Abstract
The paraventricular nucleus of the hypothalamus (PVH) contains a heterogeneous cluster of Sim1-expressing cell types that comprise a major autonomic output nucleus and play critical roles in the control of food intake and energy homeostasis. The roles of specific PVH neuronal subtypes in energy balance have yet to be defined, however. The PVH contains nitric oxide synthase-1 (Nos1)-expressing (Nos1PVH) neurons of unknown function; these represent a subset of the larger population of Sim1-expressing PVH (Sim1PVH) neurons. To determine the role of Nos1PVH neurons in energy balance, we used Cre-dependent viral vectors to both map their efferent projections and test their functional output in mice. Here we show that Nos1PVH neurons project to hindbrain and spinal cord regions important for food intake and energy expenditure control. Moreover, pharmacogenetic activation of Nos1PVH neurons suppresses feeding to a similar extent as Sim1PVH neurons, and increases energy expenditure and activity. Furthermore, we found that oxytocin-expressing PVH neurons (OXTPVH) are a subset of Nos1PVH neurons. OXTPVH cells project to preganglionic, sympathetic neurons in the thoracic spinal cord and increase energy expenditure upon activation, though not to the same extent as Nos1PVH neurons; their activation fails to alter feeding, however. Thus, Nos1PVH neurons promote negative energy balance through changes in feeding and energy expenditure, whereas OXTPVH neurons regulate energy expenditure alone, suggesting a crucial role for non-OXT Nos1PVH neurons in feeding regulation.
Keywords: body weight, energy balance, hypothalamus, oxygen consumption, oxytocin, satiety
Introduction
The paraventricular nucleus of the hypothalamus (PVH) is the major autonomic output area of the hypothalamus and is critical for energy homeostasis. Loss of one copy of single-minded 1 (Sim1), a key transcription factor regulating PVH development, disrupts PVH maturation and function, resulting in hyperphagic obesity with associated glucose dysregulation in mammals (Michaud et al., 1998; Holder et al., 2000). Similar metabolic derangements also result from electrolytic destruction of the PVH (Gold, 1973; Sims and Lorden, 1986). In addition, the PVH serves as an important regulatory center for peptidergic signals and physiologic parameters known to modulate food intake, including leptin, melanocortins, and dehydration (Cowley et al., 1999; McKinley and Johnson, 2004). Indeed, the melanocortin pathway is essential for energy balance in mammals and is directly linked to PVH function. Leptin-responsive neurons in the arcuate nucleus project to the PVH, a site of dense melanocortin receptor expression, and release melanocortin agonists and antagonists/inverse agonists to modulate PVH function (Cowley et al., 1999; Kishi et al., 2003). Consistent with its role in feeding, the PVH sends dense projections to hindbrain regions such as the nucleus of the solitary tract (NTS) and parabrachial nucleus (PBN) to modulate feeding behaviors (Sawchenko and Swanson, 1982; Fulwiler and Saper, 1985; Blevins et al., 2004). Although PVH functions are understood in broad terms, the specific cell types within this heterogeneous structure that regulate feeding are not fully defined.
In addition to modulating feeding, a variety of data suggest that PVH neurons control energy expenditure. For example, infusion of the melanocortin receptor agonist MTII into the PVH increases energy expenditure (Cowley et al., 1999), while ablation of Sim1 neurons throughout the CNS decreases the metabolic rate (Xi et al., 2012). Furthermore, polysynaptic retrograde tracing links thermogenic brown adipose tissue (BAT) to the PVH through the sympathetic nervous system via cholinergic [choline acetyltransferase (ChAT)] preganglionic neurons in the intermediolateral column of the thoracic spinal cord (ChATIML; Bamshad et al., 1999). Similar to the PVH cells that control feeding, the neurochemical identity of the PVH neurons regulating energy expenditure via the sympathetic nervous system (SNS) has yet to be established.
Given the role of the PVH in energy balance, we sought to identify subsets of Sim1-expressing PVH (Sim1PVH) neurons that contribute to energy homeostasis and reveal their roles in food intake or energy expenditure. We discovered that PVH containing nitric oxide synthase-1 (Nos1)-expressing (Nos1PVH) neurons are a subset of Sim1PVH neurons and send dense projections to hindbrain regions important for feeding control and to the upper thoracic spinal cord that regulates sympathetic output. Moreover, Nos1PVH neuron activation regulates both feeding and energy expenditure, indicating the critical importance of Nos1PVH neurons in PVH-regulated energy balance. In addition, oxytocin-expressing PVH neurons (OXTPVH) neurons represent a subset of Nos1PVH neurons that project to and modulate ChATIML neurons, yet make only a small contribution to energy balance. These studies demonstrate discrete roles for Nos1PVH and OXTPVH neurons in energy balance, and position these neurons anatomically and functionally in the neural circuitry of energy balance.
Materials and Methods
Experimental animals.
Oxytocin-ires-Cre (OXT-iCre), Nos1-ires-Cre (Nos1-iCre), and Sim1-Cre mice were generated as described previously (Balthasar et al., 2005; Leshan et al., 2012; Wu et al., 2012). Adult male mice (8–12 weeks old) were used for all studies. OXT-iCre, Nos1-iCre, or Sim1-iCre mice were bred to a Cre-dependent GFP reporter line (Bergner et al., 2014) to fluorescently label Cre-expressing PVH subpopulations. All animals were bred and housed within our colony according to guidelines approved by the University of Michigan Committee on the Care and Use of Animals. Unless otherwise noted, mice were provided ad libitum access to food and water.
Stereotaxic injections.
OXT-iCre, Nos1-iCre, Sim1-Cre, and nontransgenic [wild-type (WT)] mice were given presurgical analgesia and anesthetized with isoflurane. Mice were placed in a digital stereotaxic frame (Model 1900, Kopf Instruments), and the skull was exposed. Intracranial injection coordinates were determined from bregma using the stereotaxic atlas of Paxinos and Franklin (2001). Viral injections were performed using a pressurized picospritzer system coupled to a pulled glass micropipette [coordinates from bregma: anteroposterior, −0.500; mediolateral, ±0.220; dorsoventral (D/V), −4.800]. For tract-tracing experiments, 50–150 nl of the adenoviral synatophysin–mCherry terminal tracer (Ad-iN/syn-mCherry; Opland et al., 2013) was unilaterally injected into Sim1-Cre, Nos1-iCre, or OXT-iCre mice. Additionally, stereotaxic injection of Red Retrobeads (RRs; Lumafluor) was performed in the NTS of Sim1-Cre and Nos1-iCre mice with a Cre-dependent GFP reporter (lox-GFP). Control mice also received unilateral injection of RRs to determine PVH–NTS connections using OXT peptide staining. For NTS injections, mice were anesthetized and placed in the digital stereotax. The fourth ventricle was identified and used as a geographic landmark to determine the site of injection. A glass micropipette was lowered into the site (D/V, −0.630) and ∼25 nl of RRs was injected. For functional analysis of PVH neurons, bilateral PVH injections of AAV-hM3Dq-mCherry (AAV-hM3Dq, purchased from the University of North Carolina Vector Core, Chapel Hill, NC) were performed in Sim1-Cre (50 nl/side), Nos1-iCre (50 nl/side), and OXT-iCre (75 nl/side) mice. To control for viral transduction, nontransgenic (WT) mice also received bilateral injections of AAV-hM3Dq (75 nl/side). Mice injected with the Ad-iN/syn-mCherry tracer were individually housed for 5 d following injection to allow for viral transduction and protein transport before perfusion, whereas mice injected with RRs were perfused after 7 d following injections. Mice injected with AAV-hM3Dq were allowed to recover for 7 d following surgery before further experiments were performed.
Food intake measurements.
Following recovery, Nos1-iCre, OXT-iCre, Sim1-Cre, and WT mice with bilateral PVH AAV-hM3Dq injections were given PBS (intraperitoneally) for 3 consecutive days to allow for injection acclimatization. Before assessment, mice were fasted during the light cycle (9:00 A.M. to 6:00 P.M.) and had ad libitum access to water. Mice were then injected with vehicle [10% (2-hydroxypropyl)-β-cyclodextrin; catalog #C0926, Sigma) at the onset of feeding (6:00 P.M.) and food intake was measured at 2, 4, and 16 h (overnight) postinjection. The following day, mice were injected with clozapine-N-oxide (CNO) at the onset of feeding (0.3 mg/kg in 10% β-cyclodextrin), and food intake was measured at 2, 4, and 16 h following injection.
