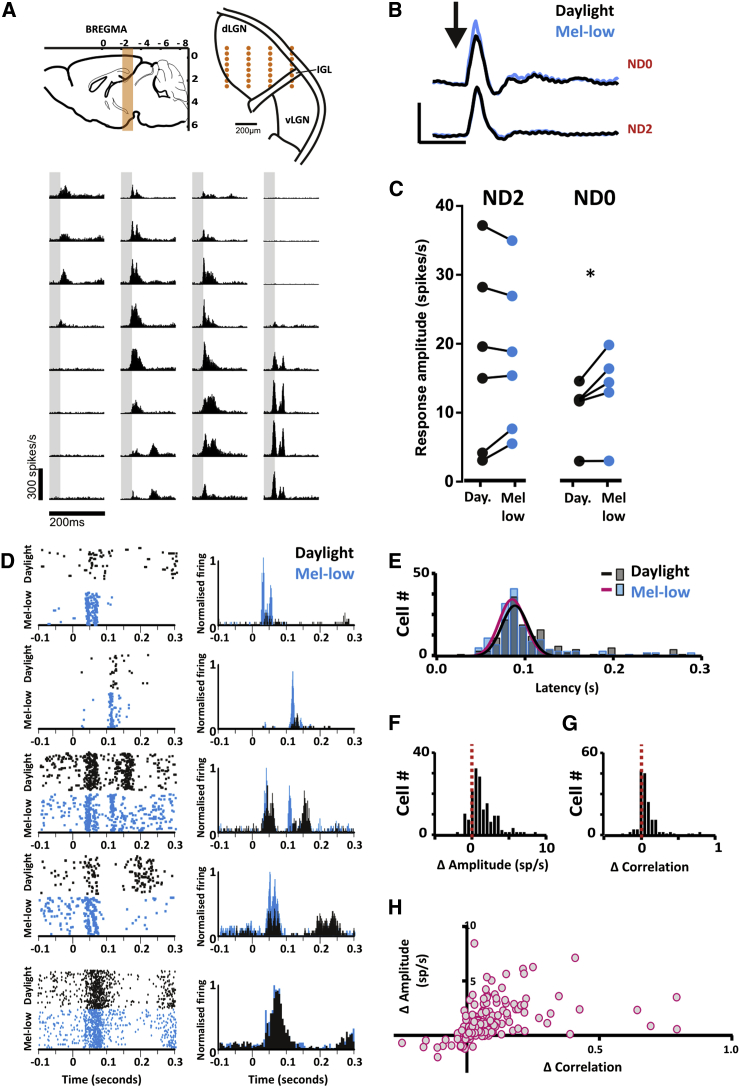
Figure 3.
Melanopsin-Dependent Changes in the dLGN Response to Flash Stimuli
(A and B) Responses to daylight and mel-low flashes (50 ms flash at 1 Hz, 200 repeats) in contralateral dLGN were recorded using multielectrode probes. (A) Top: targeted region in sagittal (left) and coronal (right) views, with projected recording site positions superimposed in orange. Bottom: representative multiunit responses recorded in the dLGN of an Opn1mwR mouse. Histograms show mean changes in firing rate detected at each of 32 recording sites (shown in gray). (B) Changes in multiunit activity averaged across multiple recording sites for a representative Opn1mwR mouse in daylight and mel-low conditions (black and blue lines, respectively), at full (ND0) and 100 times reduced (ND2) irradiance. Scale bars, 250 ms (x); 10 spikes/s (y).
(C) Mean change in multiunit firing rate over the 200 ms following flash onset for recording sites across the dLGN of six Opn1mwR mice was significantly different under daylight (black) and mel-low (blue) at ND0 but not ND2 (paired t tests). See also Figure S2D. Responses of representative single units reveal that the change in global response amplitude between mel-low and daylight conditions at high irradiance (ND0) are associated with alterations in response properties for many single units. At left are peri-event rasters for 200 repeats, with associated peri-event time histograms to the right (flash onset at time zero).
(D) Responses of representative single units reveal that the change in global response amplitude between mel-low and daylight conditions at high irradiance (ND0) are associated with alterations in response properties for many single units. At left are peri-event rasters for 200 repeats, with associated peri-event time histograms to the right (flash onset at time zero).
(E–H) The distribution of response latencies for single units (E) reveals a slight change in timing between conditions but no significant alteration in response synchrony between mel-low and daylight conditions (p > 0.05 for F-test comparison of Gaussians fitted to the two distributions, R2 > 0.8). Distributions of differences in response amplitude (F) and trial-to-trial reproducibility (G; quantified as Pearson’s correlation coefficient) for single units between conditions (mel-low − daylight) reveal that a large fraction of units showed increases in response amplitude and reliability in the mel-low condition. There was a significant correlation between these two features (Pearson’s correlation coefficient R2 = 0.16, p < 0.0001) across the population of units (H). Data shown are from 161 single units isolated in six mice.
