Abstract
Background
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) provide an effective treatment option for selected patients with colorectal peritoneal metastasis with encouraging survival results. Many different drug combinations and HIPEC regimens including bidirectional, i.e. synchronous intravenous and intraperitoneal, drug application have been used. However, there is still no standardization of the HIPEC regimen.
Methods
Between 05/2007 and 04/2010 190 patients underwent CRS and HIPEC at the University Hospital Regensburg. Thirty-two patients with peritoneal metastasis arising from colorectal or appendiceal cancer underwent complete macroscopic cytoreduction (CC-0/1) and bidirectional HIPEC and completed at least 3-year follow-up. Twenty patients received oxaliplatin-based (OX) and twelve patients received irinotecan-based HIPEC (IRI). Group-specific perioperative morbidity and 3-year survival has been determined.
Results
The grade 3/4 morbidity rate according to CTCAE v4 was 35.0% in the OX group vs. 33.3% in the IRI group (p = 1.000). There was no perioperative mortality in both groups. Median survival was 26.8 months (95% CI 15.7-33.1 months) in the IRI group and has not yet been reached in the OX group during a median follow-up of 39.4 months. Three-year survival rates were 65.0% in the OX group vs. 41.7% in the IRI group (p = 0.295).
Conclusions
The morbidity and toxicity rates of bidirectional irinotecan-based and oxaliplatin-based HIPEC are comparable. Nevertheless, in the absence of contraindications oxaliplatin-based HIPEC might be preferred due to the positive trend regarding 3-year and median survival.
Keywords: Peritoneal carcinomatosis, HIPEC, Irinotecan, Oxaliplatin, Morbidity, Survival
Background
The combined treatment concept consisting of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) performed in specialized centers has shown to be a safe and efficient additive therapeutic option for selected patients with colorectal peritoneal metastasis [1–3]. One prospective randomized controlled phase III trial and several prospective and retrospective reports provide evidence for improved long-term survival for CRS and HIPEC as an integrative part of an interdisciplinary treatment regimen [4–9]. In the Dutch RCT the median survival of patients who underwent CRS and HIPEC was 22 months vs. 12.6 months in the control group with systemic chemotherapy only. In the subgroup analysis of patients after complete macroscopic cytoreduction (CC-0/1) median survival increased to 42.9 months [8, 9]. As in most other reported studies and series a mitomycin C (MMC)-based HIPEC regimen has been used for peritoneal perfusion. Based on the results of modern systemic polychemotherapy regimens such as FOLFOX or FOLFIRI for patients with metastatic colorectal cancer oxaliplatin and irinotecan have also been used for peritoneal perfusion. Data first published from the French groups suggest that oxaliplatin-based HIPEC after complete macroscopic cytoreduction may further improve survival of patients with colorectal peritoneal metastasis [10, 11]. The addition of intraperitoneal irinotecan to the bidirectional oxaliplatin-based HIPEC regimen did not lead to improved overall or relapse-free survival [12]. Nevertheless, as irinotecan is considered to be the second most effective agent for the treatment of patients with colorectal cancer [13, 14], bidirectional irinotecan-based HIPEC might be a promising alternative treatment regimen for patients with disease progression or intolerable adverse events under oxaliplatin-based chemotherapy as well as patients with good response under previous systemic chemotherapy with irinotecan. However, conclusive data from randomized controlled trials is still missing and numerous different HIPEC regimens are used for treatment of colorectal peritoneal metastasis [15]. Cytostatic agents, drug dosage and duration of perfusion are still a matter of debate.
In the present study we retrospectively analyzed morbidity, mortality and 3-year survival of thirty-two patients with peritoneal metastasis arising from colorectal or appendiceal cancer who received either bidirectional oxaliplatin-based or irinotecan-based HIPEC after complete macroscopic cytoreduction.
Methods
Between May 2007 and April 2010 190 patients underwent cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for various peritoneal surface malignancies at the University Hospital Regensburg. Thirty-two patients with synchronous or metachronous peritoneal metastasis arising from colorectal or appendiceal cancer received bidirectional HIPEC after complete macroscopic cytoreduction (CC-0/1). Twenty patients received oxaliplatin-based HIPEC and twelve patients received irinotecan-based HIPEC. All patients had histologically proven peritoneal carcinomatosis arising from colorectal or appendiceal adenocarcinoma. Patients with disseminated peritoneal adenomucinosis (DPAM) or peritoneal mucinous carcinomatosis of intermediate features (PMCA-I) as well as patients with incomplete macroscopic cytoreduction (CC-2 or CC-3) were excluded from the present study.
Data has been analyzed retrospectively. The retrospective analysis from a database without the use of patients’ personal data was exempted from approval by the Ethics Committee at the Regensburg University. Nevertheless, CRS and HIPEC are recommended for selected patients by the German S3-guideline for the treatment of colorectal cancer [16]. Moreover, the bidirectional oxaliplatin-based HIPEC regimen has been approved by the ethic committee in the context of our prospective multicenter phase II COMBATAC trial (ClinicalTrials.gov Identifier: NCT01540344) [17] and is recommended as one of the standard HIPEC protocols for patients with colorectal peritoneal metastasis by the German Peritoneal Surface Malignancy Group. The individual reasons for the replacement of oxaliplatin by irinotecan in the IRI group are summarized in Table 1. The safety of intraperitoneal application of irinotecan has been proven in several published studies [12, 18, 19]. However, due to the lack of consistent data there are still no national and/or international standards for HIPEC regimens in patients with colorectal peritoneal metastasis [15].