Energy expenditure measurements.
Energy expenditure was measured using the Comprehensive Laboratory Monitoring System (CLAMS, Columbus Instruments) in the University of Michigan Small Animal Phenotyping Core to obtain multiparameter analysis, including open circuit calorimetry and activity via optical beam breaks. AAV-hM3Dq-injected mice were acclimatized to the sealed chambers for 2 d with free access to food and water. The experimentation room had 12 h dark/light cycles (6:00 P.M. to 6:00 A.M.), and the temperature was maintained at 20–23°C. On experimental days (Day 3 and Day 4), food was removed at 9:00 A.M., and vehicle (Day 3) or CNO (Day 4, 0.3 mg/kg) was injected at 11:00 A.M. Food was then replaced at the onset of the dark cycle (6:00 P.M.). Although measurements [oxygen consumption (VO2), carbon dioxide production, and spontaneous motor activity] were performed throughout the duration of the experiment, the data shown are the averaged VO2 or activity over the 4 h following injection of vehicle or CNO.
Intrascapular temperature measurements.
The University of Michigan Small Animal Phenotyping Core placed temperature transponders (IPTT-300 model with corresponding DAS-7007R reader, Bio Medic Data Systems) in the intrascapular subcutaneous tissue directly above brown adipose tissue under isoflurane anesthesia. Mice were allowed to recover for 14 d before testing. On the day of testing, food was removed from the cages at 9:00 A.M. Two hours later, mice with PVH-directed AAV-hM3Dq injections were injected with vehicle or CNO, and temperatures were recorded before injection and at 15, 30, 60, and 120 min following injection.
Perfusion and immunohistochemistry.
For tract-tracing experiments, mice were perfused 5 d (Ad-iN/syn-mCherry) or 7 d (RRs) after intracranial injection. At the end of the neuronal activation studies, mice with bilateral AAV-hM3Dq injections were treated with either vehicle or CNO and perfused 90 min later, as described previously (Münzberg et al., 2007). Briefly, mice were deeply anesthetized with an overdose of pentobarbital (150 mg/kg, i.p.) and transcardially perfused with sterile PBS followed by 10% neutral buffered formalin or 4% paraformaldehyde (for perfusions with spinal cord removal). Brains and spinal cords were removed, post-fixed, and dehydrated in 30% sucrose before sectioning into 30 μm slices on a freezing microtome (Leica). Coronal brain sections were collected in four representative sections, whereas longitudinal thoracic spinal cord sections were collected in three representative sections and stored at −20°C. For Fos immunohistochemistry (IHC), free-floating brain and spinal cord sections were pretreated with 30% H2O2 to remove endogenous peroxidase activity, and then were blocked with normal goat or donkey serum and incubated in primary antibody overnight (rabbit anti-cFos 1:10,000; PC38, Calbiochem). Detection of primary antibody was performed by the avidin-biotin/diaminobenzidine (DAB) method (Biotin-SP-conjugated Donkey Anti-Rabbit, 1:200, Jackson ImmunoResearch; ABC kit, Vector Laboratories; DAB reagents, Sigma). hM3Dq and ChAT were detected using primary antibodies against red fluorescent protein (RFP; rat 1:2000, Allele Biotechnology) and ChAT (spinal cords only; goat, 1:500; AB144P, Millipore), respectively, followed by secondary immunofluorescence detection with donkey anti-rat-Alexa Fluor 568 or donkey anti-goat-Alexa Fluor 488 (1:200, Invitrogen). For PVH colocalization experiments, IHC immunostaining was performed using primary antibodies for GFP (rabbit, 1:20,000, Invitrogen A6455), nNOS1 (sheep, 1:2500; Herbison et al., 1996; provided by Dr. Vincent Prevot, Inserm, Lille, France), or oxytocin (rabbit, 1:2500; catalog #T-4084, Peninsula Laboratories). For tract-tracing experiments, immunostaining was performed using primary antibodies for RFP, GFP, or oxytocin.
Immunoblotting of BAT UCP1 protein.
Sim1-Cre, OXT-iCre, and WT mice with bilateral PVH AAV-hM3Dq injections were given either vehicle or CNO and 90 min later mice were anesthetized with pentobarbital, and BAT was removed, frozen on dry ice, and stored at −80°C. Tissue was homogenized in protein lysis buffer (10% SDS, 1 m Tris, pH 6.8, 12.7 mm EDTA) with metal beads in a Bullet blender for 30 min. Samples were clarified by centrifugation, and protein concentration was quantified by BCA assay (catalog #23225, Thermo Scientific). Lysates were diluted to equal protein concentration in lysis buffer plus 1× NuPage SDS buffer (Invitrogen) with 2.5% 2-mercaptoethanol. Samples were boiled for 5 min and loaded on an SDS gradient polyacrylamide gel (Invitrogen), and separated by electrophoresis. Proteins were transferred to Immobilon PVDF membranes (Millipore), and Ponceau staining of membranes was used to confirm equal protein loading between lanes. Membranes were blocked in 5% milk for 1 h at room temperature and then incubated with primary antibodies (goat anti-UCP1, 1:2000, catalog #SC-6528, Santa Cruz Biotechnology; rat anti-α-tubulin, 1:1000, catalog #MA1-80017, Thermo Scientific) and appropriate HRP-conjugated secondary antibodies (1:5000 dilution in 5% milk, IgG peroxidase; GE Healthcare) in 5% BSA. Super Signal enhanced chemiluminescence (Pierce) was used for visualization by autoradiography, and bands were quantified by densitometry.
Statistical analysis.
Paired t tests, unpaired t tests, or repeated-measures two-way ANOVAs were calculated using GraphPad Prism. Significance was determined at p < 0.05.
Results
nNos1 (Nos1) expression defines a PVH subpopulation
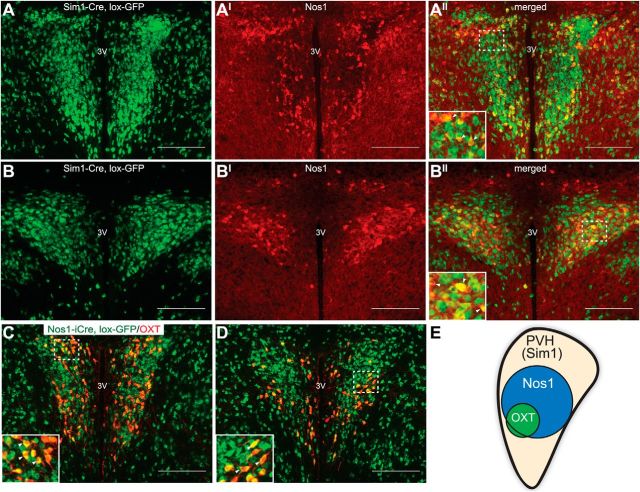
Nos1 in situ hybridization (Allen Brain Atlas) and Nos1-iCre, lox-GFP reporter mice demonstrate the existence of NOS1-containing neurons in the PVH. To determine whether NOS1 peptide marks a neuronal subset of the entire PVH, we stained brains from Sim1-Cre, lox-GFP reporter mice for GFP and NOS1 immunoreactivity (IR) and found that all Nos1PVH neurons express Sim1, but not all Sim1PVH neurons express NOS1 (Fig. 1A,B). PVH cell counts in Sim1-Cre, lox-GFP brain slices immunostained for Nos1 peptide (n = 5) revealed that Nos1PVH neurons account for ∼21% of the Sim1PVH field. To identify some potential Nos1PVH subtypes, we also investigated the overlap between Nos1PVH and OXTPVH neurons. Using Nos1-iCre, lox-GFP sections, we found that almost all OXTPVH neurons (∼90%, n = 3) contain GFP, whereas only 16% (n = 3) of Nos1-iCrePVH neurons contain OXT peptide. This confirms that OXTPVH neurons are a subset of Nos1PVH neurons (Fig. 1C,D). This establishes that Nos1PVH neurons represent a discrete subset of Sim1PVH neurons and that most OXTPVH neurons lie within the Nos1PVH field (Fig. 1E). Given the importance of Sim1PVH neurons in energy balance regulation and the limited information regarding the roles of specific PVH subtypes in this regulation, we investigated the potential contributions of Nos1PVH and OXTPVH neurons to the control of energy balance parameters.
Figure 1.
