Abstract
The abuse of synthetic psychoactive substances known as “designer drugs,” or “new psychoactive substances” (NPS), is increasing at an alarming rate. NPS are purchased as alternatives to traditional illicit drugs of abuse and are manufactured to circumvent laws regulating the sale and use of controlled substances. Synthetic cathinones (i.e., “bath salts”) and synthetic cannabinoids (i.e., “spice”) are two types of NPS that have received substantial media attention. Although low recreational doses of bath salts or spice compounds can produce desirable effects, high doses or chronic exposure often leads to dangerous medical consequences, including psychosis, violent behaviors, tachycardia, hyperthermia, and even death. Despite the popularity of NPS, there is a paucity of scientific data about these drugs. Here we provide a brief up-to-date review describing the mechanisms of action and neurobiological effects of synthetic cathinones and cannabinoids.
Introduction
Over the past few years, there has been an alarming increase in the abuse of synthetic psychoactive substances known as “designer drugs” or “legal highs” (Rosenbaum et al., 2012; Johnson et al., 2013; Nelson et al., 2014). These substances are purchased as alternatives to traditional illicit drugs of abuse and are manufactured to intentionally circumvent the laws regulating the sale and use of controlled substances. Countries of the European Union have adopted the term “new psychoactive substances” (NPS) to denote this category of emerging drugs, and we will use this nomenclature here (Brandt et al., 2014). NPS are synthesized by clandestine chemists who hijack the medical and patent literature to identify compounds targeting specific transporters or receptors implicated in the effects of psychoactive drugs (Collins, 2011; Lewin et al., 2014). Internet sales and marketing have made NPS easily available on a global scale. Synthetic cathinones (i.e., “bath salts”) and synthetic cannabinoids (i.e., “spice”) are two types of NPS that have received substantial media attention. Synthetic cathinones produce amphetamine- or cocaine-like subjective effects by activating monoamine systems in the brain and periphery (Baumann et al., 2013a; De Felice et al., 2014), whereas synthetic cannabinoids produce marijuana-like effects by activating the endocannabinoid system (Fattore and Fratta, 2011; Wiley et al., 2014a). Low recreational doses of bath salts or spice compounds produce the expected desirable effects, but high doses or chronic exposure can lead to dangerous medical consequences, including psychosis, violent behaviors, tachycardia, hyperthermia, and even death (Prosser and Nelson, 2012; Hermanns-Clausen et al., 2013; Kronstrand et al., 2013).
Because of the public health risks posed by NPS, the governments of many countries, including the United States, have passed legislation to ban the sale, possession, and use of specific synthetic cathinones and cannabinoids (Drug Enforcement Administration, 2011, 2013; German et al., 2014). Unfortunately, such legislation has fostered the emergence of new “replacement” analogs that are manufactured to skirt regulatory control, and this trend is expected to continue (Shanks et al., 2012; Seely et al., 2013). Very little information is available regarding the mechanisms of action, pharmacological effects, and toxicological profile for most NPS. The present brief review is based upon the minisymposium, “Bath Salts, Spice and Related Designer Drugs: The Science Behind the Headlines,” presented at the Society for Neuroscience annual meeting held in Washington, DC in 2014. Here we aim to provide the most up-to-date information about the pharmacology of synthetic cathinones and cannabinoids, with a specific focus on the neurobiology of these agents.
“Baths salts” cathinones interact with monoamine transporters
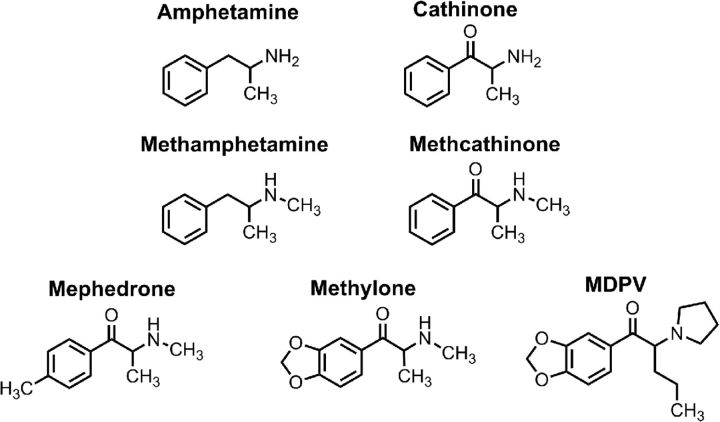
Psychoactive “bath salts” first appeared in the recreational drug marketplace of the United States during late 2010. By early 2011, there was a dramatic spike in reports of bath salts overdose to poison control centers and an influx of patients admitted to emergency departments with toxic exposures (Spiller et al., 2011; Ross et al., 2012). Patients intoxicated with bath salts can display severe symptoms, including psychosis, hallucinations, agitation, tachycardia, hypertension, and hyperthermia, often accompanied by combative or violent behaviors. Forensic analysis of bath salts products revealed the presence of three main synthetic cathinones, depicted in Figure 1: 4-methyl-N-methylcathinone (mephedrone), 3,4-methylenedioxy-N-methylcathinone (methylone), and 3,4-methylenedioxypyrovalerone (MDPV) (Spiller et al., 2011; Shanks et al., 2012). These compounds are structurally related to the parent compound cathinone, which is a naturally occurring β-keto amphetamine with known psychostimulant properties (Schechter and Glennon, 1985; Kalix and Glennon, 1986). Some bath salts powders consist of relatively pure preparations of one synthetic cathinone, but others contain mixtures of two or more different cathinones, along with adulterants, including caffeine, lidocaine, or piperazines (Davies et al., 2010; Zawilska and Wojcieszak, 2013). Like other stimulant drugs, synthetic cathinones target monoamine transporters expressed on nerve cells and other cell types (Hadlock et al., 2011; López-Arnau et al., 2012; Martínez-Clemente et al., 2012). Monoamine transporters are members of the SLC6 solute carrier family of proteins that mediate the sodium-dependent uptake of monoamine neurotransmitters, and there are specific transporters for norepinephrine (NET), dopamine (DAT), and 5-HT (SERT) (Kristensen et al., 2011). Drugs that interact with transporters can be divided into two types: (1) amphetamine-like substrates or (2) cocaine-like blockers (Rothman and Baumann, 2003; Sitte and Freissmuth, 2010). Both types of drugs increase extracellular concentrations of monoamines, but substrates induce transporter-mediated inward currents (i.e., depolarization) and transmitter efflux (i.e., release), whereas blockers do not. Additionally, the transporter selectivity for a given drug is predictive of behavioral effects because drugs that are selective for DAT are powerful locomotor stimulants, but drugs selective for SERT are not (Rothman and Baumann, 2006; Howell and Kimmel, 2008).