Melanopsin-Driven Modulation of the Cone Flash ERG
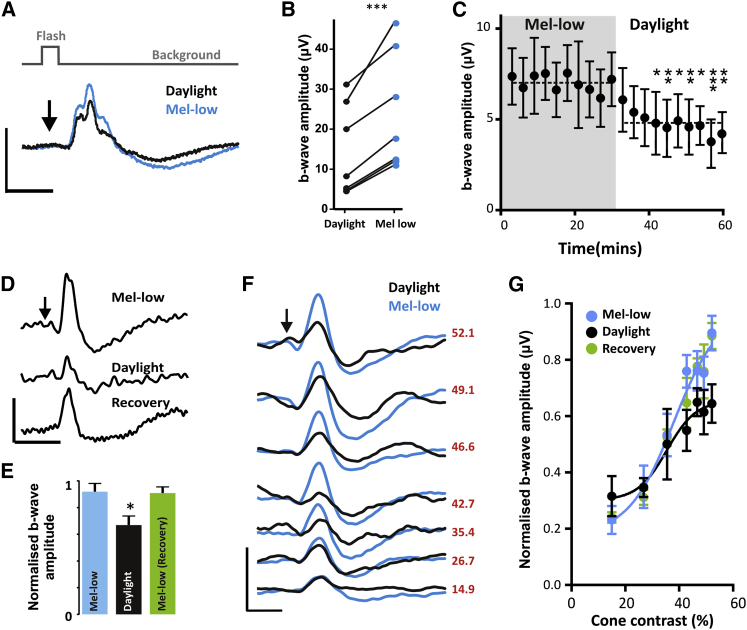
Having validated daylight and mel-low stimuli, we continued to present them to Opn1mwR mice at a high, melanopsin-active irradiance. ERG b-wave amplitude was reproducibly enhanced in the mel-low condition (Figure 2B). This change built up gradually over several minutes following transition from mel-low to daylight backgrounds (Figure 2C) and was reversible (Figures 2D and 2E). By changing our spectra to produce flash stimuli representing a range of increases in effective cone photon flux, we described contrast-response relationships under background and mel-low conditions for this flash ERG. Behavioral contrast sensitivity has recently been reported to be impaired in Opn4−/− mice [27]; however, we did not find an equivalent effect of dynamic modulations in melanopsin activity. Thus, b-wave amplitude was greater across most contrasts in the mel-low condition, indicating increased response gain, but no change in contrast sensitivity per se (Figures 2F and 2G).
Figure 2.
Melanopsin-Dependent Changes in the Cone ERG
(A and B) ERG traces (A) from a representative Opn1mwR mouse to 200 repeats of 1 Hz, 50 ms daylight (black traces) or mel-low (blue traces) flashes at maximum intensity (irradiances as in Figure 1D). Arrow indicates time of flash. Scale bars, 100 ms (x); 40 μV (y). The increase in b-wave amplitude apparent in the mel-low condition in this representative trace was observed in all seven Opn1mwR mice for which data were recorded (B, black symbols represent b-wave amplitude in daylight condition and blue circles in mel-low condition; paired t test, p > 0.001).
(C) The change in ERG b-wave occurred gradually following the switch from mel-low to daylight conditions. Data show mean ± SEM of b-wave amplitude to 180 repeats of the 1 Hz flash stimulus in seven mice. These data were collected in parallel with those for Opn4−/−;Opn1mwR data shown in Figure S2A and compared by two-way ANOVA (significant effects of genotype and genotype-time interactions [p < 0.05]; post hoc Bonferroni multiple comparisons tests against Opn1mwR at time zero revealed significant differences for daylight recordings after approximately 9 min [∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001]).
(D and E) Transitions from mel-low to daylight and then back again confirmed that the changes in response were reversible; shown for ERG traces from a representative mouse (D) and for normalized b-wave amplitude from six mice (E; mean ± SEM; one-way ANOVA with a post hoc Bonferroni multiple comparisons test: mel-low versus daylight, p < 0.05; mel-low versus mel-low [recovery], p > 0.05).
(F and G) The contrast response relationship differed between daylight and mel-low conditions. ERG traces (F) from a representative mouse exposed to variants of daylight and mel-low stimuli in which the increase in effective cone excitation of the flash (spectra 2 and 4) was altered (Michelson contrasts provided at right) while maintaining rod and melanopsin isoluminance. Scale bars, 100 ms (x); 20 μV (y). Population contrast response relationships (G) were produced by plotting mean ± SEM b-wave amplitude (normalized to 1 = the maximum recorded for that mouse under any condition; n = 6) against flash cone contrast revealed differences between conditions (F-test comparisons of sigmoidal fits to data; curves for mel-low and daylight conditions are significantly different [p < 0.05], but those for mel-low and mel-low [recovery] are not [p = 0.93]).
Melanopsin-Driven Changes in Visual Response Extend to the dLGN
We next recorded responses of neurons in the dLGN, which allowed us to determine whether these changes were propagated beyond the retina. Using multichannel electrodes, we recorded responses to daylight and mel-low flash paradigms across the contralateral dLGN (Figure 3A). To determine whether the change in ERG b-wave amplitude had a simple correlate in the dLGN response, we computed the mean change in firing of multiunit activity across the dLGN of each mouse. We found changes in this parameter across the experimental conditions very similar to those observed in the ERG. Thus, flash response amplitude was reduced for daylight versus mel-low in Opn1mwR animals at high backgrounds, but not in any of the control conditions (Figures 3B and 3C; Figures S2A–S2C).
To explore the origins of this alteration in global response amplitude, we turned to examining responses at the single-unit level. Of 272 single units isolated from seven mice, 161 responded to the flash with, in all cases, increased firing. Many units showed qualitative differences in response to mel-low and daylight conditions (Figure 3D). In some cases, the temporal profile of stimulus evoked spikes changed (see Figure S3 for analysis of this feature). However, such changes in timing could not explain the change in global response amplitude (Figure 3B), as the predominant peak in firing was no less synchronous across the dLGN population in the daylight than mel-low condition (Figure 3E). Instead, we found that the increase in global response amplitude in mel-low could be attributed to a similar change at the single-unit level, with >75% of units showing greater light-evoked firing in this condition (Figure 3F). Surveying responses to individual trials (Figure 3D) indicated that a predominant origin for this effect might be changes in trial-to-trial reproducibility. Indeed, there was an increase in response reproducibility mel-low (paired t test, p < 0.0001; Figure 3G) that correlated strongly with mean response amplitude (Pearson’s correlation coefficient R2 = 0.16, p < 0.0001; Figure 3H). These effects were absent in melanopsin knockout mice (Figures S2D and S2E).
