Abstract
Development requires tissue growth as well as cell diversification. To address how these processes are coordinated, we analyzed the development of molecularly distinct domains of neural progenitors in the mouse and chick neural tube. We show that during development these domains undergo changes in size that do not scale with changes in overall tissue size. Our data show that domain proportions are first established by opposing morphogen gradients and subsequently controlled by domain specific regulation of differentiation rate, but not differences in proliferation rate. Regulation of differentiation rate is key to maintaining domain proportions while accommodating both intra- and interspecies variations in size. Thus the sequential control of progenitor specification and differentiation elaborates pattern without requiring that signalling gradients grow as tissues expand.
In most developing tissues, the spatiotemporal pattern of gene expression is generated by gene regulatory networks controlled by morphogens (1-3). This happens at the same time as the tissue grows. Understanding how growth and cell fate specification are coordinated within a tissue to determine the relative proportions of different cell types is of central importance. In some tissues, such as the Drosophila wing disc (4), early embryo (5, 6) and Xenopus gastrula (7, 8) the scaling of the morphogen gradient with tissue size determines the underlying pattern. Whether similar strategies apply to all tissues is unclear.
In the vertebrate neural tube the dorsoventral pattern of neural progenitors is specified by 13 spatially distinct transcriptional states (9). These are encoded by the combinatorial expression of transcription factors in response to opposing morphogen gradients – ventrally secreted Shh and dorsally secreted BMP and Wnt (Fig. 1A). In mouse, the graded activation of Gli effectors downstream of Shh undergoes temporal adaptation after reaching a peak at day E9 (10, 11), hence it does not correlate with tissue size. Moreover, Gli activity levels are not constant at the boundaries of the target genes Nkx2.2 and Olig2 over time (11). While the transcriptional network is at least in part responsible for this lack of correspondence between Shh signalling and target gene boundary positions (11), anisotropic tissue growth might also affect progenitor domain sizes.
Figure 1. Quantification of neural tube pattern and size over time.
A) Markers used to partition the neural tube: floorplate FP (Arx+), motor neuron progenitors pMN (Olig2+), ventral interneuron progenitors p3 (marked by the absence of Olig2 and Arx), intermediate interneuron progenitors pI (absence of Olig2 and Pax3), dorsal interneuron progenitors pD (Pax3+). B) E10.5 transverse section and markers used for measuring the number of progenitors. Domains outlined in white. Sox2 was used to determine the basal extent. Scale bars, 50μm. C) Schematic of neuroepithelium organization illustrating different apicobasal lengths of the progenitor nuclei (grey) layer. D) Dorsoventral positions of indicated boundaries relative to the ventral midline. Time (hours post headfold stage, hph) corresponds to twice the number of somites. Upper x-axis, embryonic day, (Table S1). E) The dorsoventral boundary positions relative to total dorsoventral length change over time. F) Mean apicobasal length of the Sox2+ progenitor layer. G) Mean number of Sox2+ nuclei per hemi-section in each domain based on the markers in B, representing dorsoventral domain size in units of cells. H) Number of progenitors in G relative to the total. Error bars, SEM. Sample sizes, Table S2.
During development neural progenitors proliferate and this is counterbalanced by their terminal differentiation into neurons, which migrate out of the epithelial progenitor layer (12) (Fig. 1A-C). As a result, the neural tube grows anterioposteriorly, dorsoventrally and apicobasally, but it is not known how much growth occurs in each dimension. Here we assess the contribution of morphogen signalling and anisotropic growth to pattern formation and define how pattern adapts to size variability between individuals by measuring the growth characteristics of mouse and chick embryos.
Pattern does not scale with tissue size
To characterize how pattern is specified relative to neural tube growth and Shh signaling, we focused on five progenitor domains that partition the dorsoventral axis (Fig. 1A, B). We collected transverse sections from the forelimb level of mouse embryos adjacent to somites 7-10 between E8 (~10 hours post headfold stage (hph) and E11.5 (~90hph, the onset of gliogenesis (13)).
During this period the dorsoventral length of the neural tube increases ~4-fold and the domain boundary positions shift with respect to the ventral midline (Fig. 1D). Before E9 these shifts result, at least in part, from the progressive induction of progenitor domains (11, 14). Moreover, the relative positions of boundaries (as a fraction of the total length) are not constant, but also change (Fig. 1E). This could result from changes in cell shape, size or number. Indeed, the thickness of the single-cell layer of Sox2-positive (Sox2+) progenitors changes over time in a domain-specific manner (Fig. 1F), suggesting regulated changes in cell shape. Nevertheless, all progenitors attach to the apical surface, hence the number of Sox2+ nuclei per section provides a measure of dorsoventral size in units of cells, independent of cell shape (Fig. 1C, G, fig. S1). The relative sizes of progenitor domains measured in this way change during development (Fig. 1H), showing that dorsoventral pattern does not change linearly with the overall neural tube size.
In systems where pattern scales with growing tissue size, domain proportions are also preserved between individuals of varying size (4, 7, 8). To study how neural tube pattern accommodates variations in size between individuals, we used a mouse Minute strain that is heterozygous for a deletion in Rpl24 (15), a large ribosomal subunit component. These smaller Minute mice have no apparent locomotion defects, yet they have 19±10% fewer progenitors than wildtype littermates at E11.5 (Fig. 2A, B, D) and they produce 60±7% fewer postmitotic neurons (Student’s T-test p<0.05, Fig. 2E). While the neural tube size of Rpl24(Bst)/+ embryos is noticeably smaller than controls after 30hph, the progenitor domain proportions follow a similar pattern of development (Fig. 2F, cf. 95% confidence intervals) and at 90hph the mice end up with a similarly patterned, albeit smaller, neural tube.