Figure 1.
Chemical structures of synthetic cathinones found in bath salts NPS and their relationship to the plant-derived compound cathinone.
Several research groups have examined the interaction of synthetic cathinones with monoamine transporters using a variety of methods. In rat brain synaptosomes, mephedrone and methylone are nonselective transporter substrates, thereby evoking the release of preloaded [3H]neurotransmitters at DAT, NET, and SERT (Baumann et al., 2012). The effects of mephedrone and methylone in synaptosomes mimic those of the illicit drug 3,4-methylenedioxymethamphetamine (MDMA). By contrast, MDPV is a transporter blocker that potently inhibits [3H]neurotransmitter uptake at DAT and NET, with little effect at SERT (Baumann et al., 2013b). Importantly, MDPV is 50 and 10 times more potent than cocaine as a blocker at DAT and NET, respectively. Although studies in synaptosomes provide the advantage of high-throughput drug screening in native tissue, experiments in cells allow more detailed assessment of drug–transporter interactions. Assay systems using human transporters expressed in HEK293 cells show that mephedrone, methylone, and other ring-substituted cathinones are substrates at hDAT, hNET, and hSERT, whereas MDPV is a potent blocker at hDAT and hNET only (Eshleman et al., 2013; Simmler et al., 2013, 2014). Thus, results from human transporters are consistent with results from rat brain synaptosomes. Perhaps the most sophisticated method for examining drug–transporter interactions involves the measurement of transporter-mediated ionic currents using voltage-clamp techniques in cells expressing human transporters (Sonders et al., 1997; Sitte et al., 1998; De Felice et al., 2014). An electrophysiological signature provides definitive information about the mechanism of drug action at the molecular level. Because substrates are translocated through the transporter along with sodium ions, these agents produce transporter-mediated inward currents. Blockers bind to the transporter but are not translocated, so these agents produce outward currents (due to block of an endogenous leak current). As specific examples, amphetamine and mephedrone induce hDAT-mediated inward currents, whereas cocaine and MDPV induce outward currents (Cameron et al., 2013a, b; Kolanos et al., 2013). Together, the data from several lines of evidence agree that ring-substituted cathinones, such as mephedrone and methylone, are nonselective transporter substrates. MDPV is not a transporter substrate, most likely because the drug molecule is too large to fit through the transporter, but acts as a potent blocker at DAT and NET with minimal activity at SERT.
Synthetic cathinones induce stimulant effects in laboratory rodents
Consistent with their activity as transporter substrates and blockers, synthetic cathinones increase monoamine transmission in laboratory rodents. In vivo microdialysis studies in rats demonstrate that mephedrone and methylone elevate extracellular concentrations of dopamine and 5-HT in the nucleus accumbens, whereas MDPV increases dopamine without affecting 5-HT (Kehr et al., 2011; Baumann et al., 2012, 2013b; Wright et al., 2012). All synthetic cathinones investigated to date produce dose-dependent stimulation of motor activity when administered to rats or mice (Lisek et al., 2012; López-Arnau et al., 2012; Marusich et al., 2012, 2014; Baumann et al., 2013b; Fantegrossi et al., 2013; Gatch et al., 2013), probably due to enhancement of dopamine transmission. Most studies agree that MDPV is 3–10 times more potent than mephedrone or methylone as a locomotor stimulant. As noted above, bath salts products may contain a number of different cathinones, yet MDPV is the chief compound found in blood and urine from fatal cases of bath salts overdose in the United States (Spiller et al., 2011; Murray et al., 2012; Kesha et al., 2013; Wyman et al., 2013). This intriguing observation suggests that MDPV is the chief culprit involved with adverse effects of bath salts. It is tempting to speculate that potent blockade of DAT by MDPV is responsible for neurological symptoms and hyperthermia in bath salts overdose cases, whereas blockade of NET could underlie cardiovascular stimulation. Determining the pharmacokinetics and metabolism of synthetic cathinones in rodent models is essential because clinical data are limited to isolated forensic cases. Mephedrone and methylone are extensively metabolized in the rat (Kamata et al., 2006; López-Arnau et al., 2013; Martínez-Clemente et al., 2013), but the potential bioactivity of various identified metabolites has not been well studied. It is noteworthy that methylone and MDPV possess a 3,4-methylenedioxy ring-substitution akin to the illicit drug MDMA, a compound that displays nonlinear kinetics in rodents and humans (de la Torre et al., 2000; Kolbrich et al., 2008; Baumann et al., 2009; Fantegrossi et al., 2009; Concheiro et al., 2014). The phenomenon of nonlinear kinetics is characterized by greater-than-predicted plasma concentrations of MDMA due to autoinhibition of drug metabolism (Heydari et al., 2004; de la Torre et al., 2004). Similar to MDMA, MDPV is metabolized by O-demethylenation, thereby producing the ring-hydroxylated metabolites, 3,4-dihydroxypyrovalerone and 4-hydroxy-3-methoxypyrovalerone (Meyer et al., 2010; Strano-Rossi et al., 2010; Anizan et al., 2014). 3,4-Dihyhdroxypyrovalerone is a potent blocker of DAT and NET (Meltzer et al., 2006), so this metabolite could contribute significantly to the in vivo pharmacology of MDPV. 4-Hydroxy-3-methoxypyrovalerone is a long-lasting metabolite that may prove useful for forensic validation of MDPV exposure in human subjects (Anizan et al., 2014). Whether methylone and MDPV display nonlinear kinetics similar to MDMA is an unresolved issue that warrants investigation. No controlled clinical–laboratory studies with synthetic cathinones have been performed, and such studies are needed to understand the complex pharmacology of these drugs in humans.