Table 1.
Characteristics of patients with irinotecan-based HIPEC
| Patient | Previous syst. CTx | Previous syst. OX | Recurrent PM | Rationale for irinotecan-based HIPEC |
|---|---|---|---|---|
| Patient 1 | yes | yes | yes | Oxaliplatin-associated peripheral neuropathy |
| Patient 2 | yes | yes | no | 2ndline irinotecan-based systemic chemothera-py after disease progression |
| Patient 3 | yes | no | no | Systemic irinotecan-based chemotherapy with response |
| Patient 4 | yes | yes | no | Systemic irinotecan-based chemotherapy with response |
| Patient 5 | yes | no | no | Systemic irinotecan-based chemotherapy with response |
| Patient 6 | yes | no | no | Systemic irinotecan-based chemotherapy with response |
| Patient 7 | yes | no | no | Systemic irinotecan-based chemotherapy with response |
| Patient 8 | yes | yes | no | Oxaliplatin-associated peripheral neuropathy |
| Patient 9 | yes | no | no | Systemic irinotecan-based chemotherapy with response |
| Patient 10 | yes | yes | no | Progressive disease under oxaliplatin-based systemic chemotherapy |
| Patient 11 | yes | yes | yes | Progressive disease under oxaliplatin-based systemic chemotherapy |
| Patient 12 | yes | no | no | Systemic irinotecan-based chemotherapy with response |
CTx = chemotherapy; PM = peritoneal metastasis.
All patients included in the present retrospective study at least completed a 3-year follow-up period. The median follow-up time including events of death was 37.8 months (range 7-51).
Morbidity and toxicity were classified using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.02) of the U.S. National Cancer Institute. Perioperative mortality was defined as death within 30 days after surgery or in-hospital mortality in case of hospital stay longer than 30 days.
Cytoreductive surgery
Cytoreductive surgery consists of numerous surgical and peritonectomy procedures depending on the extent of peritoneal tumor dissemination that was determined by the intraoperative calculation of the Peritoneal Cancer Index (PCI) [20, 21]. Operating procedures were performed as described previously [22, 23]. After complete macroscopic cytoreduction (CC-0/1) one inflow drainage, three outflow drainages and two temperature probes were placed in the abdomen to allow the application of HIPEC.
Hyperthermic intraperitoneal chemotherapy
In all patients bidirectional HIPEC with additional intravenous application of 5-FU at a concentration of 400 mg/sqm body surface and folinic acid at a concentration of 20 mg/sqm body surface about 30 minutes prior to peritoneal chemoperfusion was performed in closed abdomen technique. Abdominal perfusion was started with a total volume of 3 l sodium chloride 0.9% over the inflow drainage using a roller pump system with heat exchanger (ThermaSolutions Inc., Netherlands). Cytostatic agents were added after the temperature in Douglas pouch reached at least 40°C and perfusion was continued for 30 minutes keeping an intraperitoneal temperature of 41-43°C. In the OX group the abdominal cavity was perfused with oxaliplatin at a concentration of 300 mg/sqm body surface and in the IRI group with 300 mg/sqm body surface irinotecan for 30 minutes, respectively.
Statistics
Kaplan-Maier survival analysis was performed. P-values were calculated using T-test, Chi square and Log rank test as applicable. A two-sided p-value <0.05 was defined to be statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Windows version 19 (SPSS Inc., IBM Corporation, USA).
Results
Patients’ characteristics
The mean age of patients was 54 years (range 20-68) with 53 years (range 20-68) in the OX group and 54 years (range 42-66) in the IRI group. Fourteen patients were female and eighteen male. The distribution of American Society of Anaesthesiologists (ASA) scores was 9.4% for ASA I, 71.9% for ASA II and 18.8% for ASA III, respectively.
Patient and tumor characteristics including localization of the primary tumor are summarized in Table 2. The OX group includes a higher number of patients with peritoneal metastasis from appendiceal adenocarcinoma (p = 0.139) and a lower number with primary colon carcinoma (p = 0.073) compared to the IRI group. Nevertheless, the differences were not statistically significant. Moreover, there was a not significant higher number of lymph node negative primary tumors (p = 0.066) and well differentiated adenocarcinomas (G1, p = 0.130) in the OX group. In one patient in the IRI group the T and N status of the primary tumor was not documented.
Table 2.
