Abstract
The infectivity of trachoma-inclusion conjunctivitis (TRIC) organisms (TW-5) was enhanced by pretreatment of HeLa cell monolayers before inoculation with diethylaminoethyl (DEAE)-dextran (30 μg/ml) and poly-l-lysine (10 μg/ml) and inhibited by dextran sulphate (250 μg/ml), fetuin (4%), ovomucoid (5%), N-acetyl neuraminic acid (0.5%), and Cholera vibrio neuraminidase (100 U/ml). The infectivity of lymphogranuloma venereum organisms (434) was not affected by DEAE-dextran, fetuin, and neuraminidase, was slightly inhibited by poly-l-lysine, and was inhibited by dextran-sulphate, ovomucoid, and N-acetyl neuraminic acid. The study suggested that sialic acid residues on the cell surface may be specific receptors for TRIC organisms. The receptors for TRIC organisms (TW-5 and TW-3) could be specifically blocked with inactivated (56 C for 30 min) TRIC organisms at the ratio of one live to 100 inactivated TRIC organisms, but not by inactivated lymphogranuloma venereum (434) or influenza virus (A2/Jap 305).
Full text
PDF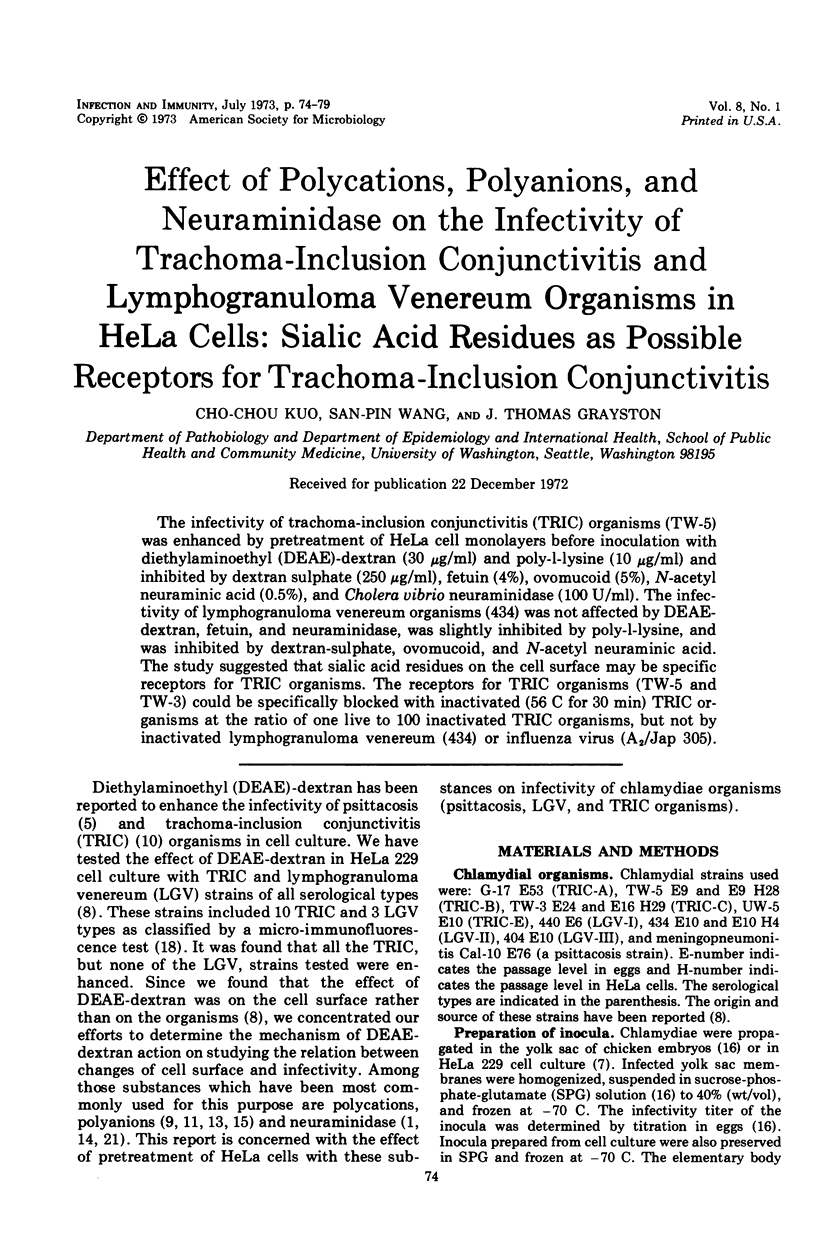


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose E. J. Electrophoretic behaviour of cells. Prog Biophys Mol Biol. 1966;16:241–265. doi: 10.1016/0079-6107(66)90008-3. [DOI] [PubMed] [Google Scholar]
- Banks J., Eddie B., Schachter J., Meyer K. F. Plaque formation by Chlamydia in L cells. Infect Immun. 1970 Mar;1(3):259–262. doi: 10.1128/iai.1.3.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURNESS G., GRAHAM D. M., REEVE P. The titration of trachoma and inclusion blennorrhoea viruses in cell cultures. J Gen Microbiol. 1960 Dec;23:613–619. doi: 10.1099/00221287-23-3-613. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. J. Enhancing effect of DEAE-Dextran on inclusion counts of an ovine Chlamydia (Bedsonia) in cell culture. Aust J Exp Biol Med Sci. 1970 Apr;48(2):207–213. doi: 10.1038/icb.1970.20. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M. The continuous passage of agents of trachoma in cell culture. I. Characteristics of TW-3 and Bour strains of trachoma cultivated in serial passage in HeLa 229 cells. J Infect Dis. 1966 Jun;116(3):390–399. doi: 10.1093/infdis/116.3.390. [DOI] [PubMed] [Google Scholar]
- Kuo C., Wang S., Grayston J. T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972 Mar;125(3):313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota T. R., Nichols R. L. Infection of cell cultures by trachoma agent: enhancement by DEAE-dextran. J Infect Dis. 1971 Oct;124(4):419–421. doi: 10.1093/infdis/124.4.419. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. A membrane effect of basic polymers dependent on molecular size. Nature. 1967 Aug 26;215(5104):934–936. doi: 10.1038/215934a0. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967 Mar;32(3):737–750. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- Simmons R. L., Rios A., Ray P. K. Immunogenicity and antigenicity of lymphoid cells treated with neuraminidase. Nat New Biol. 1971 Jun 9;231(23):179–181. doi: 10.1038/newbio231179a0. [DOI] [PubMed] [Google Scholar]
- WANG S. P., GRAYSTON J. T. EGG INFECTIVITY ASSAY OF TRACHOMA VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:587–591. doi: 10.3181/00379727-115-28978. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wallis C., Morales F., Powell J., Melnick J. L. Plaque enhancement of enteroviruses by magnesium chloride, cysteine, and pancreatin. J Bacteriol. 1966 May;91(5):1932–1935. doi: 10.1128/jb.91.5.1932-1935.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. A potency test for trachoma vaccine utilizing the mouse toxicity prevention test. Am J Ophthalmol. 1967 May;63(5 Suppl):1443–1454. doi: 10.1016/0002-9394(67)94130-x. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Antigenic variation in influenza virus. Biology and chemistry. Prog Med Virol. 1971;13:271–338. [PubMed] [Google Scholar]
- Weiss L., Mayhew E., Ulrich K. The effect of neuraminidase on the phagocytic process in human monocytes. Lab Invest. 1966 Aug;15(8):1304–1309. [PubMed] [Google Scholar]