Neuronal Nos1 marks a subset of PVH neurons. A, B, IHC for NOS1 peptide (red) in the PVH of Sim1-Cre, lox-GFP reporter mice (lox-GFP, green) identifies Nos1PVH neurons as a Sim1PVH neuronal subset. C, D, OXTPVH neurons are contained within the Nos1PVH population (green), as shown by expression of OXT (red) in sections from Nos1-iCre, lox-GFP mice. E, A model of neurochemically defined cell types within the PVH. Dashed boxes indicate regions that are digitally enlarged and shown as insets. Arrowheads indicate representative overlapped cell-bodies. Scale bar, 200 μm. 3V, Third ventricle.
Nos1PVH neurons send dense projections to hindbrain regions important for satiety
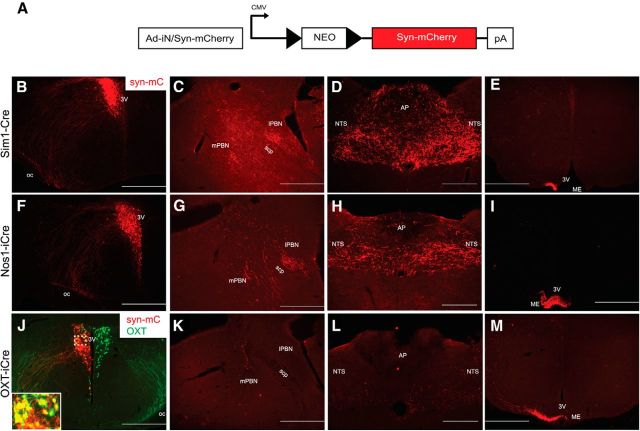
As hindbrain regions [e.g., NTS/dorsal motor nucleus of the vagus (DMV), PBN, and specifically PVH → NTS neuronal projections] have been implicated in feeding regulation, we characterized efferent projections from defined PVH subpopulations to the NTS/DMV and the PBN. We used Sim1PVH neurons as a reference group to establish projection targets for comparison with Nos1PVH and OXTPVH subsets. Sim1-Cre mice were unilaterally injected with a Cre-dependent Ad-iN/syn-mCherry tracer (Fig. 2A), which traffics predominantly to synaptic terminals and preferentially identifies projection terminals as opposed to axons of passage. We observed dense Sim1PVH neuron-derived mCherry-IR (Fig. 2B) in hindbrain regions important for satiety, including the medial PBN (mPBN) and lateral PBN (lPBN; Fig. 2C) and the NTS (Fig. 2D). Similar mCherry-IR was observed in these hindbrain regions from Nos1PVH neurons injected with Ad-iN/syn-mCherry (Fig. 2F–H). In contrast, we detected very little OXTPVH-derived syn-mCherry in the PBN (Fig. 2K) or NTS (Fig. 2L). Unilateral PVH injections of Ad-iN/syn-mCherry in OXT-iCre mice demonstrated syn-mCherry expression (Fig. 2J, red) only in OXTPVH neurons (Fig. 2J, green), confirming the fidelity of the Cre-dependent Ad-iN/syn-mCherry virus. As expected, Sim1PVH, Nos1PVH, and OXTPVH neurons also sent dense projections to the median eminence, reflecting the parvocellular PVH subpopulation projections that influence pituitary function and the magnocellular projections that release their contents directly from the posterior pituitary into the systemic circulation (Fig. 2E,I,M).
Figure 2.
Nos1PVH neurons project to hindbrain regions important for satiety. A, A Cre-dependent synaptophysin-mCherry viral vector allows for anterograde tracing of projections in the CNS. B–E, Unilateral PVH-specific injections of Ad-iN/syn-mCherry in Sim1-Cre mice (B) identify projections to the parabrachial nucleus (C), nucleus of the solitary tract (D), and the median eminence (ME; E). F–I, Stereotaxic injection of Ad-iN/syn-mCherry in the PVH of Nos1-iCre mice (F) demonstrates similar projections to the PBN (G), NTS (H), or ME (I). J–M, In contrast, injection of Ad-iN/syn-mCherry in the PVH of OXT-iCre mice (J) reveals few projections to either the PBN (K) or NTS (L), though projections to the ME are readily apparent (M). J, Sections were costained for OXT peptide (green) to show the fidelity of the Cre-dependent virus. Dashed boxes indicate regions that are digitally enlarged and shown as insets. Scale bars: D, H, L, 200 μm; all others, 500 μm. 3V, Third ventricle; scp, superior cerebellar peduncle; AP, area postrema; cc, central canal; ME, median eminence.
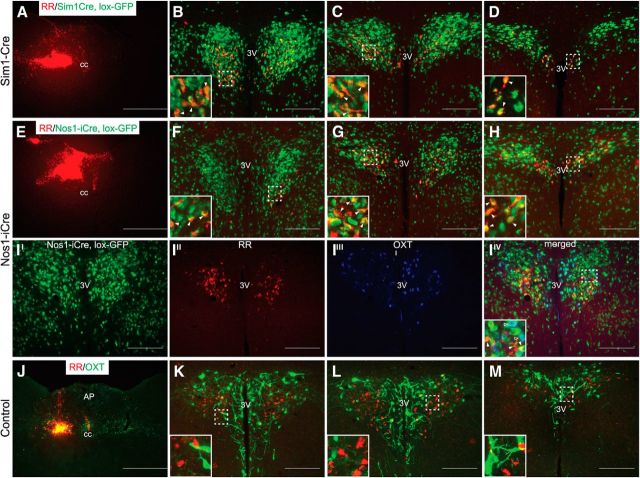
To better characterize the PVH neurons that project to the NTS/DMV and potentially affect feeding behaviors, we injected fluorescent latex microspheres (RRs, Lumafluor) unilaterally into the NTS of Sim1-Cre, lox-GFP, Nos1-iCre, lox-GFP, and wild-type mice (Fig. 3A,E,J, respectively). RRs are preferentially taken up by presynaptic terminals at the site of injection and undergo retrograde transportation back to the cell body, thus allowing neuron identification by autofluorescence. As might be expected, all RR-labeled PVH neurons from Sim1-Cre, loxGFP animals coexpress GFP, suggesting that all PVH → NTS projections originate from Sim1PVH neurons (Fig. 3B–D). Retrograde labeling from the NTS/DMV in Nos1-iCre, lox-GFP mice also demonstrates extensive (but not complete) overlap between NTS-projecting RR-labeled neurons and Nos1PVH neurons (Fig. 3F–H). Since previous reports suggest the presence of OXTPVH projections to the NTS/DMV and essentially all OXTPVH neurons are within the Nos1-iCrePVH field, we costained Nos1-iCre, lox-GFP mice with NTS RR injections for OXT peptide (Fig. 3II–IIV). As expected, OXT-IR is seen within Nos1PVH neurons (Fig. 3IIV, green, open arrowheads); however, NTS-injected RRs predominantly labeled Nos1PVH neurons that do not contain OXT (white arrowheads). In agreement with these findings, very few OXTPVH cell bodies were labeled with NTS-injected RRs (Fig. 3J–M) in separate control mice, even though OXT peptide (green) was detectable in the NTS/DMV at the site of the RR injection (Fig. 3J). This suggests that much of the OXT-IR in the NTS identifies fibers of passage, as opposed to synaptic terminals. Nevertheless, it is the non-OXT Nos1PVH neurons that comprise the bulk of the NTS-projecting Nos1PVH neurons.
Figure 3.
Retrograde labeling of PVH neurons from the NTS. A–H, RRs were injected in the hindbrains of in Sim1-Cre, lox-GFP (A) or Nos1-iCre, lox-GFP (E) mice to identify NTS projecting Sim1PVH (B–D) and Nos1PVH (F–H) neurons. I, Additional sections from Nos1-iCre, lox-GFP mice (II) with hindbrain RR injections (same injection shown in E) were stained for OXT peptide (IIII) and show that RR-labeled Nos1 neurons (III) do not contain OXT peptide (IIV, white arrowheads). OXT neurons only colocalize with Nos1, but not RR (IIV, open arrowheads). J–M, Furthermore, RR injected in the NTS of control mice (J) do not colocalize with OXT peptide (green) in the PVH (J–M). At the site of injection, beads appear in both green and red channels due to bead intensity (note yellow injection site). Immunohistochemistry identifies OXT peptide (green) expression near the injection site (J). Dashed boxes indicate regions that are digitally enlarged and shown as insets. Arrowheads indicate representative overlapped cell-bodies. Scale bars: A, E, J, 500 μm; all others, 200 μm. AP, Area postrema; cc, central canal; 3V, third ventricle.