Patient and tumor characteristics
| Overall | OX | IRI | p-value | |
|---|---|---|---|---|
| Number of patients [n] | 32 | 20 | 12 | |
| I. Patient characteristics | ||||
| Mean age (range) [y] | 54 (20-68) | 53 (20-68) | 54 (42-66) | 0.842 |
| Sex [n] - male | 14 (43.8%) | 9 (45.0%) | 5 (41.7%) | |
| - female | 18 (56.3%) | 11 (55.0%) | 7 (52.8%) | 1.000 |
| ASA score [n] | ||||
| - ASA I | 3 (9.4%) | 2 (10.0%) | 1 (8.3%) | 1.000 |
| - ASA II | 23 (71.9%) | 14 (70.0%) | 9 (75.0%) | 1.000 |
| - ASA III | 6 (18.8%) | 4 (20.0%) | 2 (16.7%) | 1.000 |
| II. Localization of primary tumor | ||||
| Appendix | 11 (34.4%) | 9 (45.0%) | 2 (16.7%) | 0.139 |
| Colon | 7 (21.9%) | 2 (10.0%) | 5 (41.7%) | 0.073 |
| Sigma | 10 (31.3%) | 7 (35.0%) | 3 (25.0%) | 0.702 |
| Rectum | 4 (12.5%) | 2 (10.0%) | 2 (16.7%) | 0.620 |
| III. Initial TNM classification | ||||
| T2 | 1* (3.2%) | 1 (5.0%) | 0** | 1.000 |
| T3 | 10* (32.3%) | 6 (30.0%) | 4** (36.4%) | 1.000 |
| T4 | 20* (64.5%) | 13 (65.0%) | 7** (63.7%) | 1.000 |
| N0 | 16* (51.6%) | 13 (65.0%) | 3** (27.3%) | 0.066 |
| N1 | 5* (16.1%) | 2 (10.0%) | 3** (27.3%) | 0.317 |
| N2 | 10* (32.3%) | 5 (25.0%) | 5** (45.5%) | 0.423 |
| M1 | 22 (68.8%) | 14 (70.0%) | 8 (66.7%) | 1.000 |
| G1 | 5 (15.6%) | 5 (25.0%) | 0 | 0.130 |
| G2 | 11 (34.4%) | 7 (35.0%) | 4 (33.3%) | 1.000 |
| G3 | 16 (50.0%) | 8 (40.0%) | 8 (66.7%) | 0.273 |
| IV. Further tumor characteristics | ||||
| Mean PCI (range) | 13 (2-28) | 13 (4-28) | 12 (2-28) | 0.630 |
| Synchronous PM | 16 (50.0%) | 10 (50.0%) | 6 (50.0%) | 1.000 |
| Metachronous PM | 10 (31.3%) | 6 (30.0%) | 4 (33.3%) | 1.000 |
| Recurrent disease | 6 (18.8%) | 4 (20.0%) | 2 (16.7%) | 1.000 |
| Liver metastasis | 4 (12.5%) | 1 (5.0%) | 3 (25.0%) | 0.271 |
| V. Medical history | ||||
| Previous abdominal surgery | 14 (43.8%) | 9 (45.0%) | 5 (41.7%) | 1.000 |
| Previous oncologic surgery | 24 (75.0%) | 14 (70.0%) | 10 (83.3%) | 0.676 |
| Previous CRS and HIPEC | 5 (15.6%) | 3 (15.0%) | 2 (16.7%) | 1.000 |
| Previous chemotherapy | 28 (87.5%) | 16 (80.0%) | 12 (100%) | 0.271 |
| Previous systemic oxaliplatin | 17 (53.1%) | 11 (55.0%) | 6 (50%) | 1.000 |
*n = 31, **n = 11.
The mean PCI was 13 (range 2-18). 50% of the patients had synchronous and 31.3% metachronous peritoneal metastasis. Six patients showed recurrent disease (18.8%) and four patients had liver metastases at the time of surgery (12.5%). Fourteen patients had previous abdominal surgery (43.8%) and 24 patients (75.0%) already underwent oncologic abdominal surgery for primary tumor or metastasis. Five patients had previous CRS and HIPEC (15.6%). There were no significant differences between the two groups.
Most patients (87.5%) already received systemic chemotherapy during the course of their disease consisting of different chemotherapy regimens. More than half of the patients (53.1%) had previous oxaliplatin-based systemic chemotherapy.
Operative and perioperative data
Operative and perioperative data is summarized in Table 3. The mean operating time was 348 minutes (range 149-586) with 337 minutes (range 149-586) in the OX group and 366 minutes (range 200-557) in the IRI group, respectively (p = 0.497). The mean blood loss was 271 ml (range 100-600), and the mean number of anastomoses was 1.19 (range 0-3). There were no significant differences between the two groups.
Table 3.
Operative and perioperative data
| Overall | OX | IRI | p-value | |
|---|---|---|---|---|
| Number of patients [n] | 32 | 20 | 12 | |
| I. Operative data | ||||
| Mean operating time [min] | 348 (149-586) | 337 | 366 | 0.497 |
| Mean blood loss [ml] | 271 (100-600) | 257 | 280 | 0.760 |
| Mean no. of anastomoses | 1.19 | 1.21 | 1.17 | 0.899 |
| II. Perioperative data | ||||
| Median stay on ICU [d] | 1 (0-6) | 1 (0-5) | 1.5 (0-6) | 0.332 |
| Median hospital stay [d] | 15.5 (9-42) | 15 (9-38) | 15.5 (8-42) | 0.863 |
| Morbidity °3/4 [n] | 11 (34.4%) | 7 (35.0%) | 4 (33.3%) | 1.000 |
| In-hospital mortality [n] | 0 | 0 | 0 | 1.000 |
| 30-day mortality [n] | 0 | 0 | 0 | 1.000 |
| Revision surgery [n] | 3 (9.4%) | 1 (5.0%) | 2 (16.7%) | 0.540 |
The detailed surgical and peritonectomy procedures are summarized in Table 4. There were no statistically significant differences regarding surgery between the two groups.