Changes in Spatial Frequency Preference
A predominant effect of switching from mel-low to daylight conditions was thus a reduction in trial-to-trial reproducibility of flash responses. This result is perhaps counterintuitive, insofar as one might have expected visual performance to be improved under the daylight spectrum, which approximates the mouse’s experience of natural conditions. A possible explanation for this apparent paradox is that the reduction in reproducibility of responses to the full-field flash reflects a change in preference toward more complex visual stimuli. To explore this possibility, we modified a digital mirror device projector (DLP LightCommander, Logic PD), replacing the intrinsic light engine with our own multispectral light source. This allowed us to render back-projected, structured images in the same mel-low versus daylight spectra.
We first looked at spatial response properties by mapping receptive fields (RFs) in each condition. As expected, the spatial location of RF centers was invariant between the two conditions (Figure 4A). As previously reported [28, 29], there was substantial diversity in RF size between units (Figure 4B. Nonetheless, we did not find a single example of a significant change in the size of RF centers between mel-low and daylight (p > 0.05 for F-test comparisons of Gaussians fitted to spike-triggered averages to bars presented in vertical and horizontal orientations). In common with reports for mice dLGN [28, 29], however, we were unable to map robust surround components of the RF (Figure 4C). Because of this, we used an additional approach to determine spatial feature preference and presented contrast inverting gratings over a range of spatial frequencies.
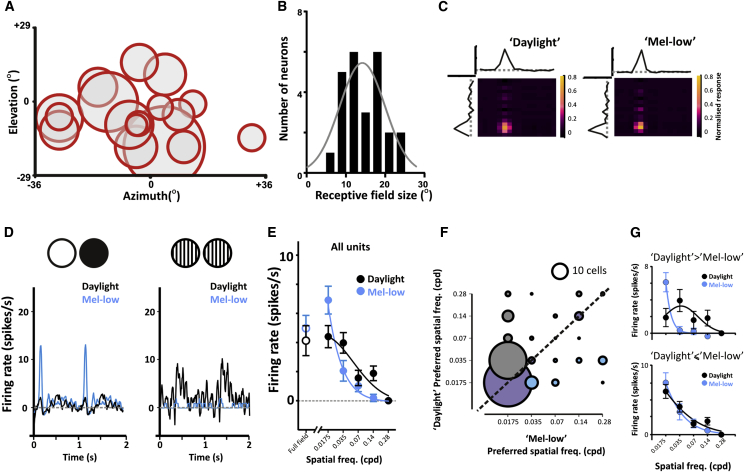
Figure 4.
Spatial Tuning Properties Are Modulated According to Melanopsin Excitation
(A–C) Spatial receptive field mapping. RFs single dLGN units were mapped under both daylight and mel-low conditions using a white noise presentation of black-and-white horizontal and vertical bars upon a gray background. (A) The location of spatial RFs for 17 cells from 4 Opn1mwR mice (circles show half-maximum width of Gaussian fits of spike-triggered averages in each dimension). (B) RF diameter of these units (half-maximum bandwidth of Gaussian) ranged from 5.6° to 23°. (C) Spatial RFs mapped under both daylight and mel-low conditions for a representative unit. Heatmaps (scale is normalized response) show response to bars in each orientation under each condition, derived spike triggered averages plotted above and to the left. Note the similarity in RF position in each condition.
(D–G) Many units that failed to respond to the full-field flash under the daylight spectrum responded well to spatially structured stimuli. (D) Mean peri-event histograms of baseline-subtracted firing rate to 200 presentations of full-field flashes (left) and 40 presentations of inverted gratings (0.035 cpd) in a representative unit under daylight (black) and mel-low (blue) conditions. Flashes occurred at time 0 and 1 and grating inversions every 0.5 s starting at zero. Spatial frequency tuning was examined by recording responses to 1 Hz inverting gratings at four orientations, at five different spatial frequencies (0.035–0.56 cpd) in mel-low and daylight conditions. (E) Mean modulation in firing rate for individual cells when the grating in preferred orientation revealed a shift in the spatial frequency tuning between mel-low and daylight condition. Responses to full-field stimuli plotted for reference (50 ms flashes at 1 Hz). (F) Distribution of preferred spatial frequency (maximum response) for single units in each condition; circle width scaled to represent number of cells in each group (circle size for 10 cells shown above for comparison). Blue circle show cells with higher preferred spatial frequency in mel-low; gray cells show higher preferred spatial frequency in daylight, and lilac cells with no change. (G) The mean response at different spatial frequencies from (E) is replotted for those cells showing a higher preferred spatial frequency in daylight (gray circles in F; top graph), or the remaining cells (lilac and blue circles in F; bottom graph). Data in (E) and (G) are fitted with partial Gaussian curves. Data shown indicate mean ± SEM.