Nos1PVH and OXTPVH neurons project to the spinal cord
Since sympathetic outflow promotes energy expenditure and the PVH is implicated in energy expenditure regulation, we also investigated whether Nos1PVH and OXTPVH neurons project to hindbrain and spinal cord regions important for SNS control (Cowley et al., 1999; Xi et al., 2012). The raphe pallidus (RPa) is an important hindbrain region controlling BAT function and energy expenditure (Bamshad et al., 1999; Morrison, 1999; Cano et al., 2003). We found few syn-mCherry terminals originating from Sim1PVH (Fig. 4A), Nos1PVH (Fig. 4B), or OXTPVH (Fig. 4C) neurons in the RPa. To determine whether Sim1PVH, Nos1PVH, and OXTPVH neurons project to the preganglionic, sympathetic output neurons of the thoracic spinal cord, we also examined longitudinal spinal cord sections from Sim1-Cre, Nos1-iCre, or OXT-iCre mice following unilateral PVH Ad-iN/syn-mCherry injections. We identified robust syn-mCherry tracer in thoracic spinal cord regions originating from Sim1PVH (Fig. 4D), Nos1PVH (Fig. 4E), and OXTPVH neurons (Fig. 4F). Syn-mCherry-IR is localized in close proximity to neurons expressing ChAT in the ChATIML, suggesting potential Sim1PVH, Nos1PVH, and OXTPVH neuronal connections with and regulation of IML preganglionic sympathetic neurons (Fig. 4D′,E′,F′).
Figure 4.
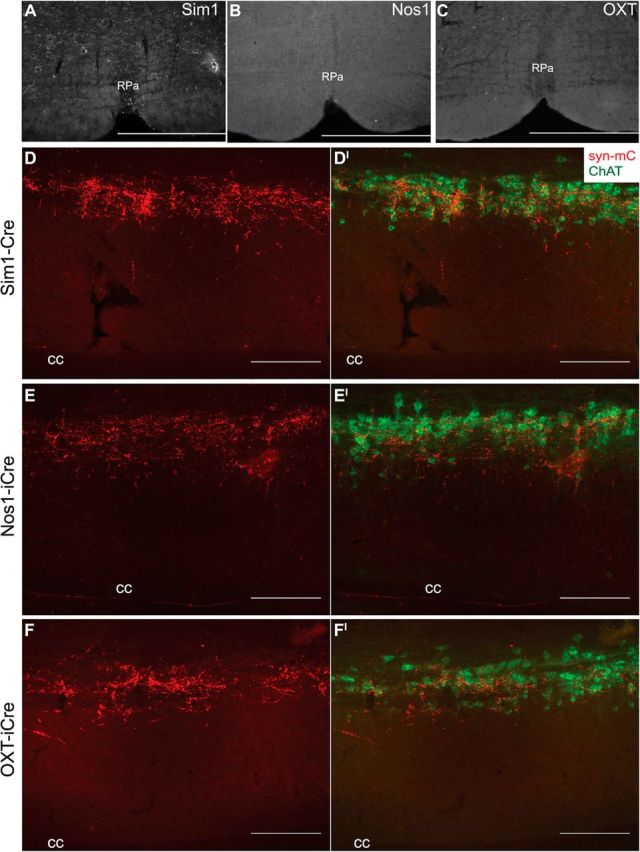
Nos1PVH and OXTPVH neurons project to preganglionic neurons in the spinal cord. A–C, Sim1-Cre (A), Nos1-iCre (B), or OXT-iCre (C) mice with unilateral Ad-iN/syn-mCherry injections (red) show few projections to the RPa. D–F, In contrast, Sim1PVH (D), Nos1PVH (E), and OXTPVH (F) all innervate thoracic spinal cord regions in close proximity to cholinergic neurons of the intermediolateral column expressing ChAT (green; D′, E′, F′). Scale bars: A–C, 500 μm; D–F, 200 μm. cc, Central canal.
Temporal control of PVH neuronal subpopulations
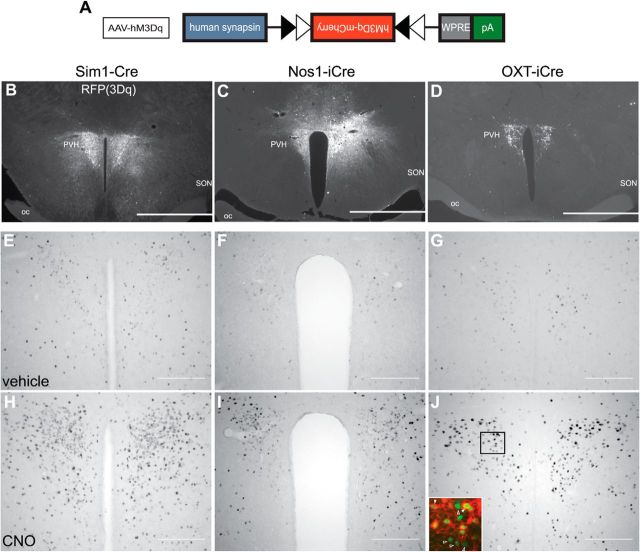
Having established the anterograde projection targets for Sim1PVH, Nos1PVH, and OXTPVH neurons, we tested the physiologic effects of acute activation of these PVH neurons on feeding and energy expenditure. To selectively activate these PVH subsets, we used Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) technology (Alexander et al., 2009). The hM3Dq DREADD is a modified human muscarinic receptor designed to couple with stimulatory Gq-proteins. Binding of an otherwise inert, synthetic ligand, CNO, activates neurons expressing hM3Dq. This system has been engineered to be Cre recombinase-dependent to achieve cell-specific control (Fig. 5A). Thus, site-specific injection of a Cre-dependent adeno-associated virus (AAV)-hM3Dq allows for remote and temporal activation only of neurons that express Cre recombinase. As for our tracing studies, we used Sim1-Cre mice to target the majority of PVH neurons and to establish an upper threshold of PVH “output capacity” upon DREADD activation followed by Nos1PVH and OXTPVH subset activation. As a control for DREADD injection, bilateral PVH injections of AAV-hM3Dq into WT mice were also performed.
Figure 5.
DREADDs allow for remote and temporal control of PVH neuronal activity. A, Diagram of the Cre-dependent hM3Dq DREADDs expression vector. B–D, Expression of AAV-hM3Dq-mCherry in the PVH of Sim1-Cre (B), Nos1-iCre (C), or OXT-iCre (D) mice is detected by IHC. E–G, Sim1-Cre (E), Nos1-iCre (F), or OXT-iCre (G) mice with bilateral AAV-hM3Dq injections demonstrate little nuclear Fos immunoreactivity after treatment with vehicle. H–J, In contrast, PVH neurons expressing hM3Dq are activated following injection of CNO. OXTPVH neuronal activation leads to nuclear Fos expression (J, inset, green) not only in hM3Dq-expressing OXTPVH neurons (J, inset, red, closed arrowheads), but also in neighboring non-OXTPVH neurons (open arrowheads). Scale bars: B–D, 1 mm; E–J, 200 μm. oc, Optic chiasm.
Although AAV-hM3Dq was primarily limited to the PVH of Sim1-Cre-injected mice, there was a small amount of AAV-hM3Dq expression in Sim1-Cre+ areas in the anterior hypothalamus (Fig. 5B). Similarly, while injections in Nos1-iCre mice were targeted for the PVH, some Nos1-iCre+ neurons in the thalamus also expressed hM3Dq (Fig. 5C). Importantly, any injected Nos1-iCre animals that expressed hM3Dq in peri-PVH areas implicated in feeding (i.e., dorsomedial hypothalamus) were excluded from the analysis. PVH injections of AAV-hM3Dq were restricted to OXTPVH neurons, as Cre expression in OXT-iCre mice is limited to the PVH and supraoptic nucleus (SON; Fig. 5D). Using nuclear Fos staining as an indicator of neuronal activation, vehicle injection caused little PVH activation in Sim1-Cre (Fig. 5E), Nos1-iCre (Fig. 5F), or OXT-iCre (Fig. 5G) mice with bilateral PVH AAV-hM3Dq injections. In contrast, hM3Dq-injected mice treated with CNO before perfusion demonstrated a marked increase in nuclear Fos staining in transduced PVH neurons (Fig. 5H–J). Specifically, CNO stimulated PVH nuclear Fos expression in Sim1-Cre+AAV-hM3Dq mice [176.5 ± 6.5 vs 51.0 ± 6.0 (vehicle); n = 2 each; unpaired t test, t(2) = 14.2, p = 0.005), Nos1-iCre+AAV-hM3Dq mice (200.7 ± 39.0 vs 36.0 ± 5.0 (vehicle); vehicle, n = 2; CNO, n = 3; unpaired t test, t(3) = 3.3, p = 0.047] and OXT-iCre+AAV-hM3Dq mice [130 ± 14 vs 63.0 ± 5.1 (vehicle); n = 4 each; unpaired t test, t(6) = 4.4, p = 0.005]. This demonstrates that cell-specific DREADD expression allows for temporal control of PVH neuron activity. Nuclear Fos was also apparent in non-Cre-expressing cells, suggesting that PVH subtypes can activate neighboring PVH cells via local connections (Fig. 5J, inset; Boudaba et al., 1996; Ziegler and Herman, 2000). We detected no hM3Dq expression or CNO-dependent activation in the PVH of wild-type control mice (data not shown).