Table 4.
Peritonectomy and surgical procedures
| Overall | OX | IRI | p-value | |
|---|---|---|---|---|
| Number of patients [n] | 32 | 20 | 12 | |
| Greater omentectomy | 24 | 17 | 7 | 0.204 |
| Upper right peritonectomy | 21 | 14 | 7 | 0.703 |
| Upper left peritonectomy | 7 | 5 | 2 | 0.683 |
| Parietal peritonectomy | 9 | 6 | 3 | 1.000 |
| Pelvic peritonectomy | 22 | 15 | 7 | 0.438 |
| Small bowel resection | 19 | 12 | 7 | 1.000 |
| Colonic resection | 17 | 11 | 6 | 1.000 |
| Rectal resection | 20 | 13 | 7 | 0.999 |
| Cholecystectomy | 9 | 6 | 3 | 1.000 |
| Liver resection | 5 | 1 | 4 | 0.053 |
| Gastric resection | 3 | 1 | 2 | 0.540 |
| Splenectomy | 6 | 3 | 3 | 0.647 |
| Ovarectomy | 2 | 0 | 2 | 0.133 |
| Hysterectomy | 3 | 1 | 2 | 0.540 |
| Vesical resection | 2 | 1 | 1 | 1.000 |
| Loop ileostomy | 4 | 3 | 1 | 1.000 |
| Overall number of peritonectomy procedures | 83 | 57 | 26 | 0.104 |
| Overall number of visceral resections | 86 | 49 | 37 | 0.142 |
The median hospital stay was 15 days (range 9-38) in the OX group and 15.5 days (range 8-42) in the IRI group, respectively (p = 0.863). The median stay on ICU was one day (range 0-6) in both groups (p = 0.332).
Morbidity and mortality
The overall grade 3/4 morbidity rate according to CTCAE v4.02 was 34.4% with 35.0% in the OX group and 33.3% in the IRI group, respectively (p = 1.000). Postoperative complications are summarized in detail in Table 5. In the OX group two patients developed pleural effusion requiring intervention. Moreover, ileus, intraabdominal abscess, bowel perforation, lung embolism, and cardiac arrhythmia were observed. In the IRI group ileus, postoperative bleeding, wound infection and pneumonia occurred. There was no documented hematological toxicity requiring intervention in both groups. Three patients (9.4%), one patient in the OX group (5.0%) and two patients in the IRI group (16.7%), required revision surgery due to postoperative complications (p = 0.540). Reasons for re-operation were postoperative bleeding, small bowel perforation and extensive wound infection.
Table 5.
Postoperative complications grade 3/4
| Overall | OX | IRI | p-value | |
|---|---|---|---|---|
| Number of patients [n] | 32 | 20 | 12 | |
| Pleural effusion | 2 (6.3%) | 2 (10.0%) | 0 | 0.516 |
| Pneumonia | 1 (3.1%) | 0 | 1 (8.3%) | 0.375 |
| Lung embolism | 1 (3.1%) | 1 (5.0%) | 0 | 1.000 |
| Bowel perforation | 1 (3.1%) | 1 (5.0%) | 0 | 1.000 |
| Ileus | 2 (6.3%) | 1 (5.0%) | 1 (8.3%) | 1.000 |
| Postoperative bleeding | 1 (3.1%) | 0 | 1 (8.3%) | 0.375 |
| Wound infection | 1 (3.1%) | 0 | 1 (8.3%) | 0.375 |
| Intraabdominal abscess | 1 (3.1%) | 1 (5.0%) | 0 | 1.000 |
| Cardiac arrhythmia | 1 (3.1%) | 1 (5.0%) | 0 | 1.000 |
Perioperative morbidity defined as 30-day or in-hospital mortality (depending on the length of hospital stay) was 0% in both groups.
Survival analysis
The overall 2-year and 3-year survival rates were 68.8% and 56.3%, respectively (Figure 1A). The group-specific 2-year survival rates were 70.0% in the OX group and 66.7% in the IRI group (p = 0.846). The 3-year survival rates reached 65.0% in the OX group and 41.7% in the IRI group (p = 0.295). This difference was not statistically significant (Figure 1B). In the OX group median survival has not yet been reached during follow-up. The median follow-up time including events of death in this group of patients was 39.4 months (range 7.2-51.1). The IRI group showed a median survival of 26.8 months (95% CI 15.7-33.1 months).Subgroup analysis showed a 3-year overall survival rate of 72.7% in 11 patients with appendiceal primary compared to 47.6% in 21 patients with peritoneal metastasis arising from colonic, sigmoid or rectal cancer (Figure 2, p = 0.213). After three years 68.8% of patients with negative initial lymph node status survived (n = 16) compared to 46.7% of patients with positive lymph nodes (n = 15) at time of first diagnosis (p = 0.231). There was also no statistically significant difference regarding 3-year survival rates depending on the histological grading. Overall 3-year survival rates were 60.0% for patients with well differentiated primary tumors (G1, n = 5), 54.5% for patients with moderately differentiated primary tumors (G2, n = 11) and 53.6% for patients with poorly differentiated primary tumors (G3, n = 16), respectively (p =0.998).