Synthetic cathinones have abuse liability in rodent models
Recently, the addictive potential of synthetic cathinones has been investigated in rodent models. In preclinical abuse liability studies, the intravenous self-administration model is considered the “gold standard” because of its high degree of face and predictive validity (Watterson et al., 2013). From a neurobiological perspective, stimulation of mesolimbic dopamine transmission is a key mechanism underlying drug self-administration behavior (Willuhn et al., 2010; Espana and Jones, 2013). In the self-administration procedure, rats fitted with indwelling jugular catheters are placed in operant chambers equipped with two levers. Presses on the “active” lever result in a computer-controlled intravenous drug infusion and simultaneous presentation of a stimulus complex (light + tone). Presses on the other lever (i.e., “inactive”) produce no consequences but are recorded as a measure of nonspecific behaviors. Comparisons between MDPV and methylone in the self-administration model are of interest because these drugs have structural similarity but differ in their mechanism of action and selectivity for DAT versus SERT (see above). For MDPV, rats readily acquire self-administration across a range of doses (0.05–0.5 mg/kg/infusion) (Aarde et al., 2013a; Watterson et al., 2014). Under a progressive ratio schedule of reinforcement, in which the number of lever presses for drug infusion increases exponentially with each successive infusion, MDPV breakpoints are positively correlated with drug dose (Watterson et al., 2014). Moreover, MDPV breakpoints are similar to those produced by methamphetamine at the same dose (0.05 mg/kg/infusion). Escalation of MDPV intake is observed at 0.1 and 0.2 mg/kg/infusion doses across 6 h access sessions. This escalation of intake is similar in magnitude to that observed with 0.05 mg/kg/infusion of methamphetamine and is typically seen with other drugs that support compulsive use in humans (e.g., cocaine and heroin). For methylone, rats display self-administration across a range of doses (0.1–0.5 mg/kg/infusion) (Watterson et al., 2012). As with MDPV, a positive relationship between methylone dose and breakpoints is observed under progressive ratio conditions. However, unlike MDPV, escalation of drug intake is not observed with methylone. These data suggest that the serotonergic effects of methylone may dampen certain reinforcing properties of the drug. Nevertheless, under some conditions, rats will self-administer methylone to the point of convulsions and death, highlighting the dangers of synthetic cathinone use and corroborating reports of methylone-associated deaths in humans (Cawrse et al., 2012; Pearson et al., 2012). Overall, the available evidence shows that MDPV and methylone are readily self-administered by laboratory rats, and these findings agree with reports showing that mephedrone is also self-administered by rats (Hadlock et al., 2011; Aarde et al., 2013b; Motbey et al., 2013). Escalation of drug intake, a cardinal feature of drug addiction, is observed in laboratory rodents self-administering certain synthetic cathinones. Together, these preclinical studies suggest a high abuse potential for synthetic cathinones, such as MDPV, methylone, and mephedrone.
“Spice” cannabinoids interact with the endocannabinoid system
Plant-derived phytocannabinoids (e.g., Δ9-tetrahydrocannabinol [THC]) and synthetic cannabinoids produce their psychoactive effects through activation of the endocannabinoid system in the brain. This neuromodulatory system is comprised of two identified receptors (CB1 in the brain and periphery, and CB2 primarily in the periphery), at least two endogenous ligands (anandamide and 2-arachidonoyl glycerol), and associated synthetic and metabolic enzymes (for review, see Pertwee, 2008). Discovered in the late 1980s and early 1990s (Devane et al., 1988, 1992; Matsuda et al., 1990), the endocannabinoid system contributes to a number of physiological and pathological processes, including appetite, pain, mental illness, reward, and neurodegenerative diseases (Berry and Mechoulam, 2002; Mechoulam et al., 2002; Walker and Huang, 2002; van der Stelt and Di Marzo, 2003; Di Marzo et al., 2004). Development of the first research cannabinoid compounds that would later appear in products confiscated from drug users also occurred during this time span (Huffman et al., 1994; Lainton et al., 1995), although illicit diversion of these compounds did not occur until the early 2000s. The primary goals of the original research were to identify and differentiate structural properties of the newly discovered CB1 and CB2 receptors (Huffman, 2000, 2005; Huffman and Padgett, 2005; Huffman et al., 2005), but the purpose of currently available cannabinoid NPS is to achieve a marijuana-like intoxication. Compared with marijuana, however, abuse of these synthetic substances is associated with a higher prevalence of severe adverse effects, such as hypertension, tachycardia, hallucinations, agitation, seizures, and panic attacks that often require immediate medical care (Seely et al., 2012; Fantegrossi et al., 2014).