The inverting gratings revealed responses to a wide range of spatial frequencies in both conditions (Figures 4D and 4E). As a population, however, there was a tendency for larger-amplitude responses to finer gratings in the daylight condition. The origin of this effect was revealed by comparing the preferred spatial frequency of cells in mel-low and daylight (Figure 4F). Although many (42/112) cells showed maximal responses to the same spatial frequency in both conditions, there was an overall tendency for cells to prefer higher spatial frequencies in daylight compared to mel-low (Wilcoxon matched-pairs signed rank test, p < 0.05). Notably, those cells switching spatial frequency preference also had the largest reduction in full-field flash response (1.7 ± 1.2 versus 7.5 ± 1.6 spikes/s in daylight and mel-low, respectively; paired t test, p < 0.001) and accounted for all the change in spatial frequency tuning between conditions (Figure 4G). The most common effect (displayed by 51% of units changing) was a shift from preferring the lowest frequency (0.0175 cycles per degree [cpd]) to 0.035 cpd in the daylight condition. This optimal grating size, which equates to a visual angle of ∼14°, is smaller than the calculated RF center of these cells, but would be predicted to provide good contrast between the RF center and neighboring points of visual space. Such behavior could be readily explained by strengthening of an inhibitory surround to the RF. In any event, these data show that many dLGN neurons adjust spatial frequency tuning between mel-low and daylight conditions. For a substantial fraction this entails a fundamental realignment from responding most to full-field stimuli to preferring spatial patterns. Together, these changes provide a simple explanation for the surprising reduction in responses to full-field flashes in the daylight condition.
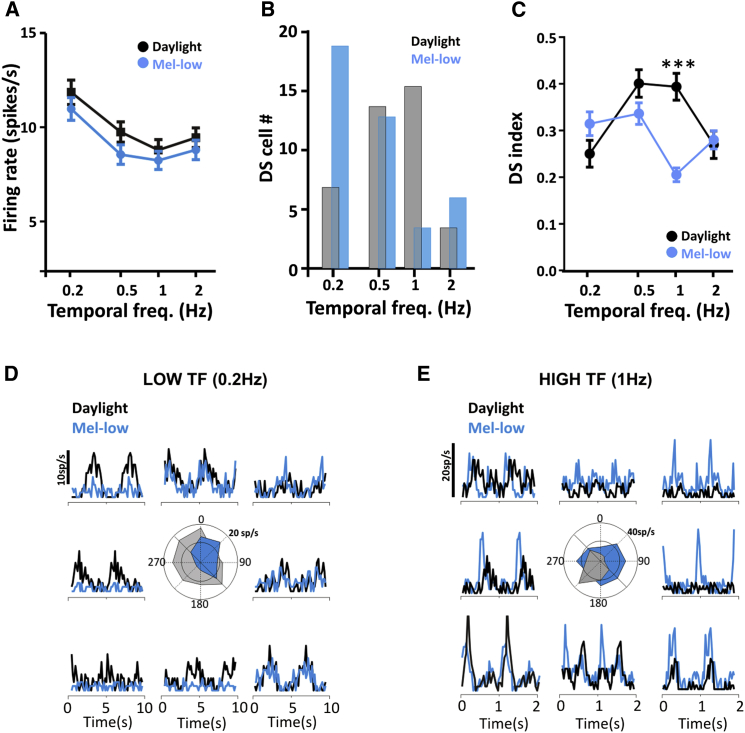
Changes in Temporal Frequency Tuning
We next asked whether the changes in temporal profile of responses to full-field flashes observed upon switching from mel-low to daylight conditions (see Figures 3D and S3) could reflect alterations in temporal frequency preference of dLGN units and therefore examined responses to drifting gratings at different temporal frequencies (spatial frequency fixed at 0.035 cpd over eight directions of motion). More than 80% of cells responding to drifting gratings responded to movements in all directions. Response amplitude increased across all temporal frequencies under the daylight condition (Figure 5A), consistent with the view that responses to stimuli with appropriate spatial structure are improved in this condition (Figure 4). However, there was no difference in temporal frequency tuning under mel-low and daylight spectra. Nor could we discern any relationship between responses to the full-field flash and drifting gratings to explain the changes in the temporal profile of flash responses between these conditions (Figures S3C–S3E).
Figure 5.
Changes in Temporal Frequency Tuning among Direction-Sensitive Units
(A) Temporal frequency tuning was assessed by recording responses to drifting gratings (0.035 cpd) moving in eight orientations, at four temporal frequencies (0.2–2 Hz) in both daylight and mel-low conditions. Across all temporal frequencies, response amplitude (mean ± SEM peak firing rate; for all light-responsive units, n = 117 from five mice) was higher in the daylight condition, but there was no change in the relative effectiveness of different frequencies between daylight and mel-low. Two-way repeated-measures ANOVA, significant effects of frequency (p < 0.0001) and condition (p = 0.036), but not of the frequency-condition interaction (p > 0.05).
(B–E) A subset of cells showed direction sensitivity at one or more temporal frequencies. (B) The number of direction-sensitive cells as a function of grating temporal frequency in daylight (gray) and mel-low (blue) conditions, revealing a switch to more abundant direction selectivity for higher temporal frequencies in the daylight condition. This was apparent also at the single-unit level, with examples of cells switching the temporal frequency at which they were most direction sensitive. (C) The DS index was compared for all cells that were classed as direction sensitive in any condition, at any frequency (data shown indicate mean ± SEM). This again revealed a significant change in the temporal frequency tuning of cell responses in mel-low and daylight conditions, with repeated-measures two-way ANOVA revealing significant increase in DS index at 1 Hz in the daylight condition (p < 0.001). (D and E) Representatives of such behavior, with peristimulus rate histograms and associated polar plots depicting response amplitude for movement in each of eight directions differing markedly under daylight (black) and mel-low (blue) conditions. The temporal frequency (TF) at which the grating was presented is provided above. (D) A cell with selectivity for direction of slow moving gratings (0.2 Hz) under the mel-low but not day-light condition. (E) A cell showing direction sensitivity at higher frequencies in daylight but not mel-low.