Figure 1.
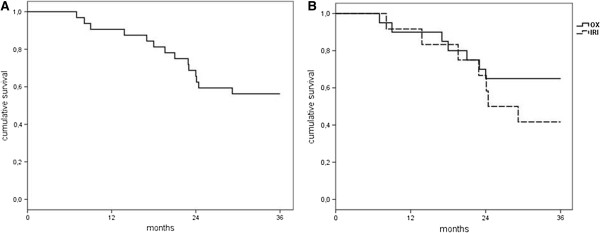
Kaplan-Meier survival analysis. A - Overall survival. n =32. 2-year survival rate 68.8%, 3-year survival rate 56.3%. B - group-specific overall survival. 2-year survival rates: 70.0% (OX) and 66.7% (IRI), 3-year survival rates: 65.0% (OX) and 41.7% (IRI). p =0.295 (n.s.). OX = oxaliplatin-based HIPEC, IRI = irinotecan-based HIPEC.
Figure 2.
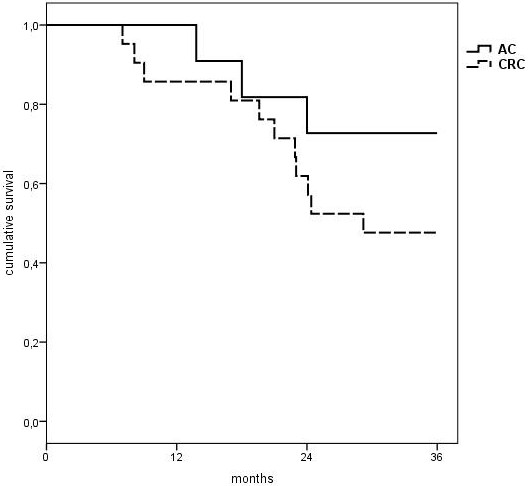
3-year survival depending on primary tumor. Overall survival of patients with appendiceal cancer (n = 11) vs. patients with colorectal cancer (n = 21) as primary tumor. 3-year survival rates: 72.7% (AC) and 47.6% (CRC). p = 0.213 (n.s.). AC = appendiceal cancer, CRC = colorectal cancer.
Discussion
The multimodality treatment concept of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is associated with significant rates perioperative morbidity. Beyond patient factors such as comorbidities the operative risk of CRS depends on the extent of surgery and the distinctive features of the performed surgical procedures. HIPEC may cause additional chemotherapy-related complications and toxicity. In the literature morbidity rates in specialized peritoneal carcinomatosis centers range from 23% to 45% depending on the assessment and definition of perioperative complications [3, 4, 7, 24–27]. Quenet et al. reported an in increase of the morbidity rate from 34.9% to 52.4% by adding intraperitoneal irinotecan to an oxaliplatin-based bidirectional HIPEC regimen [12]. In the present study the overall grade 3/4 morbidity rate was 34.4%. Although pharmacokinetic studies on heated intraperitoneal oxaliplatin reported dose absorption rates from 40% to 68% within 30 minutes perfusion [28, 29], we found no hematologic toxicity requiring intervention neither in the OX nor in the IRI group. This result is consistent with previous observations [30, 31]. Nevertheless, Elias et al. reported a haematological toxicity rate of 11% after bidirectional HIPEC with intraperitoneal oxaliplatin plus irinotecan [18]. In a systematic review including numerous different HIPEC regimens the mean overall rate of hematologic toxicity was 5.6% [32]. The low chemotherapy-related morbidity in our series may be caused by the concentration of 300 mg/sqm body surface in comparison to 460 mg/sqm body surface in the French series. In a recently published study of oxaliplatin pharmacokinetics during bidirectional HIPEC the oxaliplatin dose has been reduced from 460 mg/sqm body surface to 360 mg/sqm body surface after the first 17 patients due to toxicity [33].
In the present series three patients had to be re-operated for perioperative complications (9.4%). In the literature revision surgery is reported for 8.2% to 14% of patients that underwent CRS and HIPEC [3, 25, 26, 34]. The recently published data from the American College of Surgeons National Surgical Quality Improvement Program showed a re-operation rate of 10% [35]. There was no perioperative mortality in the present study. In a systematic review of 155 articles published by Chua et al. the mean mortality rate was 2.9% ranging from 0% to 17% [32]. In the American College of Surgeons National Surgical Quality Improvement Program hospitals overall morbidity rate was 2% [35]. Hospital stay and stay on ICU did not differ between the two groups in our series and were comparable to published data [3, 7, 9, 24, 30, 34, 35].