Direct activation of PVH neurons alters food intake
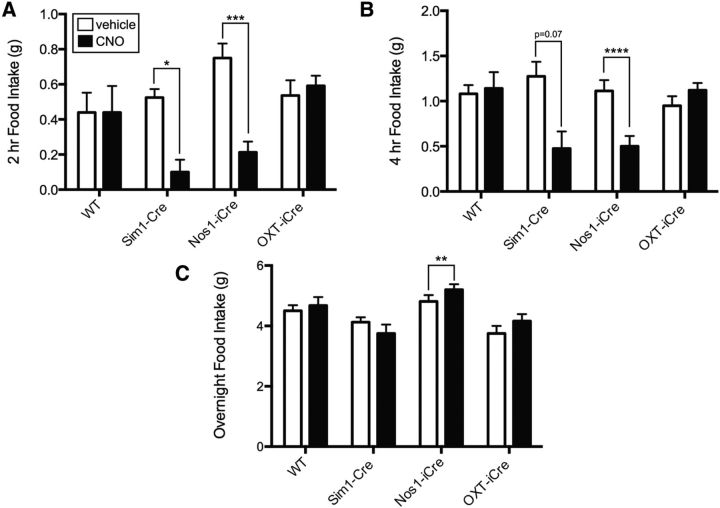
Based on our anterograde tracing of PVH subsets, we hypothesized that activation of Sim1PVH and Nos1PVH neurons would alter feeding (via projections to hindbrain regions). To test this hypothesis, we injected AAV-hM3Dq bilaterally into the PVH of Sim1-Cre, Nos1-iCre, and OXT-iCre mice, and treated these animals with CNO (0.3 mg/kg) at the onset of dark cycle feeding. Body weights (±SEM) of the animals used in these studies are as follows: Sim1-Cre, 22.6 ± 1.6 g (n = 4); Nos1-iCre, 25.6 ± 0.7 g (n = 8); OXT-iCre, 22.8 ± 0.8 g (n = 10); WT, 24.1 ± 0.8 g (n = 4). Wild-type controls with bilateral PVH AAV-hM3Dq injections showed no change in feeding behavior in response to CNO compared with vehicle injections, demonstrating that neither viral transduction nor CNO alone altered food intake (Fig. 6A, first panel). Activation of Sim1PVH neurons robustly suppressed feeding compared with vehicle control (Fig. 6A, second panel; paired t test, t(3) = 5.0, p = 0.015). As hypothesized, Nos1-iCre mice with bilateral AAV-hM3Dq injections also ate significantly less following CNO activation of Nos1PVH neurons compared with vehicle (Fig. 6A, third panel; paired t test, t(7) = 7.9, p < 0.0001). Interestingly, the 2 h suppression of food intake following activation of Sim1PVH and Nos1PVH neurons is comparable (t(10) = 1.0, p = 0.35, unpaired t test), suggesting that the anorexia associated with Sim1PVH activation is mediated largely via Nos1PVH neurons. The anorectic effect of Nos1PVH activation persists through 4 h of refeeding (paired t test, t(7) = 11.2, p < 0.0001; there is a similar trend for Sim1PVH neuron activation; Fig. 6B), although total overnight food intake (16 h) approximates that of controls (Fig. 6C). In contrast to Sim1PVH and Nos1PVH neurons, activation of OXTPVH neurons had little effect on 2 or 4 h food intake (Fig. 6A,B, fourth panel). Thus, Nos1PVH neuron activation suppresses feeding to a similar extent as Sim1PVH neurons, suggesting a major role for Nos1PVH neurons in the control of food intake. Furthermore, since activation of the OXT-containing subset of Nos1PVH neurons fails to blunt feeding, the activation of non-OXT Nos1PVH neurons must be required for this effect.
Figure 6.
Acute activation of Nos1PVH neurons suppresses feeding. WT+AAV-hM3Dq, Sim1-Cre+AAV-hM3Dq, Nos1-iCre, or OXT-iCre+AAV-hM3Dq mice were injected with vehicle (white bars) or CNO (black bars). A, Activation of Sim1PVH or Nos1PVH neurons decreases 2 h food intake whereas activation of OXTPVH neurons had no effect on food intake. B, Activation of Nos1PVH neurons also significantly decrease 4 h food intake, and Sim1PVH neuronal activation shows a similar trend, though this is not significant. C, Cumulative overnight food intake (16 h) is not altered by activation of PVH neuronal subsets. Average values ± SEM are shown. *p < 0.05, ***p < 0.01, ****p < 0.0001 compared with vehicle values (WT, n = 5; Sim1-Cre, n = 4; Nos1-iCre, n = 8; OXT-iCre, n = 10). Significance was determined using two-tailed paired t test.
Activation of PVH neurons increases Fos expression in sympathetic output neurons and promotes energy expenditure
The role of the PVH in modulating energy expenditure has received less attention than its contribution to feeding regulation. Given the robust spinal cord projections from Sim1PVH, Nos1PVH, and OXTPVH neurons, we determined the effect of DREADD-mediated activation of these PVH neural subsets on nuclear Fos expression in preganglionic, sympathetic output neurons and overall oxygen consumption. First, we treated Sim1-Cre+AAV-hM3Dq, Nos1-iCre+AAV-hM3Dq, or OXT-iCre+AAV-hM3Dq mice with vehicle (Fig. 7A,C,E) or CNO (Fig. 7B,D,F) and analyzed thoracic spinal cord sections for nuclear Fos accumulation in ChATIML neurons. Acute activation of Sim1PVH, Nos1PVH, and OXTPVH neurons all appeared to increase nuclear Fos expression in ChATIML neurons relative to vehicle control. To estimate this effect, we determined the percentage of ChATIML neurons containing nuclear Fos immunoreactivity in thoracic spinal cord sections from Sim1-Cre+AAV-hM3Dq mice in response to CNO versus vehicle. Activation of Sim1PVH neurons showed a trend toward increased nuclear Fos expression in ChATIML neurons with 42.6 ± 7.2% (CNO-treated) versus 16.1 ± 5.4% (vehicle-treated) ChATIML neurons counted (n = 2 each; unpaired t test, t(2) = 2.9, p = 0.099). These data thereby suggest a potential neuroanatomical pathway for the regulation of sympathetic output and energy expenditure.
Figure 7.