These drifting gratings did, however, reveal another change in feature selectivity between mel-low and daylight conditions. As previously reported [29], a subset of units preferred for movement in a particular direction (direction sensitive; see Experimental Procedures). Under both mel-low and daylight spectra, these direction-sensitive cells accounted for ∼16% of the total. For all such cells, the degree of response suppression for motion in the null direction varied according to the temporal frequency of the stimulus (representative examples shown in Figures 5D and 5E). Under the mel-low condition the prevalence of direction sensitivity was highest for the slowest movement (0.2 Hz; corresponding to grating motion of 6°/s), while under daylight this occurred at 1 Hz (29°/s; Figures 5B–5D). This switch to preferring higher frequencies was also apparent in comparisons of direction selectivity (DS) index (Figure 5E). These data therefore indicate that, for at least a subset of direction-sensitive cells, the change in melanopsin effective photon flux drives an alteration in preferred speed of motion.
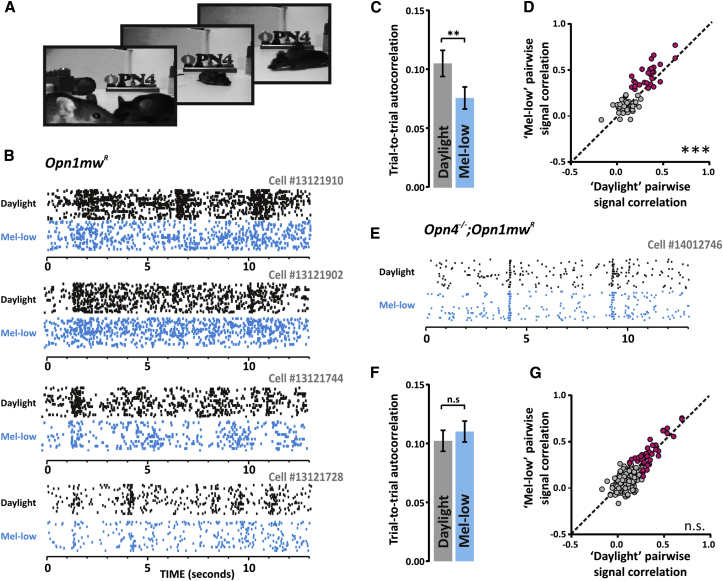
Melanopsin-Dependent Changes in Response to Natural Movies
Having described differences in feature preference under daylight versus mel-low spectra using artificial stimuli, we finally set out to determine their implication for the dLGN’s ability to encode naturalistic stimuli. To this end, we projected movies of a natural scene (mice moving around an open arena; Figure 6A) in each condition. This short movie (30 s) was presented repeatedly over a 30 min period, enabling us to identify many units (∼30% of all cells) with highly reproducible firing patterns across multiple presentations (see Supplemental Experimental Procedures, Natural Movie Correlation Analyses). We found many instances in which the firing pattern of units from Opn1mwR mice was reliable for multiple presentations of the movie under either mel-low or daylight spectra, but diverged substantially between the two conditions (Figure 6B). These were absent from recordings in Opn4−/−;Opn1mwR animals (Figure 6E).
Figure 6.
Responses to Natural Scenes in Daylight and Mel-Low Conditions
(A) Example frames from a short natural movie (mice moving around an open arena in horizontal view) presented to Opn1mwR and Opn4−/−;Opn1mwR mice.
(B) Raster plots for four representative units from Opn1mwR mice exposed to 50 repeats of the movie under either daylight (black) or mel-low (blue) spectra.
(C) The trial-to-trial reproducibility of firing patterns of individual units (estimated by Pearson’s correlation coefficient; autocorrelation) was significantly higher in the daylight condition (paired t test, p < 0.01; n = 28 from four Opn1mwR mice).
(D) The similarity in firing pattern between pairs of units from the same mouse under mel-low and daylight conditions is depicted as signal correlation (Pearson’s correlation coefficient; 28 pairs from four Opn1mwR mice). Signal correlation was significantly reduced under the daylight spectrum (paired t test, p < 0.001). This was especially apparent for those pairs of units showing high correlation (>0.25) in either or both conditions, the pink data points.
(E–G) The response of melanopsin knockout mice to the movie was similar in daylight and mel-low spectra. (E) Raster plot of firing of a representative Opn4−/−;Opn1mwR unit in response to the movie under daylight (black) and mel-low (blue) conditions. At the population level (33 single units from three mice), there was no difference in autocorrelation (F) or signal correlation (G) between daylight and mel-lo conditions. Data shown indicate mean ± SEM.
Based upon the improvement in trial-to-trial reproducibility of responses to artificial stimuli with appropriate spatial structure under the daylight spectrum (Figure 4C), we expected to see a similar increase in response reliability for the naturalistic movies in this condition. We therefore computed the trial-to-trial correlation for each unit under each condition (“autocorrelation”; see Experimental Procedures), and found that single-unit responses were indeed more reliable enhanced in the daylight condition (Figure 6C). Importantly, this effect was absent in Opn4−/−;Opn1mwR animals (Figure 6F).