The safety and efficacy of intravenous oxaliplatin and irinotecan in combination with 5-FU and folinic acid has been demonstrated in numerous studies [36–43]. Both cytostatic agents are considered to be part of the standard treatment of advanced colorectal cancer. Nevertheless, systemic treatment is less efficient in patients with peritoneal metastasis [44]. Franko et al. compared the outcome of 2,095 patients enrolled onto two prospective randomized clinical trials evaluating systemic chemotherapy for patients with CRC with (pcCRC) and without peritoneal metastasis (non-pcCRC) and reported median survival of 12.7 months in the pcCRC group (n = 364) vs. 17.6 months in the non-pcCRC group. In this analysis infusional oxaliplatin-based chemotherapy was superior to irinotecan in first line therapy of pcCRC patients [14].
Based on the successful use of systemic oxaliplatin, 5-fluorouracil and folinic acid in patients with mCRC bidirectional HIPEC with intraperitoneal oxaliplatin and intravenous 5-FU/folinic acid has been used within the multimodality concept of CRS and HIPEC in patients with peritoneal metastasis arising from CRC. Elias et al. reported promising results with a median survival of 62.7 months and a 5-year survival rate of 51% [11]. In a prospective phase II study published by Hompes et al. the 2-year overall survival rate reached 88.7% and the median disease-free survival (DFS) was 19.8 months [45]. Consistent with this data the 3-year survival rate in our series was 65.0%. In contrast to the results for systemic treatment using the FOLFOXIRI protocol published by Falcone et al. [38] addition of intraperitoneal irinotecan to the bidirectional oxaliplatin-based HIPEC regimen did not lead to improved overall or relapse-free survival. Quenet et al. reported a median overall survival 47 months and a 5-year survival rate of 42.4% [12]. The survival data of a phase I trial combining irinotecan with mitomycin C for HIPEC is not yet available [19].
In the present retrospective analysis the rationale for intraperitoneal irinotecan was response to systemic irinotecan-based chemotherapy in seven patients, disease progression under oxaliplatin-based systemic chemotherapy in three and oxaliplatin-associated peripheral polyneuropathy ≥ grade 3 in two patients. Median overall survival was 26.8 months and 3-year survival rate 41.7% in the IRI group suggesting a negative trend compared to the OX group as well as most published survival data after complete macroscopic cytoreduction and HIPEC with other cytostatic agents or combinations [8, 9, 11, 45]. Nevertheless, in a recently published retrospective analysis of 95 patients Hompes et al. reported a median overall survival of 37.1 months after oxaliplatin-based HIPEC and 26.5 months for the mitomycin C-based HIPEC after complete macroscopic cytoreduction [46]. This observation is supported by the data published from the American Society of Peritoneal Surface Malignancies showing a median overall survival of 32.7 months in patients treated with complete macroscopic cytoreduction and MMC-based HIPEC vs. 31.4 months in patients after CC-0/1-resection and oxaliplatin-based HIPEC [47]. Due to the small number of patients in the present series the survival difference between the two groups was not statistical significant (p = 0.295) and therefore the relevance of this observation is limited. Moreover, there were a statistically not significant lower number of patients with appendiceal cancer and G1 differentiation as well as more patients with positive lymph node status in the IRI group. In a retrospective study Elias et al. reported a statistically significant improved overall 5-year survival rate of 63% for patients with peritoneal metastasis arising from appendiceal adenocarcinoma in comparison to colon (29.7%) and rectal cancer (37.9%). In the multivariate analysis positive lymph node status was an independent negative prognostic factor (p = 0.001) [48]. This observation has been confirmed by other published data [49, 50]. Nevertheless, Jimenez et al. reported a median survival of 47 months and a 5-year survival rate of 41% after CRS and HIPEC in 125 patients with histologically proven peritoneal carcinomatosis (PMCA) arising from appendiceal cancer [51]. This survival data is comparable to the survival rates published for patients with colorectal peritoneal metastasis. However, in the present series the subgroup analysis of tumor origin, grading and lymph node status showed no significant differences.
Conclusions
In conclusion, our data show that both bidirectional HIPEC regimens may be used with comparable low mortality and acceptable morbidity in a specialized peritoneal carcinomatosis center. Published data and the positive trend regarding the overall 3-year survival rate in the present series support oxaliplatin-based HIPEC as the first choice treatment regimen. Nevertheless, in our opinion irinotecan-based HIPEC should still be considered as a promising alternative in patients with tumor progression or intolerable toxicity under chemotherapy with oxaliplatin. However, comparative prospective randomized trials are necessary to determine the best treatment regimen regarding morbidity, mortality and particularly long-term oncological outcome.
Consent
Written informed consent was obtained from the patient for inclusion into the database and publication of the data.