The broad structural diversity of cannabinoid agonists was recognized early, as observed in the array of structurally distinct classes of compounds that produce cannabinoid activity, including tetrahydrocannabinols, bicyclic cannabinoids, aminoalkylindoles, and anandamide analogs. WIN55212-2, an aminoalkylindole, was the template for synthesis of the original series of indole-derived synthetic cannabinoids (Eissenstat et al., 1995; Wiley et al., 2011). Subsequent studies reported an orderly structure–activity relationship between structural variation in these molecules (e.g., length and branching of the chain) and cannabinoid binding (Huffman et al., 1994; Lainton et al., 1995; Aung et al., 2000) and, when tested, in vivo cannabimimetic activity (Wiley et al., 1998, 2012a, b, 2014a). Interestingly, some of the compounds in this series with the best CB1 receptor affinities are the ones that were identified in earliest “spice” products, demonstrating that clandestine chemists are mining the scientific literature to guide manufacture.
Yet, despite similarity to THC in much of its pharmacology, WIN55212-2 differs from other classes of cannabinoids in its molecular interactions with cannabinoid receptors, having at least one unique site of attachment that is not shared by other cannabinoid agonists or by the CB1 antagonist rimonabant (Petitet et al., 1996; Song and Bonner, 1996). In addition, WIN55212-2 has higher affinity for peripheral cannabinoid CB2 receptors than for CB1 receptors in the brain (Showalter et al., 1996), a property it shares with many of the synthetic cannabinoids that have been identified in confiscated NPS (Huffman and Padgett, 2005; Manera et al., 2008). Because the physiological functions of CB2 receptors are not completely understood, it is possible that agonist action at these receptors may modulate the pharmacological profile of WIN55212-2 and other indoles. In addition, WIN55212-2 and many other indole-derived cannabinoids are full agonists at CB1 receptors, compared with the weak partial agonist activity of THC (Atwood et al., 2011; Brents et al., 2011, 2012; Chimalakonda et al., 2012). Finally, several lines of research have suggested that WIN55212-2 (and perhaps other structurally related synthetic cannabinoids) interacts with noncannabinoid receptors in the brain (Breivogel et al., 2001; Hájos et al., 2001; Monory et al., 2002). These actions may, in turn, modulate in vivo drug effects, leading to unexpected psychoactive effects and peripheral toxicities.
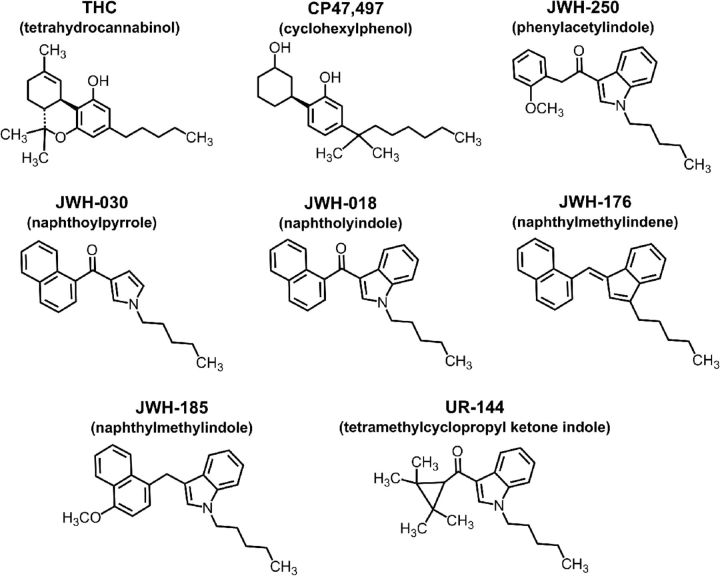
As of 2009, the most prevalent synthetic cannabinoids identified in spice or herbal incense products were classified into seven structural groups, as depicted in Figure 2: naphthoylindoles (e.g., JWH-018, JWH-073, AM-2201), naphthylmethylindoles (JWH-185), naphthoylpyrroles (JWH-030), naphthylmethylindenes (JWH-176), phenylacetylindoles (JWH-250, RCS-4), cyclohexylphenols (CP47,497), and tetrahydrocannabinols (Δ9-THC, HU-210) (European Monitoring Centre for Drugs and Drug Addiction, 2010); however, increased legal restriction has resulted in exploitation of new structural motifs from the scientific literature or led to the invention of new structures. For example, after the United States Drug Enforcement Administration banned JWH-018 and other naphthoylindoles, novel tetramethylcyclopropyl ketone indoles (UR-144, XLR-11) started being identified in confiscated products (Fig. 2). The sheer number of possible combinations of structural constituents is staggering and complicates prediction of which NPS are likely to be included in the next round of “product.”
Figure 2.
Chemical structures of synthetic cannabinoids found in spice NPS and their relationship to the plant-derived compound THC.