A couple of features of the response to artificial stimuli suggest that the visual representation of visual scenes across dLGN neurons could be more diverse (richer) in the daylight condition: changes in spatial frequency tuning (Figure 4) indicate that a subset of dLGN neurons provide finer spatial resolution of visual features; and alterations in the behavior of direction-sensitive units (Figure 5) imply that this aspect of motion is tracked over a wider range of velocities. To determine whether responses across the population were indeed more varied in the daylight condition, we computed the correlation between mean firing response patterns of pairs of units from each animal (“signal correlation”). To limit the risk of sampling bias, we did not restrict our analysis to units with equivalent spatial RFs or related feature selectivity; our only constraint was that the units included had an RF centered within the projection screen and that they responded consistently to at least some aspect of the movie (see Experimental Procedures). As might be predicted, therefore, we found substantial variability in the signal correlation between pairs of units within a single animal. Nevertheless, even in this unfiltered data set there was a significant increase in signal correlation in the mel-low compared to daylight condition in Opn1mwR animals (Figure 6D). This effect was enhanced if one restricted analysis to those pairs with relatively high pairwise correlations in either condition (Pearson’s linear correlation coefficient > 0.25). Once again, Opn4−/−;Opn1mwR animals lacked this change (Figure 6G). We conclude that melanopsin-driven adjustments in the visual response allow the visual code to provide a richer, less redundant representation of natural scenes.
Discussion
Here, we have applied the concepts of metamerism and receptor silent substitution to compare responses to visual stimuli presented under conditions that differ only in their effective photon flux for melanopsin. The first condition (daylight) approximates the mouse’s experience of natural daylight, while in the second condition (mel-low), those wavelengths to which melanopsin is most responsive, were selectively depleted. Switching between daylight and mel-low conditions resulted in substantial alterations in visual responses in the retina and dLGN. These were caused by fine changes in stimulus selectivity in spatial and temporal dimensions at the single-neuron level. Such changes in feature preference were associated with quantitative improvements in the dLGN’s ability to encode natural scenes when presented in the daylight condition.
To reveal the impact of melanopsin on conventional vision, it was necessary to devise a method of selectively modulating the activity of melanopsin in an animal with a fully functional visual system. Our approach adopts the concept of metamerism: that stimuli differing markedly in spectral composition can appear indistinguishable for one or more classes of photoreceptor. In this case, our mel-low and daylight conditions are designed to appear equivalent for cones. One feature of the approach is that it cannot reveal any contribution of ipRGCs to adaptation at lower irradiances when their activity depends upon rods and cones. Our data thus likely underestimate the contribution of ipRGCs to retinal physiology.
While our conclusions do not require that the rate of photon capture by all cones is absolutely identical under mel-low and daylight conditions (a practical impossibility), it is important that they are sufficiently similar as to make any difference in cone response fall below the detection limits of our methods. We are confident that this is the case. We have designed these stimuli based upon extensive measurements of the in vivo spectral sensitivity of mouse cones [25, 30, 31]. Moreover, both ERG and dLGN responses are indistinguishable between mel-low and daylight conditions when working at light levels below those favored by melanopsin and under all conditions in mice lacking melanopsin. Finally, the changes in response properties we observe build up over several minutes of exposure to the new background (Figure 2), consistent with a gradual, melanopsin-dependent adjustment in visual response, but not with a fundamental difference in the cone experience of the two conditions, which should be apparent from the very first presentation of the new stimulus.
What functional advantage could be gained by adjusting vision according to a melanopsin-dependent assessment of irradiance? Light adaptation in visual circuits involves changes in the behavior of individual synapses and the nature and extent of connections between pairs of neurons. One function of this adaptation is to conserve the visual code against changes in irradiance; another is to adjust circuitry to take advantage of irradiance-dependent increases in signal reliability and/or changes in photoreceptor temporal resolution [1, 2, 32, 33, 34]. However, these two features are in some ways contradictory. Viewed from the perspective of a circuit element charged with achieving the second of these goals, the fact that light adaptation upstream in the circuit minimizes the impact of changes in background light levels on incoming signals makes it more difficult to accurately assess irradiance. Basing adaptation instead on an independent measure of irradiance not subject to the same light adaptation processes, as shown here for melanopsin, is one solution to this problem. If such a general mechanism were to explain the influence of melanopsin in the mouse retina, one might expect to find independent irradiance codes also in other visual systems. Indeed, this seems to be the case. Thus, in fish and other lower vertebrates, a range of non-rod, non-cone photopigments are expressed widely in inner retinal neurons [35, 36, 37, 38]. Meanwhile, in Drosophila, a recent publication reports regulation of vision by cryptochrome, the fly’s version of an irradiance measurement system [39].
The simplest effect we observe is smaller responses to a full-field flash in the daylight condition. This is apparent in the ERG and in the LGN at both population and single-unit levels. Using spatially structured stimuli reveals that this effect represents a widespread shift in spatial frequency tuning. Many dLGN units that respond well to full-field flashes and gratings at the lowest spatial frequencies in mel-low switch to preferring higher spatial frequencies under daylight (∼0.035 cpd). Future work will be required to define the changes in visual circuits underlying these effects. There are multiple reports of luminance- and/or irradiance-dependent changes in spatial frequency preference (although generally at lower light levels than studied here [1, 2, 40, 41, 42]). Where elucidated, mechanisms for such changes involve alterations in the inhibitory surround provided by retinal horizontal and amacrine cells. These cell types therefore seem likely targets of melanopsin control. Inhibitory amacrine cells are also responsible for establishing direction selectivity [43, 44], implicating changes in their activity also as a likely origin of the melanopsin-dependent change in temporal frequency tuning of direction sensitive units that we observe (Figure 5).
While our ERG data reveal that at least some melanopsin-driven changes in visual response originate with the retina, our study does not preclude a contribution of central processing within the brain. These retinal changes could themselves involve feedback via centrifugal histaminergic [45, 46] and/or serotonergic [47] projections. Moreover, thalamic and thalamocortical circuits allow plenty of opportunity for fine-tuning visual responses. It may therefore be that some of the changes in visual feature selectivity we see in the dLGN reflect an impact of melanopsin-based assessments of irradiance within the brain itself. These could extend to melanopsin-driven increases in arousal or attention, although any such effect would likely be specific to vision as responses to another sensory stimulus were indistinguishable under the two lighting conditions (Figure S4).