Abbreviations
- 5-FU
Fluorouracil
- ASA
American Society of Anesthesiologists
- CA19-9
Carbohydrate antigen 19-9
- CC
Completeness of cytoreduction
- CEA
Carcinoembryonic antigen
- CI
Confidence interval
- CRC
Colorectal cancer
- CRS
Cytoreductive surgery
- CT
Computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DPAM
Disseminated peritoneal adenomucinosis
- FA
Folinic acid
- FOLFIRI
Folinic acid + fluorouracil + irinotecan
- FOLFOX
Folinic acid + fluorouracil + oxaliplatin
- FOLFOXIRI
Folinic acid + fluorouracil + irinotecan + oxaliplatin
- HIPEC
Hyperthermic intraperitoneal chemotherapy
- mCRC
Metastatic colorectal cancer
- MMC
Mitomycin C
- OS
Overall survival
- pcCRC
Peritoneal carcinomatosis of CRC
- PCI
Peritoneal Cancer Index
- PFS
Progression-free survival
- PM
Peritoneal metastases
- PMCA-I
Peritoneal mucinous carcinomatosis of intermediate features
- QoL
Quality of life
- RCT
Randomized controlled trial.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GG drafted the manuscript. MG and MKS provided the survival data. SAL, PP and HJS revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Gabriel Glockzin, Email: gabriel.glockzin@ukr.de.
Michael Gerken, Email: michael.gerken@ukr.de.
Sven A Lang, Email: sven.lang@ukr.de.
Monika Klinkhammer-Schalke, Email: monika.klinkhammer-schalke@ukr.de.
Pompiliu Piso, Email: pompiliu.piso@barmherzige-regensburg.de.
Hans J Schlitt, Email: hans.schlitt@ukr.de.
References
- 1.Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, Schlag P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16(4 Suppl 6):83–97. [PubMed] [Google Scholar]
- 2.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 3.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer. 2010;116(24):5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 4.Pilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, Lise M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10(5):508–513. doi: 10.1245/aso.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91(6):747–754. doi: 10.1002/bjs.4473. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AA, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11(2):178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 9.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 10.Quenet F, Claus C, Roca L, Gauthey A, Essissen M, Ychou M, Rouanet P, Saint-Aubert B. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from digestive tract cancer--New management with oxaliplatin plus irinotecan: A feasibility study in 37 patients. J Clin Oncol. 2008;26:Suppl. [Google Scholar]
- 11.Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, Bonastre J. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 12.Quenet F, Goere D, Mehta SS, Roca L, Dumont F, Hessissen M, Saint-Aubert B, Elias D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254(2):294–301. doi: 10.1097/SLA.0b013e3182263933. [DOI] [PubMed] [Google Scholar]
- 13.Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1407–1412. doi: 10.1016/S0140-6736(98)03085-2. [DOI] [PubMed] [Google Scholar]
- 14.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2(2):68–75. doi: 10.4251/wjgo.v2.i2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pox C, Aretz S, Bischoff SC, Graeven U, Hass M, Heussner P, Hohenberger W, Holstege A, Hubner J, Kolligs F, Kreis M, Lux P, Ockenga J, Porschen R, Post S, Rahner N, Reinacher-Schick A, Riemann JF, Sauer R, Sieg A, Scheppach W, Schmitt W, Schmoll HJ, Schulmann K, Tannapfel A, Schmiegel W. S3-guideline colorectal cancer version 1.0. Z Gastroenterol. 2013;51(8):753–854. doi: 10.1055/s-0033-1350264. [DOI] [PubMed] [Google Scholar]
- 17.Glockzin G, Rochon J, Arnold D, Lang SA, Klebl F, Zeman F, Koller M, Schlitt HJ, Piso P. A prospective multicenter phase II study evaluating multimodality treatment of patients with peritoneal carcinomatosis arising from appendiceal and colorectal cancer: the COMBATAC trial. BMC Cancer. 2013;13:67. doi: 10.1186/1471-2407-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias D, Goere D, Blot F, Billard V, Pocard M, Kohneh-Shahri N, Raynard B. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol. 2007;14(6):1818–1824. doi: 10.1245/s10434-007-9348-1. [DOI] [PubMed] [Google Scholar]
- 19.Cotte E, Passot G, Tod M, Bakrin N, Gilly FN, Steghens A, Mohamed F, Glehen O. Closed abdomen hyperthermic intraperitoneal chemotherapy with irinotecan and mitomycin C: a phase I study. Ann Surg Oncol. 2011;18(9):2599–2603. doi: 10.1245/s10434-011-1651-1. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 21.Glehen O, Gilly FN. Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am. 2003;12(3):649–671. doi: 10.1016/s1055-3207(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 22.Glockzin G, Ghali N, Lang SA, Agha A, Schlitt HJ, Piso P. Peritoneal carcinomatosis. Surgical treatment, including hyperthermic intraperitoneal chemotherapy. Chirurg. 2007;78(12):1100–1110. doi: 10.1007/s00104-007-1419-0. [DOI] [PubMed] [Google Scholar]
- 23.Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7(1):5. doi: 10.1186/1477-7819-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, Francois Y, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10(8):863–869. doi: 10.1245/aso.2003.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, Steves MA, Sugarbaker PH. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6(8):790–796. doi: 10.1007/s10434-999-0790-0. [DOI] [PubMed] [Google Scholar]