Synthetic cannabinoids display complex pharmacokinetics and metabolism
In addition to the obvious risk of using compounds with unknown pharmacological properties, synthetic cannabinoids have an additional set of potential risks associated with exposure to pyrolytic byproducts of the compound or the plant material on which it is sprayed (because smoking is the normal route of administration) (Kavanagh et al., 2013), biotransformation to active metabolites, and shared metabolic pathways with commonly used medications, which may lead to drug–drug interactions. Despite the popular use of these substances, little is known about their pharmacokinetics or their in vivo pharmacology and toxicology. The few published pharmacokinetic studies have shown that, like phytocannabinoids, synthetic cannabinoids are highly lipophilic and the parent compounds readily cross the blood–brain-barrier and are distributed to areas high in CB1 receptor density (Dhawan et al., 2006; Wiebelhaus et al., 2012). Whereas THC has only one major psychoactive Phase I metabolite (11-hydroxy-THC) (Huestis et al., 1992), metabolism of synthetic cannabinoids can proceed via several pathways, resulting in multiple metabolites. Earlier studies reported that several Phase I hydroxylated metabolites of AM2201 (Chimalakonda et al., 2012), JWH-018 (Brents et al., 2011), and JWH-073 (Brents et al., 2012) retain high affinity for CB1 receptors, often higher than that of THC. In addition, these metabolites exhibit a range of intrinsic efficacies at the CB1 receptor, from neutral antagonists, to partial agonists, to full agonists. Importantly, in vivo studies demonstrate that these metabolites retain biological effects consistent with their in vitro profiles, in some cases suggesting that they continue to penetrate the brain, or are perhaps generated within the brain. In vitro studies using human recombinant P450 enzymes identified CYP2C9 and CYP1A2 as major isoforms responsible for the generation of these hydroxylated metabolites, which can be detected in human blood and urine as glucuronic acid conjugates (Chimalakonda et al., 2011; Patton et al., 2013). Mechanistic studies have shown that selective inhibitors of CYP2C9 (sulfaphenazole) and CYP1A2 (α-naphthoflavone) block oxidation of JWH-018 and AM2201 in human liver microsomes (Chimalakonda et al., 2013), further demonstrating the importance of these P450 isoforms in the detoxification of these compounds. The in vitro evidence suggests that individuals with certain allelic variants for these enzymes might be more likely to experience increased toxicity following the use of synthetic cannabinoids. Urinary elimination of synthetic cannabinoids or their metabolites may contribute to the kidney toxicity that has been observed with some of these compounds (Sobolevsky et al., 2010; Moran et al., 2011; Centers for Disease and Prevention, 2013).
Given their shared metabolism via P450 isoforms, combined use of synthetic cannabinoids and various prescription medications may result in adverse drug–drug reactions. Commonly prescribed drugs, such as valproic acid and sertraline potently inhibit CYP2C9, whereas drugs, such as ciprofloxacin and fluvoxamine, strongly inhibit CYP1A2. Additionally, CYP2C9 is a major polymorphic enzyme (Paine et al., 2006) and is responsible for the metabolism of a number of clinically important drugs, such as warfarin, phenytoin, tolbutamide, losartan, and ibuprofen. More than five allelic variants have been identified, including two “loss of function” variants (CYP2C9*4 and CYP2C9*5) (Seng and Seng, 2008). Similarly, CYP1A2 is responsible for the metabolism of numerous psychiatric medications, including olanzapine, clozapine, haloperidol, thioridazine, imipramine, clomipramine, fluvoxamine, and tacrine (Shirley et al., 2003) but is well conserved without common functional polymorphisms (Hiratsuka, 2012). Hence, the possibility of drug–drug interactions is a serious consideration using synthetic cannabinoids.
Synthetic cannabinoids produce THC-like stimulus effects in animal models
To the extent that synthetic cannabinoids have been evaluated in vivo, they share the capacity of THC to produce a tetrad of diagnostic effects: locomotor suppression, antinociception, hypothermia, and catalepsy (Wiley et al., 2014a). Synthetic cannabinoids also engender THC-like discriminative stimulus effects in rodents and nonhuman primates (Wiley et al., 1995; Wiley et al., 1998). Unlike the situation with synthetic cathinones, a robust model of intravenous THC self-administration in rodents has not been established, although self-administration has been reported in squirrel monkeys (Tanda et al., 2000; Justinova et al., 2003). WIN55212-2 self-administration has been reported in rodents (Fattore et al., 2001; Deiana et al., 2007); however, its usefulness as a screening tool for identification of synthetic cannabinoids likely to be abused has been disputed (Lefever et al., 2014). Consequently, THC discrimination in animal models remains the most pharmacologically selective method for assessing the likelihood that novel compounds will produce marijuana-like subjective effects in humans (Balster and Prescott, 1992). Animals used in drug discrimination learn to associate the interoceptive cues produced by THC or vehicle with one of two responses. For example, to receive food reinforcement, the animal must press a lever on the right side of the test chamber if an injection of THC was given before the session. If a THC injection was not given (e.g., vehicle injection), the animal must respond on the left lever to receive food. After the discrimination is established, other compounds are administered to determine whether they produce interoceptive cues similar to those produced by THC. Early research found that a number of naphthoylindoles, naphthylmethylindoles, and naphthoylpyrroles produce cannabinoid effects in rats or rhesus monkeys trained to discriminate THC or CP55940 from vehicle (Wiley et al., 1995, 1998). Later studies confirmed these results with other naphthoylindoles (JWH-018, JWH-073, and AM5983) (Järbe et al., 2010, 2011, 2014; Ginsburg et al., 2012; Marusich et al., 2013) and extended them to other classes of indole-derived cannabinoids, including phenylacetylindoles (Vann et al., 2009; Järbe et al., 2011) and tetramethylcyclopropyl ketone indoles (UR-144, XLR-11) (Wiley et al., 2013). In studies where more than one compound has been evaluated, the rank order of drug potency parallels CB1 affinity, with compounds showing the highest affinity for CB1 also showing the highest potency in drug discrimination. Reversal by the CB1 antagonist rimonabant provides further verification that CB1 receptors mediate the THC-like discriminative stimulus effects of synthetic cannabinoids (Järbe et al., 2011; Ginsburg et al., 2012). In the context of a THC discrimination procedure, cross-tolerance of THC and synthetic cannabinoids (CP55940, JWH-018, and JWH-073) has also been demonstrated in rhesus monkeys chronically treated with THC (Hruba et al., 2012). More recently, cross-substitution of THC and synthetic cannabinoids has been reported in separate groups of rodents trained to discriminate THC or JWH-018 from vehicle (Wiley et al., 2014b). To the limited extent to which they have been assessed, sex differences in the discriminative stimulus effects of synthetic cannabinoids are not apparent (J.L.W. and J.A.M., unpublished data). Finally, the duration of action of some synthetic cannabinoids in drug discrimination has been reported to be shorter than that of THC (Ginsburg et al., 2012), suggesting that the synthetic drugs may be administered more frequently with resultant enhancement of abuse potential.