Our findings add to the growing evidence that the sensory requirements of ipRGCs should influence lighting design [48]. Most incandescent and fluorescent lighting is long wavelength biased compared to natural light and thus deficient in those wavelengths to which melanopsin is most sensitive, effectively recapitulating our mel-low condition. While the degree to which melanopsin excitation is reduced in our experimental mel-low condition is probably several times greater than in most artificial lighting, these data do imply that choosing light sources that more closely approximate melanopsin’s experience of daylight may bring qualitative improvements in visual performance.
Experimental Procedures
In Vivo Electrophysiology
Animal care was in accordance with the UK Animals, Scientific Procedures, Act (1986). ERGs and dLGN responses were recorded concurrently from 13 Opn1mwR and eight Opn4−/−;Opn1mwR male mice (aged 3–6 months) anesthetized with urethane (1.6 g/kg, 30% w/v; Sigma-Aldrich). dLGN recordings employed a 32-channel probe (A4x8-5mm-50-200-413; Neuronexus). Recording methods as previously reported [49]. In addition, a separate set of mice (six Opn1mwR and three Opn4−/−;Opn1mwR males) was used to record responses in the dLGN to spatially structured stimuli.
Visual Stimuli
Full-field visual stimuli were generated using three independently controlled LEDs (λmax 365, 460, and 600 nm; Cairn Research). LEDs were combined to generate two background and stimulus combinations that are summarized in Figure 1D. The approach is equivalent to that described in [48], using spectral efficiency functions available at http://lucasgroup.lab.ls.manchester.ac.uk/research/measuringmelanopicilluminance. Structured images were presented using a custom-made light source containing four independently controlled LEDs (λmax 405, 455, 525, and 630 nm; Phlatlight PT-120 Series, Luminus Devices), directed into a digital mirror device projector (DLP LightCommander).
Acknowledgments
This work was supported by grants from the European Research Council and the Biological Sciences and Biotechnology Research Council to R.J.L. We thank Jonathan Wynne for technical assistance.
Published: October 9, 2014
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2014.09.015.
Contributor Information
Annette E. Allen, Email: annette.allen@manchester.ac.uk.
Robert J. Lucas, Email: robert.lucas@manchester.ac.uk.
Supplemental Information
References
- 1.Barlow H.B., Fitzhugh R., Kuffler S.W. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J. Physiol. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J. Physiol. 1975;247:551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloomfield S.A., Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat. Rev. Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witkovsky P. Dopamine and retinal function. Doc. Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 5.Warren E.J., Allen C.N., Brown R.L., Robinson D.W. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur. J. Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey D.M., Liao H.W., Peterson B.B., Robinson F.R., Smith V.C., Pokorny J., Yau K.W., Gamlin P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 7.Tu D.C., Zhang D., Demas J., Slutsky E.B., Provencio I., Holy T.E., Van Gelder R.N. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Brown T.M., Gias C., Hatori M., Keding S.R., Semo M., Coffey P.J., Gigg J., Piggins H.D., Panda S., Lucas R.J. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berson D.M., Dunn F.A., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 10.Hattar S., Liao H.W., Takao M., Berson D.M., Yau K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provencio I., Rollag M.D., Castrucci A.M. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 12.Hattar S., Lucas R.J., Mrosovsky N., Thompson S., Douglas R.H., Hankins M.W., Lem J., Biel M., Hofmann F., Foster R.G., Yau K.W. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda S., Provencio I., Tu D.C., Pires S.S., Rollag M.D., Castrucci A.M., Pletcher M.T., Sato T.K., Wiltshire T., Andahazy M. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 14.Hankins M.W., Lucas R.J. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr. Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- 15.Sekaran S., Foster R.G., Lucas R.J., Hankins M.W. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 16.Müller L.P., Do M.T., Yau K.W., He S., Baldridge W.H. Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. J. Comp. Neurol. 2010;518:4813–4824. doi: 10.1002/cne.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joo H.R., Peterson B.B., Dacey D.M., Hattar S., Chen S.K. Recurrent axon collaterals of intrinsically photosensitive retinal ganglion cells. Vis. Neurosci. 2013;30:175–182. doi: 10.1017/S0952523813000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang D.Q., Wong K.Y., Sollars P.J., Berson D.M., Pickard G.E., McMahon D.G. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc. Natl. Acad. Sci. USA. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailes H.J., Lucas R.J. Melanopsin and inner retinal photoreception. Cell. Mol. Life Sci. 2010;67:99–111. doi: 10.1007/s00018-009-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do M.T., Yau K.W. Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnard A.R., Hattar S., Hankins M.W., Lucas R.J. Melanopsin regulates visual processing in the mouse retina. Curr. Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Dkhissi-Benyahya O., Coutanson C., Knoblauch K., Lahouaoui H., Leviel V., Rey C., Bennis M., Cooper H.M. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell. Mol. Life Sci. 2013;70:3435–3447. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao S., Chun C., Fan J., Kofron J.M., Yang M.B., Hegde R.S., Ferrara N., Copenhagen D.R., Lang R.A. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renna J.M., Weng S., Berson D.M. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat. Neurosci. 2011;14:827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smallwood P.M., Olveczky B.P., Williams G.L., Jacobs G.H., Reese B.E., Meister M., Nathans J. Genetically engineered mice with an additional class of cone photoreceptors: implications for the evolution of color vision. Proc. Natl. Acad. Sci. USA. 2003;100:11706–11711. doi: 10.1073/pnas.1934712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szél A., Röhlich P., Caffé A.R., van Veen T. Distribution of cone photoreceptors in the mammalian retina. Microsc. Res. Tech. 1996;35:445–462. doi: 10.1002/(SICI)1097-0029(19961215)35:6<445::AID-JEMT4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt T.M., Alam N.M., Chen S., Kofuji P., Li W., Prusky G.T., Hattar S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron. 2014;82:781–788. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grubb M.S., Thompson I.D. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J. Neurophysiol. 2003;90:3594–3607. doi: 10.1152/jn.00699.2003. [DOI] [PubMed] [Google Scholar]
- 29.Piscopo D.M., El-Danaf R.N., Huberman A.D., Niell C.M. Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 2013;33:4642–4656. doi: 10.1523/JNEUROSCI.5187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs G.H., Williams G.A. Contributions of the mouse UV photopigment to the ERG and to vision. Doc. Ophthalmol. 2007;115:137–144. doi: 10.1007/s10633-007-9055-z. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs G.H., Williams G.A., Cahill H., Nathans J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science. 2007;315:1723–1725. doi: 10.1126/science.1138838. [DOI] [PubMed] [Google Scholar]
- 32.De Valois R.L., Morgan H., Snodderly D.M. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974;14:75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- 33.Pasternak T., Merigan W.H. The luminance dependence of spatial vision in the cat. Vision Res. 1981;21:1333–1339. doi: 10.1016/0042-6989(81)90240-6. [DOI] [PubMed] [Google Scholar]
- 34.Rieke F., Rudd M.E. The challenges natural images pose for visual adaptation. Neuron. 2009;64:605–616. doi: 10.1016/j.neuron.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Cheng N., Tsunenari T., Yau K.W. Intrinsic light response of retinal horizontal cells of teleosts. Nature. 2009;460:899–903. doi: 10.1038/nature08175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies W.I., Zheng L., Hughes S., Tamai T.K., Turton M., Halford S., Foster R.G., Whitmore D., Hankins M.W. Functional diversity of melanopsins and their global expression in the teleost retina. Cell. Mol. Life Sci. 2011;68:4115–4132. doi: 10.1007/s00018-011-0785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guido M.E., Garbarino-Pico E., Contin M.A., Valdez D.J., Nieto P.S., Verra D.M., Acosta-Rodriguez V.A., de Zavalía N., Rosenstein R.E. Inner retinal circadian clocks and non-visual photoreceptors: novel players in the circadian system. Prog. Neurobiol. 2010;92:484–504. doi: 10.1016/j.pneurobio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Matos-Cruz V., Blasic J., Nickle B., Robinson P.R., Hattar S., Halpern M.E. Unexpected diversity and photoperiod dependence of the zebrafish melanopsin system. PLoS ONE. 2011;6:e25111. doi: 10.1371/journal.pone.0025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzotta G., Rossi A., Leonardi E., Mason M., Bertolucci C., Caccin L., Spolaore B., Martin A.J., Schlichting M., Grebler R. Fly cryptochrome and the visual system. Proc. Natl. Acad. Sci. USA. 2013;110:6163–6168. doi: 10.1073/pnas.1212317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrow K., Teixeira M., Szikra T., Viney T.J., Balint K., Yonehara K., Roska B. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron. 2013;78:325–338. doi: 10.1016/j.neuron.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Sagdullaev B.T., McCall M.A. Stimulus size and intensity alter fundamental receptive-field properties of mouse retinal ganglion cells in vivo. Vis. Neurosci. 2005;22:649–659. doi: 10.1017/S0952523805225142. [DOI] [PubMed] [Google Scholar]
- 42.Bisti S., Clement R., Maffei L., Mecacci L. Spatial frequency and orientation tuning curves of visual neurones in the cat: effects of mean luminance. Exp. Brain Res. 1977;27:335–345. doi: 10.1007/BF00235508. [DOI] [PubMed] [Google Scholar]
- 43.Fried S.I., Münch T.A., Werblin F.S. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- 44.Yonehara K., Balint K., Noda M., Nagel G., Bamberg E., Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- 45.Akimov N.P., Marshak D.W., Frishman L.J., Glickman R.D., Yusupov R.G. Histamine reduces flash sensitivity of on ganglion cells in the primate retina. Invest. Ophthalmol. Vis. Sci. 2010;51:3825–3834. doi: 10.1167/iovs.09-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frazão R., McMahon D.G., Schunack W., Datta P., Heidelberger R., Marshak D.W. Histamine elevates free intracellular calcium in mouse retinal dopaminergic cells via H1-receptors. Invest. Ophthalmol. Vis. Sci. 2011;52:3083–3088. doi: 10.1167/iovs.10-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Repérant J., Araneda S., Miceli D., Medina M., Rio J.P. Serotonergic retinopetal projections from the dorsal raphe nucleus in the mouse demonstrated by combined [(3)H] 5-HT retrograde tracing and immunolabeling of endogenous 5-HT. Brain Res. 2000;878:213–217. doi: 10.1016/s0006-8993(00)02706-2. [DOI] [PubMed] [Google Scholar]
- 48.Lucas R.J., Peirson S.N., Berson D.M., Brown T.M., Cooper H.M., Czeisler C.A., Figueiro M.G., Gamlin P.D., Lockley S.W., O’Hagan J.B. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen A.E., Cameron M.A., Brown T.M., Vugler A.A., Lucas R.J. Visual responses in mice lacking critical components of all known retinal phototransduction cascades. PLoS ONE. 2010;5:e15063. doi: 10.1371/journal.pone.0015063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.