- 26.Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, Deraco M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106(5):1144–1153. doi: 10.1002/cncr.21708. [DOI] [PubMed] [Google Scholar]
- 27.Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, Zeh HJ, 3rd, Bartlett DL. Multivisceral resection does Not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15(11):3065–3072. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 28.Ferron G, Dattez S, Gladieff L, Delord JP, Pierre S, Lafont T, Lochon I, Chatelut E. Pharmacokinetics of heated intraperitoneal oxaliplatin. Cancer Chemother Pharmacol. 2008;62(4):679–683. doi: 10.1007/s00280-007-0654-x. [DOI] [PubMed] [Google Scholar]
- 29.Mahteme H, Wallin I, Glimelius B, Pahlman L, Ehrsson H. Systemic exposure of the parent drug oxaliplatin during hyperthermic intraperitoneal perfusion. Eur J Clin Pharmacol. 2008;64(9):907–911. doi: 10.1007/s00228-008-0511-9. [DOI] [PubMed] [Google Scholar]
- 30.Glockzin G, von Breitenbuch P, Schlitt HJ, Piso P. Treatment-related morbidity and toxicity of CRS and oxaliplatin-based HIPEC compared to a mitomycin and doxorubicin-based HIPEC protocol in patients with peritoneal carcinomatosis: a matched-pair analysis. J Surg Oncol. 2013;107(6):574–578. doi: 10.1002/jso.23228. [DOI] [PubMed] [Google Scholar]
- 31.Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, Pignon JP, Drouard-Troalen L, Ouellet JF, Ducreux M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13(2):267–272. doi: 10.1093/annonc/mdf019. [DOI] [PubMed] [Google Scholar]
- 32.Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249(6):900–907. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- 33.Chalret du Rieu Q, White-Koning M, Picaud L, Lochon I, Marsili S, Gladieff L, Chatelut E, Ferron G. Population pharmacokinetics of peritoneal, plasma ultrafiltrated and protein-bound oxaliplatin concentrations in patients with disseminated peritoneal cancer after intraperitoneal hyperthermic chemoperfusion of oxaliplatin following cytoreductive surgery: correlation between oxaliplatin exposure and thrombocytopenia. Cancer Chemother Pharmacol. 2014;74(3):571–582. doi: 10.1007/s00280-014-2525-6. [DOI] [PubMed] [Google Scholar]
- 34.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, Edwards RP, Brown CK, Holtzman MP, Zeh HJ, Bartlett DL. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15(3):754–763. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 35.Jafari MD, Halabi WJ, Stamos MJ, Nguyen VQ, Carmichael JC, Mills SD, Pigazzi A. JAMA Surg. 2013. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the American College of Surgeons National surgical quality improvement program. [DOI] [PubMed] [Google Scholar]
- 36.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts S. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24(21):3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 38.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crino L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G, Gruppo Oncologico Nord Ovest Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 39.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 40.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Lévi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18(1):136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 41.Lee DH, Oh SY, Lee YR, Huh SJ, Yoon HH, Kim SH, Lee S, Lee JH, Kim Y, Kim HJ, Lee S, Lee JH, Kim Y, Kim HJ, Kwon HC. A Phase II Study of Modified FOLFOX4 for Colorectal Cancer Patients with Peritoneal Carcinomatosis. Cancer Res Treat. 2011;43(4):225–230. doi: 10.4143/crt.2011.43.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 43.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 44.Zani S, Papalezova K, Stinnett S, Tyler D, Hsu D, Blazer DG., 3rd Modest advances in survival for patients with colorectal-associated peritoneal carcinomatosis in the era of modern chemotherapy. J Surg Oncol. 2013;107(4):307–311. doi: 10.1002/jso.23222. [DOI] [PubMed] [Google Scholar]
- 45.Hompes D, D’Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M, Van der Speeten K, Bertrand C, Legendre H, Kerger J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (hipec) with oxaliplatin: a Belgian Multicentre Prospective Phase II Clinical Study. Ann Surg Oncol. 2012;19(7):2186–2194. doi: 10.1245/s10434-012-2264-z. [DOI] [PubMed] [Google Scholar]
- 46.Hompes D, D’Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: A comparative study. J Surg Oncol. 2014;109(6):527–532. doi: 10.1002/jso.23546. [DOI] [PubMed] [Google Scholar]
- 47.Prada-Villaverde A, Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, Gomez-Portilla A, González-Moreno S, Goodman M, Halkia E, Kusamura S, Moller M, Passot G, Pocard M, Salti G, Sardi A, Senthil M, Spiliotis J, Torres-Melero J, Turaga K, Trout R. J Surg Oncol. 2014. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. [DOI] [PubMed] [Google Scholar]
- 48.Elias D, Glehen O, Pocard M, Quenet F, Goere D, Arvieux C, Rat P, Gilly F. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg. 2010;251(5):896–901. doi: 10.1097/SLA.0b013e3181d9765d. [DOI] [PubMed] [Google Scholar]
- 49.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dube P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 51.Jimenez W, Sardi A, Nieroda C, Sittig M, Milovanov V, Nunez M, Aydin N, Gushchin V. Ann Surg Oncol. 2014. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. [DOI] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/807/prepub