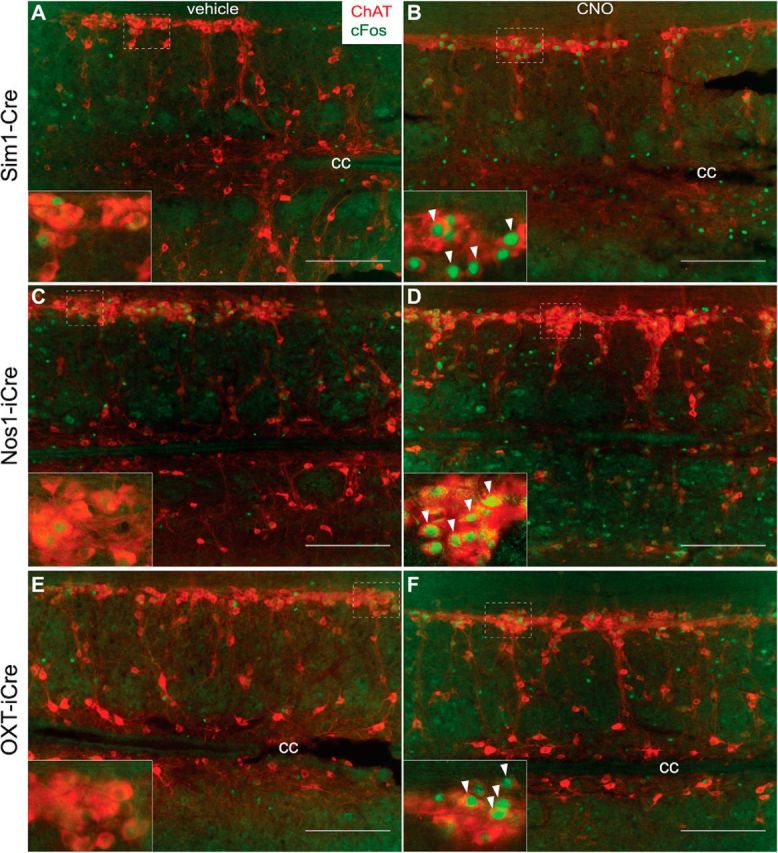
Nos1PVH and OXTPVH neurons can activate ChATIML neurons. A–F, While basal neuronal activity of ChATIML neurons is minimal in Sim1-Cre+AAV-hM3Dq (A), Nos1-iCre+AAV-hM3Dq (C), or OXT-iCre+AAV-hM3Dq (E) mice injected with vehicle, CNO-mediated activation of Sim1PVH (B), Nos1PVH (D), or OXTPVH (F) neurons increases nuclear Fos (green) in IML ChAT neurons (red). Scale bars, 200 μm. Dashed white boxes identify regions where 40× inset images were taken. Arrowheads indicate representative overlapped cell bodies and Fos-IR nuclei upon treatment with CNO. Scale bars, 200 μm. cc, Central canal.
To determine the ability of each PVH subset to modulate energy expenditure, we used metabolic cages to measure VO2 and locomotor activity in animals expressing hM3Dq in Sim1PVH, Nos1PVH, and OXTPVH neurons. Activation of Sim1PVH neurons and Nos1PVH neurons increased the average oxygen consumption in the absence of food [Fig. 8A,B, second and third panels; Sim1: paired t test; average 4 h VO2, t(3) = 8.8, p = 0.003; average 4 h VO2 lean body mass (LBM), t(3) = 11.0, p = 0.002; Nos1: average 4 h VO2, t(3) = 3.2, p = 0.05; average 4 h VO2 LBM, t(3) = 3.6 p = 0.038]. Acute activation of OXTPVH neurons also significantly increased oxygen consumption (Fig. 8A,B, fourth panel; average 4 h VO2, t(9) = 2.4, p = 0.042; average 4 h VO2 LBM, t(9) = 2.3, p = 0.05), albeit not to the extent seen with activation of Nos1PVH neurons (unpaired t test of average change in 4 h VO2, t(13) = 2.5, p = 0.029). Baseline oxygen consumption was elevated in the initial cohort of OXT-iCre+AAV-hM3Dq mice compared with other groups (one-way ANOVA of the average change in 4 h VO2, F(3,19) = 7.3, p = 0.002). To exclude the possibility that this elevated baseline O2 consumption was a property of the OXT-iCre transgenic line, as opposed to a cohort effect, oxygen consumption experiments were repeated in a second cohort of mice naive to CNO (Fig. 8C,D). Indeed, OXT-iCre+AAV-hM3Dq mice treated with vehicle had the same baseline oxygen consumption as WT and OXT-iCre mice without AAV-hM3Dq (one-way ANOVA of average change in 4 h VO2, F(2,9) = 1.0, p = 0.4), indicating that OXT-iCre mice are not metabolically compromised.
Figure 8.
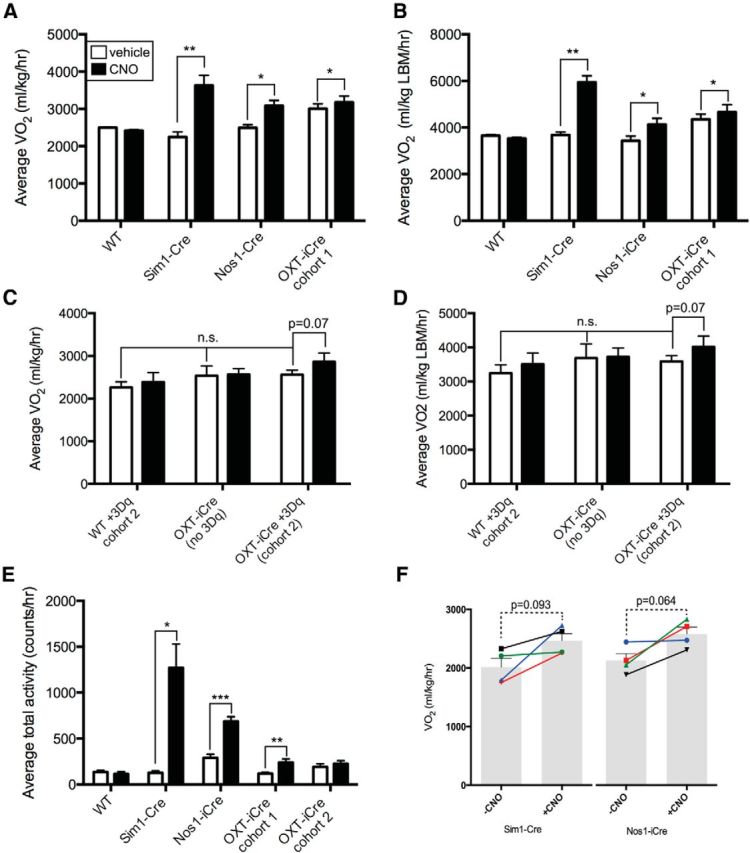
Acute activation of Nos1PVH neurons increases energy expenditure. WT+AAV-hM3Dq, Sim1-Cre+AAV-hM3Dq, and Nos1-iCre or OXT-iCre+AAV-hM3Dq mice were injected with vehicle (white bars) or CNO (black bars). A, B, E, Activation of Sim1PVH, Nos1PVH or OXTPVH neurons increases average oxygen consumption (A, B) and total activity (E) over 4 h following injection. C, D, A second cohort of CNO-naive mice also shows a trend toward increased 4 h average VO2 in response to activation of OXTPVH neurons, while baseline VO2 is unchanged compared with WT+3Dq or OXT-iCre mice without 3Dq injections (WT, n = 5; Sim1-Cre, n = 4; Nos1-iCre, n = 4; OXT-iCre cohort 1, n = 10; WT cohort 2, n = 4; OXT-iCre+3Dq cohort 2, n = 4; OXT-iCre, n = 4). F, To determine potential activity-independent changes in VO2, VO2 was determined in Sim1-Cre+3Dq and Nos1-iCre+3Dq mice before and after CNO treatment, at time points when locomotor activity was approximately matched at activity levels below a threshold value of 300 counts/h (bars indicate average values ± SEM, line segments indicate individual mice; Sim1, n = 4; Nos1, n = 4). Average values ± SEM are shown. *p < 0.05, **p < 0.01, ***p < 0.001 compared with vehicle values. Significance was determined using two-tailed paired t test within groups or unpaired t test between groups.
Interestingly, activation of any of the PVH subpopulations increased average total activity, although not equally (Fig. 8E; paired t test; Sim1+3Dq, t(3) = 4.7, p = 0.019; Nos1+3Dq, t(3) = 26.8, p = 0.0001; OXT+3Dq, t(9) = 4.3, p = 0.002). As expected, WT+AAV-hM3Dq mice showed no change in oxygen consumption or activity in response to CNO (Fig. 8A–E). Body weights (±SEM) of the animals used in these studies are as follows: Sim1-Cre, 30.5 ± 2.1 g (n = 4); Nos1-iCre, 29.4 ± 0.9 g (n = 4); OXT-iCre+3Dq cohort 1, 23.9 ± 0.6 g (n = 10); WT cohort 1, 26.3 ± 1.3 g (n = 4); OXT-iCre+3Dq cohort 2, 28.9 ± 1.3 g (n = 4); OXT-iCre (no 3Dq), 28.1 ± 1.8 g (n = 4); and WT cohort 2, 30.4 ± 1.6 g (n = 4). Therefore, Nos1PVH or OXTPVH neuron activation promotes locomotor activity and overall energy expenditure, although to a lesser extent than that seen with pan-PVH activation. Unlike the feeding effects observed with Nos1PVH activation, Nos1PVH-driven increases in VO2 and activity were significantly smaller than those seen with Sim1PVH activation (unpaired t test of average 4 h VO2, F(3,3) = 1.062, p = 0.009; unpaired t test of average 4 h activity, F(3,3) = 274.3, p = 0.023), suggesting that both Nos1PVH and non-Nos1PVH subsets of Sim1 neurons contribute to the control of energy expenditure. As increased locomotor activity may contribute to overall energy expenditure and VO2, we analyzed VO2 in Sim1-Cre+AAV-hM3Dq and Nos1-iCre+AAV-hM3Dq mice at time points when locomotor activity was approximately matched before and after CNO treatment (Fig. 8F). While this analysis revealed a trend toward increased VO2 for both Sim1-Cre+AAV-hM3Dq and Nos1-iCre+AAV-hM3Dq animals when matched for activity (Sim1-Cre, paired t test, t(3) = 2.4, p = 0.093; Nos1-iCre, paired t test, t(3) = 2.9, p = 0.064), the magnitude of the effect was small relative to the overall increase in VO2. This suggests that increased locomotor activity contributes significantly to the increased oxygen consumption observed with PVH stimulation, but that changes independent of locomotor activity may also play a role.