Conclusions
In conclusion, this brief review has summarized the latest scientific data on the neurobiology of synthetic stimulants and cannabinoids. The available information suggests that most of these NPS resemble their progenitors (i.e., stimulants and THC) in their basic pharmacology, but these substances may have unexpected toxicological effects related to factors, such as dosage, efficacy, or active metabolites, which remain largely unexplored because most mechanistic research has focused on the stimulant actions of cathinones and cannabimimetic effects of synthetic cannabinoids. Potential “off-target” sites of action for most NPS are not known. This paucity of scientific knowledge is in direct contrast to the widespread availability of peer-to-peer information and access via the Internet and other electronic media, particularly for adolescents (Wax, 2002; Castellanos et al., 2011). Despite an alarming increase in the number of people seeking medical attention following use of NPS (Spiller et al., 2011; Centers for Disease and Prevention, 2011; Harris and Brown, 2013; Helander et al., 2014), the actual incidence of use is probably still underestimated because tracking continues to be difficult, partially due to inadequacy of detecting most substances. Meanwhile, the number of new “replacement” cathinones and cannabinoids continues to grow. The challenge for scientists, clinicians, and policymakers is to discover creative and effective ways to maximize their efforts in responding to this rapidly changing drug landscape.
Footnotes
The work was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
The authors declare no competing financial interests.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013a;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013b;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anizan S, Ellefsen K, Concheiro M, Suzuki M, Rice KC, Baumann MH, Huestis MA. 3,4-Methylenedioxypyrovalerone (MDPV) and metabolites quantification in human and rat plasma by liquid chromatography-high resolution mass spectrometry. Anal Chim Acta. 2014;827:54–63. doi: 10.1016/j.aca.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47,497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–140. doi: 10.1016/S0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/S0149-7634(05)80051-X. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (+ or −)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013b;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry EM, Mechoulam R. Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol Ther. 2002;95:185–190. doi: 10.1016/S0163-7258(02)00257-7. [DOI] [PubMed] [Google Scholar]
- Brandt SD, King LA, Evans-Brown M. The new drug phenomenon. Drug Test Anal. 2014;6:587–597. doi: 10.1002/dta.1686. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. doi: 10.1124/mol.60.1.155. [DOI] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013a;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013b;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos D, Singh S, Thornton G, Avila M, Moreno A. Synthetic cannabinoid use: a case series of adolescents. J Adolesc Health. 2011;49:347–349. doi: 10.1016/j.jadohealth.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Cawrse BM, Levine B, Jufer RA, Fowler DR, Vorce SP, Dickson AJ, Holler JM. Distribution of methylone in four postmortem cases. J Anal Toxicol. 2012;36:434–439. doi: 10.1093/jat/bks046. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”: Michigan, November 13, 2010–March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:624–627. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use: multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93–98. [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011;83:6381–6388. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, James LP, Radominska-Pandya A, Moran JH. Sulfaphenazole and alpha-naphthoflavone attenuate the metabolism of the synthetic cannabinoids JWH-018 and AM2201 found in K2/spice. Drug Metab Lett. 2013;7:34–38. doi: 10.2174/187231280701131211151523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. Some new psychoactive substances: precursor chemicals and synthesis-driven end-products. Drug Test Anal. 2011;3:404–416. doi: 10.1002/dta.315. [DOI] [PubMed] [Google Scholar]
- Concheiro M, Baumann MH, Scheidweiler KB, Rothman RB, Marrone GF, Huestis MA. Nonlinear pharmacokinetics of (+/−)3,4-methylenedioxymethamphetamine (MDMA) and its pharmacodynamic consequences in the rat. Drug Metab Dispos. 2014;42:119–125. doi: 10.1124/dmd.113.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Wood DM, Smith G, Button J, Ramsey J, Archer R, Holt DW, Dargan PI. Purchasing ‘legal highs’ on the Internet: is there consistency in what you get? QJM. 2010;103:489–493. doi: 10.1093/qjmed/hcq056. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97:20–26. doi: 10.1016/j.lfs.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Fattore L, Spano MS, Cossu G, Porcu E, Fadda P, Fratta W. Strain and schedule-dependent differences in the acquisition, maintenance and extinction of intravenous cannabinoid self-administration in rats. Neuropharmacology. 2007;52:646–654. doi: 10.1016/j.neuropharm.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Deng H, Gatley SJ, Makriyannis A, Akinfeleye T, Bruneus M, Dimaio AA, Gifford AN. Evaluation of the in vivo receptor occupancy for the behavioral effects of cannabinoids using a radiolabeled cannabinoid receptor agonist, R-[125/131I]AM2233. Synapse. 2006;60:93–101. doi: 10.1002/syn.20277. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration Department of Justice. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Federal Register. 2011;76:65371–65375. [PubMed] [Google Scholar]
- Drug Enforcement Administration Department of Justice. Establishment of drug codes for 26 substances. Final rule. Federal Register. 2013;78:664–666. [PubMed] [Google Scholar]
- Eissenstat MA, Bell MR, D'Ambra TE, Alexander EJ, Daum SJ, Ackerman JH, Gruett MD, Kumar V, Estep KG, Olefirowicz EM. Aminoalkylindoles: structure–activity relationships of novel cannabinoid mimetics. J Med Chem. 1995;38:3094–3105. doi: 10.1021/jm00016a013. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘Spice’. 2010. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids.