Acute Sim1PVH activation increases intrascapular BAT temperature
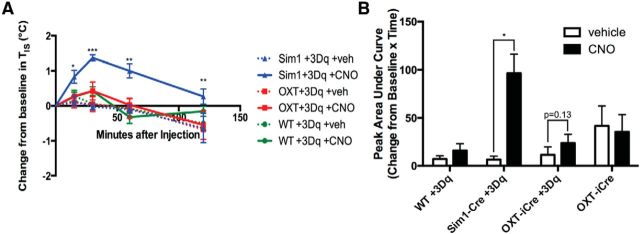
Since the activation of PVH neurons tends to increase energy expenditure in mice matched for locomotor activity, and Sim1PVH, Nos1PVH, and OXTPVH neurons send dense projections to preganglionic sympathetic ChAT neurons in the thoracic spinal cord, we hypothesized that activation of these PVH subpopulations might promote thermogenesis in addition to potentially playing a role in locomotor activation. To test this, temperature transponders were placed in the subcutaneous tissue directly above intrascapular BAT in Sim1-Cre+AAV+hM3Dq, OXT-iCre+AAV+hM3Dq, and WT+AAV+hM3Dq mice and intrascapular temperatures (TISs) were measured before and after PVH neuron activation. Body weights (±SEM) of the animals used in these studies are as follows: Sim1-Cre, 27.4 ± 1.6 g (n = 3); OXT-iCre, 28.5 ± 0.8 g (n = 4); and WT, 31.5 ± 1.2 g (n = 5). Activation of Sim1PVH neurons increased TISs when compared with baseline TISs before injection of vehicle or CNO (Fig. 9A,B; repeated-measures two-way ANOVA of change in baseline TISs, F(1,2) = 102.9, p = 0.010 with Sidak multiple-comparisons post hoc test: 15 min, t(8) = 4.2, p = 0.016; 30 min, t(8) = 8.3, p < 0.001; 60 min, t(8) = 6.5, p = 0.001; 120 min, t(8) = 5.3, p = 0.004). Additionally, activation of OXTPVH neurons display a trend of increased TIS, though this did not reach statistical significance. TIS in WT+AAV+hM3Dq mice were not altered in response to CNO administration.
Figure 9.
Acute activation of Sim1PVH neurons increases subcutaneous intrascapular temperature. Sim1-Cre+3Dq, OXT-iCre+3Dq, and WT+3Dq mice received temperature transponders in the subcutaneous intrascapular tissue directly above BAT. TIS was measured before and after vehicle or CNO administration. A, B, TIS is shown relative to baseline TIS before and after injection of vehicle or CNO (A, dashed and solid lines, respectively) and also is represented as the peak area under the curve (B). Average values ± SEM are shown. *p < 0.05, **p < 0.01, ***p < 0.001 compared with vehicle values (WT, n = 5; Sim1-Cre, n = 3; OXT-iCre, n = 4). Significance was determined using repeated-measures two-way ANOVA with Sidak multiple-comparisons post hoc test.
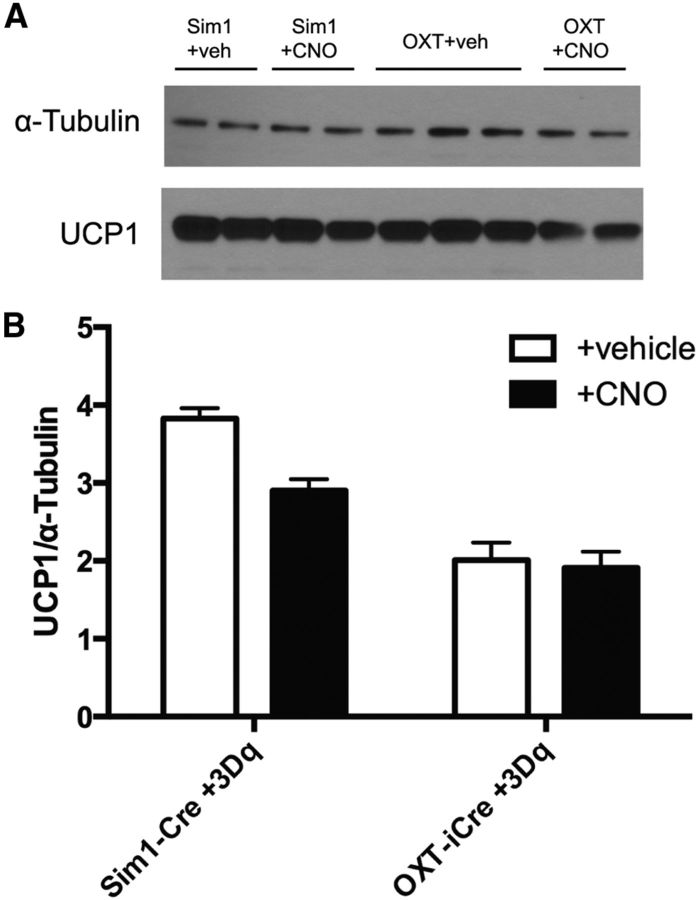
Since acute PVH activation increased TIS, we determined whether this was mediated by increased BAT uncoupling protein 1 (UCP1), the primary facilitator of BAT thermogenesis (Matthias et al., 2000). We examined UCP1 protein levels in BAT from Sim1-Cre+AAV-hM3Dq, OXT-iCre+AAV-hM3Dq, or WT+AAV-hM3Dq mice treated with either vehicle or CNO. UCP1 protein levels did not change in response to Sim1PVH or OXTPVH neuronal activation when normalized to the loading control (Fig. 10). Therefore, increases in TIS following PVH activation may be due to mechanisms that increase UCP1 activity rather than protein levels (Divakaruni et al., 2012; Richard et al., 2012).
Figure 10.
Acute activation of PVH neurons does not alter BAT UCP1 protein expression. BAT protein lysates were prepared from Sim1-Cre+3Dq and OXT-iCre+3Dq mice treated with either vehicle or CNO for 90 min. A, UCP1 protein levels are no different following Sim1PVH (left) or OXTPVH (right) activation with CNO compared with vehicle. B, Quantification of BAT UCP1 protein levels relative to the loading control, α-tubulin [Sim1+vehicle (veh), n = 2; Sim1+CNO, n = 2; OXT+veh, n = 3; OXT+CNO, n = 2].
Discussion
The importance of the PVH in feeding regulation, energy balance, and endocrine and autonomic function is well established (Sawchenko and Swanson, 1982; Cowley et al., 1999; Balthasar et al., 2005). However, a detailed understanding of the cellular and neural pathways used by the PVH to regulate these physiologic functions has been complicated by the heterogeneity of this nucleus and the inability to investigate specific PVH cell populations independently. To tackle these issues, we combined Cre-dependent viral vectors with PVH cell-specific Cre drivers to probe the function of discrete PVH neuron subsets and explore their connectivity with brain regions known to be involved in energy homeostasis.
The hypothalamic transcription factor Sim1 marks PVH neurons involved in feeding regulation. Sim1-restricted melanocortin-4 receptor (Mc4R) expression in an otherwise Mc4R-null background corrects the associated hyperphagia of Mc4R-null mice and targeted ablation of Sim1 neurons in the CNS results in hyperphagia and altered energy expenditure (Balthasar et al., 2005; Xi et al., 2012). Sim1 neurons lie in other brain areas, but most if not all PVH neurons express Sim1 (Michaud et al., 1998). We now show that acute activation of Sim1PVH neurons suppresses feeding and increases energy expenditure and activity, highlighting the ability of PVH neurons to regulate both energy balance parameters and validating our experimental system. To clarify the neurochemical identity and neural circuitry of the PVH neurons mediating these effects, we identified and used Nos1 expression to mark a specific Sim1PVH subset. Our tracing studies revealed dense Nos1PVH projections to hindbrain and spinal cord structures involved in energy balance regulation. Using a pharmacogenetic approach, we showed that activation of Nos1PVH neurons suppressed food intake at the onset of feeding to an extent that is comparable to that observed upon activation of the entire Sim1PVH field. These experiments therefore establish Nos1PVH neurons as an important Sim1PVH subset in feeding regulation.
OXTPVH neurons are a subset of Nos1PVH neurons and pharmacologic evidence has demonstrated the ability of hindbrain OXT action to suppress feeding, suggesting a role for OXTPVH neurons in anorectic signaling by the PVH (Blevins et al., 2004; Kublaoui et al., 2008; Tolson et al., 2010). In contrast, however, genetic inactivation of OXT or its receptors minimally impacts feeding, and ablation of OXT neurons in adult mice neither alters feeding nor the anorexic response to a melanocortin agonist (Takayanagi et al., 2008; Camerino, 2009; Wu et al., 2012). To determine the contribution of OXTPVH neurons to Nos1PVH-regulated feeding, we subjected OXTPVH neurons to Cre-dependent synaptic tracing and pharmacogenetic analysis. DREADD-mediated activation of OXTPVH neurons failed to suppress feeding under normal conditions. Therefore, while Nos1PVH neurons mediate a powerful anorectic signal, the OXT-expressing subset of Nos1PVH neurons cannot account for this effect, thus revealing a requisite role for non-OXT Nos1PVH neurons in food intake control.
The role of the PVH in energy expenditure regulation has received less attention than its role in food intake control. Sim1 haploinsufficiency or manipulation of Mc4R expression in Sim1 neurons alters feeding, but minimally affects energy expenditure (Michaud et al., 2001; Balthasar et al., 2005). Selective ablation of Sim1 neurons in the CNS, however, lowers oxygen consumption, suggesting a role for Sim1 neurons in the regulation of both food intake and energy expenditure (Xi et al., 2012). Indeed, recent data reveal that glutamatergic signaling in Sim1 neurons contributes to energy expenditure regulation (Xu et al., 2013). In this study, we show that direct activation of Sim1PVH neurons increases energy expenditure, and that both Nos1PVH and OXTPVH neurons contribute to this physiologic response. Using a novel anterograde viral tracing tool, we did not find significant projections to the RPa. However, we did identify dense terminals from Sim1PVH, Nos1PVH, and OXTPVH neurons in close proximity to ChATIML neurons of the thoracic spinal cord, a cholinergic preganglionic structure that regulates sympathetic output. Importantly, direct activation of Nos1PVH and OXTPVH neurons appears to stimulate ChATIML cells concomitant with increases in metabolic rate, locomotor activity, and thermogenesis. This physical connection between PVH neuron subsets and ChATIML neurons provides a potential neuroanatomical mechanism by which sympathetic output may be increased to promote energy expenditure following activation of these PVH neurons. Indeed, activation of Sim1PVH neurons increases intrascapular temperature overlying BAT. As thermogenesis requires BAT UCP1 (Cannon and Nedergaard, 2004), and there is no change in UCP1 protein expression following Sim1PVH stimulation, this effect is likely dependent on a change in UCP1 activity.
The increase in oxygen consumption upon Sim1PVH activation is more robust than that seen with activation of either Nos1PVH or OXTPVH neurons alone. Interestingly, the extent of ChATIML activation is relatively similar, suggesting that additional CNS pathways are engaged to regulate energy expenditure acutely. Only an estimated 25% of hindbrain and spinal cord-projecting PVH neurons have been neurochemically defined (Sawchenko and Swanson, 1982). Therefore, other, unidentified PVH neurons likely play important roles in modulating energy expenditure. Additional studies directed at identifying and manipulating chemically defined populations of PVH neurons will be critical in understanding the cellular and neuroanatomical pathways used by the PVH to modulate energy expenditure and achieve energy homeostasis.
The neurotransmitters by which Nos1PVH and OXTPVH neurons regulate feeding and energy expenditure remain undefined. It is likely that both neuropeptides and fast-acting neurotransmitters such as glutamate, the predominant PVH neurotransmitter, contribute to PVH-mediated energy balance. Indeed, Sim1 glutamate signaling is important for overall control of energy balance (Xu et al., 2013). Our DREADD activation studies suggest the possibility that PVH neurons regulate adjacent cells. Given the presence of local, intra-PVH glutamatergic connections, it is conceivable that PVH neuronal subsets can recruit certain neighboring cell types to affect functional outputs (Ziegler and Herman, 2000).
Overall, our dissection of PVH neuron subpopulations reveals that specific subsets of PVH neurons play distinct roles in energy balance regulation. Specifically, we reveal a role for Nos1PVH neurons in the control of feeding, and that this function requires the participation of non-OXT Nos1PVH neurons. Moreover, both Nos1PVH and the OXTPVH neurons project to sympathetic output areas of the thoracic spinal cord and are capable of increasing energy expenditure (although to a lesser extent than Sim1PVH neurons, suggesting roles for non-Nos1 Sim1PVH cells). The identification and analysis of other PVH subpopulations will be crucial to determining the molecular mechanisms by which the PVH regulates energy homeostasis.
Footnotes
Core support (Animal Phenotyping) was provided by the University of Michigan Animal Phenotyping Core. This work was supported by the National Institutes of Health (Grants F31 NS082027-01 and T32 GM 8322-22 to A.K.S.), the Michigan Diabetes Research and Training Center Pilot and Feasibility Award (NIH Grant 5P60 DK20572 to D.P.O.), and NIH P30 Grants DK089503 and DK020572 to the Animal Phenotyping Core. We thank the members of the Myers laboratory for helpful discussions and technical support; and Dr. Michael Scott and the Scott laboratory (University of Virginia, Charlottesville, VA) for training in targeted stereotaxic injections into the nucleus of the solitary tract. We also thank Dr. Sebastian Parlee, Dr. Hiroyuki Mori, and the MacDougald Laboratory (University of Michigan) for their advice and assistance with Western blots.
The authors declare no competing financial interests.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Bergner AJ, Stamp LA, Gonsalvez DG, Allison MB, Olson DP, Myers MG, Myers MG, Jr, Anderson CR, Young HM. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J Comp Neurol. 2014;522:514–527. doi: 10.1002/cne.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabó K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 2009;17:980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/S0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Humphrey DM, Brand MD. Fatty acids change the conformation of uncoupling protein 1 (UCP1) J Biol Chem. 2012;287:36845–36853. doi: 10.1074/jbc.M112.381780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Cholecystokinin-immunoreactive innervation of the ventromedial hypothalamus in the rat: possible substrate for autonomic regulation of feeding. Neurosci Lett. 1985;53:289–296. doi: 10.1016/0304-3940(85)90553-1. [DOI] [PubMed] [Google Scholar]
- Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182:488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG., Jr Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Lévy E, Mitchell GA, Himms-Hagen J, Fan CM. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12:3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Münzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Björnholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M, Jr, Leinninger G. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol Metab. 2013;2:423–434. doi: 10.1016/j.molmet.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 2001. [Google Scholar]
- Richard D, Monge-Roffarello B, Chechi K, Labbé SM, Turcotte EE. Control and physiological determinants of sympathetically mediated brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012;3:36. doi: 10.3389/fendo.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Sims JS, Lorden JF. Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav Brain Res. 1986;22:265–281. doi: 10.1016/0166-4328(86)90071-9. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30:3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, Olson DP, Tong Q. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS One. 2012;7:e45167. doi: 10.1371/journal.pone.0045167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Gandhi N, Lai M, Kublaoui BM. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS One. 2012;7:e36453. doi: 10.1371/journal.pone.0036453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, Lowell BB, Arenkiel BR, Xu Y, Tong Q. Glutamate mediates the function of melanocortin receptor 4 on sim1 neurons in body weight regulation. Cell Metab. 2013;18:860–870. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses. 2000;141:4801–4804. doi: 10.1210/endo.141.12.7949. [DOI] [PubMed] [Google Scholar]