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Jones SR. Presynaptic dopamine modulation by stimulant self-administration. Front Biosci. 2013;5:261–276. doi: 10.2741/s371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murai N, Mathúna BO, Pizarro N, de la Torre R. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine and its enantiomers in mice: pharmacokinetic considerations. J Pharmacol Exp Ther. 2009;329:1006–1015. doi: 10.1124/jpet.109.150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Delta(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN55212-2 in rats. Psychopharmacology. 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: δ(9)-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/S0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Harris CR, Brown A. Synthetic cannabinoid intoxication: a case series and review. J Emerg Med. 2013;44:360–366. doi: 10.1016/j.jemermed.2012.07.061. [DOI] [PubMed] [Google Scholar]
- Helander A, Backberg M, Hulten P, Al-Saffar Y, Beck O. Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci Int. 2014;243:C23–C29. doi: 10.1016/j.forsciint.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Heydari A, Yeo KR, Lennard MS, Ellis SW, Tucker GT, Rostami-Hodjegan A. Mechanism-based inactivation of CYP2D6 by methylenedioxymethamphetamine. Drug Metab Dispos. 2004;32:1213–1217. doi: 10.1124/dmd.104.001180. [DOI] [PubMed] [Google Scholar]
- Hiratsuka M. In vitro assessment of the allelic variants of cytochrome P450. Drug Metab Pharmacokinet. 2012;27:68–84. doi: 10.2133/dmpk.DMPK-11-RV-090. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ(9)-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342:843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids: I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Huffman JW. The search for selective ligands for the CB2 receptor. Curr Pharm Design. 2000;6:1323–1337. doi: 10.2174/1381612003399347. [DOI] [PubMed] [Google Scholar]
- Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–649. doi: 10.2174/1389557054368844. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–1411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Dai D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–566. doi: 10.1016/S0960-894X(01)80155-4. [DOI] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR. Structure–activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus functions of methanandamide and Δ(9)-THC in rats: tests with aminoalkylindoles (WIN55212-2 and AM678) and ethanol. Psychopharmacology. 2010;208:87–98. doi: 10.1007/s00213-009-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Δ(9)-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, LeMay BJ, Halikhedkar A, Wood J, Vadivel SK, Zvonok A, Makriyannis A. Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats. Psychopharmacology. 2014;231:489–500. doi: 10.1007/s00213-013-3257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Johnson RL, Portier RB. Current “legal highs.”. J Emerg Med. 2013;44:1108–1115. doi: 10.1016/j.jemermed.2012.09.147. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Kalix P, Glennon RA. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem pharmacol. 1986;35:3015–3019. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]
- Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica. 2006;36:709–723. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- Kavanagh P, Grigoryev A, Savchuk S, Mikhura I, Formanovsky A. UR-144 in products sold via the Internet: identification of related compounds and characterization of pyrolysis products. Drug Test Anal. 2013;5:683–692. doi: 10.1002/dta.1456. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J Forensic Sci. 2013;58:1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- Kolanos R, Solis E, Jr, Sakloth F, De Felice LJ, Glennon RA. “Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci. 2013;4:1524–1529. doi: 10.1021/cn4001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monitor. 2008;30:320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ, Strømgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kronstrand R, Roman M, Andersson M, Eklund A. Toxicological findings of synthetic cannabinoids in recreational users. J Anal Toxicol. 2013;37:534–541. doi: 10.1093/jat/bkt068. [DOI] [PubMed] [Google Scholar]
- Lainton JA, Huffman JW, Martin BR, Compton DR. 1-Alkyl-3-(1-naphthoyl)pyrroles: a new cannabinoid class. Tetrahedron Lett. 1995;36:1401–1404. doi: 10.1016/0040-4039(95)00016-6. [DOI] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL. Evaluation of WIN55212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol Biochem Behav. 2014;118:30–35. doi: 10.1016/j.pbb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin AH, Seltzman HH, Carroll FI, Mascarella SW, Reddy PA. Emergence and properties of spice and bath salts: a medicinal chemistry perspective. Life Sci. 2014;97:9–19. doi: 10.1016/j.lfs.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Carbo M, Pubill D, Escubedo E, Camarasa J. An integrated pharmacokinetic and pharmacodynamic study of a new drug of abuse, methylone, a synthetic cathinone sold as “bath salts.”. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:64–72. doi: 10.1016/j.pnpbp.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Manera C, Tuccinardi T, Martinelli A. Indoles and related compounds as cannabinoid ligands. Mini Rev Med Chem. 2008;8:370–387. doi: 10.2174/138955708783955935. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur J Neuropsychopharmacol. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, López-Arnau R, Carbó M, Pubill D, Camarasa J, Escubedo E. Mephedrone pharmacokinetics after intravenous and oral administration in rats: relation to pharmacodynamics. Psychopharmacology. 2013;229:295–306. doi: 10.1007/s00213-013-3108-7. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Novak SP, Blough BE, Wiley JL. Prediction and prevention of prescription drug abuse: role of preclinical assessment of substance abuse liability. Methods Rep RTI Press. 2013:1–14. doi: 10.3768/rtipress.2013.op.0014.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.016. doi: 10.1016/j.neuropharm.2014.02.016. Advance online publication. Retrieved Mar. 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Sci STKE. 2002;2002:re5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. J Mass Spectrom. 2010;45:1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, Hanoune J. Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun. 2002;292:231–235. doi: 10.1006/bbrc.2002.6635. [DOI] [PubMed] [Google Scholar]
- Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, Kornilov AM, Bratton LD, Dobrowolski PJ, Wessinger WD, Fantegrossi WE, Prather PL, James LP, Radominska-Pandya A, Moran JH. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013;27:823–836. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Bryant SM, Aks SE. Emerging drugs of abuse. Emerg Med Clin North Am. 2014;32:1–28. doi: 10.1016/j.emc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie.”. Drug Metab Dispos. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton AL, Seely KA, Chimalakonda KC, Tran JP, Trass M, Miranda A, Fantegrossi WE, Kennedy PD, Dobrowolski P, Radominska-Pandya A, McCain KR, James LP, Endres GW, Moran JH. Targeted metabolomic approach for assessing human synthetic cannabinoid exposure and pharmacology. Anal Chem. 2013;85:9390–9399. doi: 10.1021/ac4024704. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, 3rd, Garg U, Pietak BR. Three fatal intoxications due to methylone. J Anal Toxicol. 2012;36:444–451. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Petitet F, Marin L, Doble A. Biochemical and pharmacological characterization of cannabinoid binding sites using [3H]SR141716A. Neuroreport. 1996;7:789–792. doi: 10.1097/00001756-199602290-00026. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow, and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8:15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. J Med. 2012;125:854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann N Y Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Glennon RA. Cathinone, cocaine and methamphetamine: similarity of behavioral effects. Pharmacol Biochem Behav. 1985;22:913–916. doi: 10.1016/0091-3057(85)90295-3. [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233:416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Seng KC, Seng CK. The success of the genome-wide association approach: a brief story of a long struggle. Eur J Hum Genet. 2008;16:554–564. doi: 10.1038/ejhg.2008.12. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J Anal Toxicol. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- Shirley KL, Hon YY, Penzak SR, Lam YW, Spratlin V, Jann MW. Correlation of cytochrome P450 (CYP) 1A2 activity using caffeine phenotyping and olanzapine disposition in healthy volunteers. Neuropsychopharmacology. 2003;28:961–966. doi: 10.1038/sj.npp.1300123. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters: why amphetamines take two to tango. J Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZH, Bonner TI. A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Mol Pharmacol. 1996;49:891–896. [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Strano-Rossi S, Cadwallader AB, de la Torre X, Botrè F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2706–2714. doi: 10.1002/rcm.4692. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of Δ9-tetrahydrocannabinol (THC) in C57BL/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Huang SM. Endocannabinoids in pain modulation. Prostaglandins Leukot Essent Fatty Acids. 2002;66:235–242. doi: 10.1054/plef.2001.0361. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts.”. J Addict Res Ther Suppl. 2012;9 doi: 10.4172/2155-6105.S9-002. pii.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Watterson E, Olive MF. Abuse liability of novel ‘legal high’ designer stimulants: evidence from animal models. Behav Pharmacol. 2013;24:341–355. doi: 10.1097/FBP.0b013e3283641ec8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics. 2002;109:e96. doi: 10.1542/peds.109.6.e96. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126:316–323. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure–activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW, Balster RL, Thomas BF. Hijacking of basic research: the case of synthetic cannabinoids. Methods Rep RTI Press. 2011;2011 doi: 10.3768/rtipress.2011.op.0007.1111. pi.17971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012a;123:148–153. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Selley DE, Wang P, Kottani R, Gadthula S, Mahadeven A. 3-Substituted pyrazole analogs of the cannabinoid type 1 (CB(1)) receptor antagonist rimonabant: cannabinoid agonist-like effects in mice via non-CB(1), non-CB(2) mechanism. J Pharmacol Exp Ther. 2012b;340:433–444. doi: 10.1124/jpet.111.187815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014a;97:55–63. doi: 10.1016/j.lfs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA. Cross-substitution of Δ-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav. 2014b;124:C123–C128. doi: 10.1016/j.pbb.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts.”. J Anal Toxicol. 2013;37:182–185. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Wojcieszak J. Designer cathinones: an emerging class of novel recreational drugs. Forensic Sci Int. 2013;231:42–53. doi: 10.1016/j.forsciint.2013.04.015. [DOI] [PubMed] [Google Scholar]