Figure 2. Comparison of wildtype, Rpl24(Bst)/+ mouse and chick embryos.
A-C”’) Transverse sections immunostained for Pax3, Olig2, Nkx2.2, Arx. Identical magnification, scale bars 50μm. D) Progenitors per hemi-section in wildype (circles, light shades) vs Rpl24(Bst)/+ (diamonds, dark shades) mouse embryos. The larger more dorsal domains are plotted on the left panel, smaller more ventral domains on the right. E) Postmitotic neurons in Rpl24(Bst)/+ vs wildtype embryos (legend as in E), F) The progenitors per hemi-section in each domain relative to the total number change in a similar way between Rpl24(Bst)/+ and control. G) Wildype mouse (circles, light shades) vs chick (diamonds, dark shades) comparison as in F. The chick dataset was registered to mouse so the somite next to which the measurements were made was generated at equivalent Corresponding time (Table S1). D-G) Error bars, SEM. Shaded areas, 95% confidence intervals. Sample sizes, Table S2.
Furthermore, by comparing pattern at equivalent stages and anterioposterior positions, we found that the dynamics of progenitor domain proportions are also conserved between the mouse and chick neural tube (Fig. 2C, G). Together, this indicates that the dorsoventral domain proportions change during development via a conserved sequence, which is independent of the size of the animal.
Proliferation is uniform but differentiation is cell type specific
How is the temporal sequence of progenitor domain proportions controlled? Four factors control the number of progenitors in a domain: 1) cell proliferation, 2) apoptosis, 3) terminal differentiation leading to cell cycle exit and delamination, and 4) switches in cell identity, i.e. changes in gene expression that respecify one progenitor type into another. It is possible that spatially non-uniform growth, driven by non-uniform differentiation, proliferation, or apoptosis, together with a memory of the transcriptional state (11), could account for the change in proportions independent of signalling. Alternatively, switches in gene expression could increase the size of one progenitor pool at the expense of another. To distinguish between these possibilities we measured the growth parameters of the mouse spinal cord.
We measured the proliferation rate using sequential pulse labelling with two thymidine analogues, IdU and BrdU (16, 17). The fraction of Sox2+ progenitors labelled only with IdU during the 1.5h pulse (IduLI) is directly related to the proliferation rate λ (fig. S2A, B; Materials and Methods). We also measured the mitotic index (MI, the fraction of phospho-histone H3 labelled Sox2+ progenitors) in combination with progenitor domain markers (Fig. 3A, fig. S2C). The MI/IduLI ratio allows us to determine the duration of mitosis, 27.7±11.3min, and the domain-specific λ from the MI (Materials and Methods). Both approaches indicate that the proliferation rate is uniform in all domains except the Shh producing floorplate (ANOVA p>0.05 for all stages, excluding floorplate).
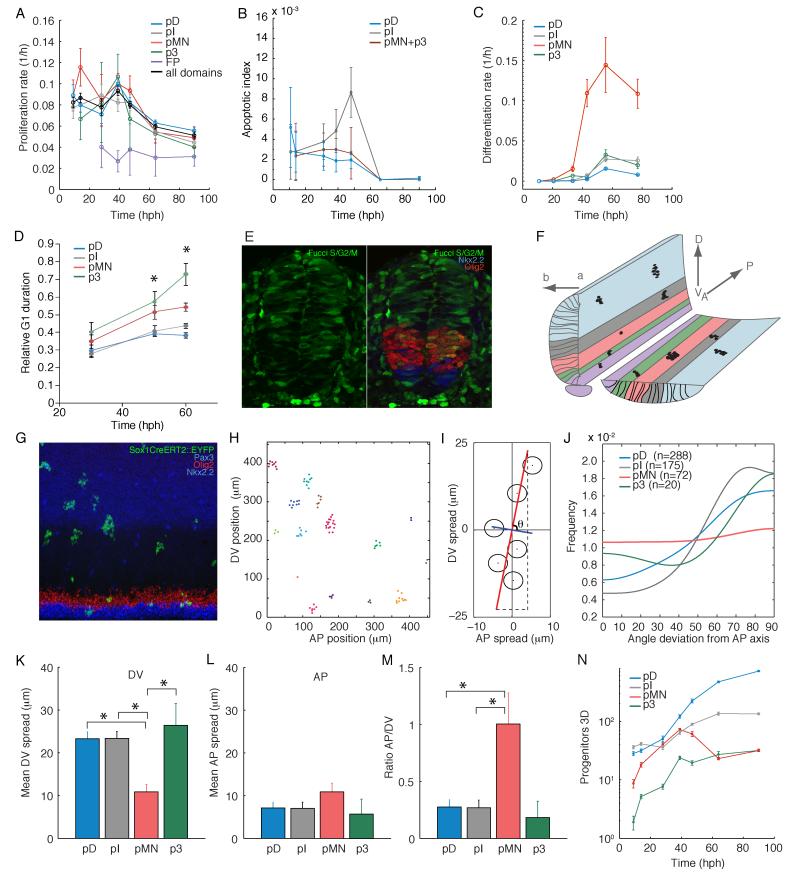
Figure 3. Mouse growth parameters.
A) The proliferation rate determined from MI in sections is similar (ANOVA p>0.05) for all domains except FP. B) Fraction of Caspase3+ Sox2+ progenitors. C) Differentiation rate determined from transverse sections. D-E) G1 length relative to the total cell cycle length (D) estimated from the fraction of S/G2/M-Fucci negative progenitors (E). Asterisks, one-way ANOVA across domains p<0.05. F) Neural tube geometry and flat mount preparation. Colors as in Fig. 1A, labelled cells, black. G) Maximum projection though a flat mount, imaged between somites 6-13. Dorsal up, anterior left. Clones marked by EYFP expression. H) Coordinates of the EYFP+ cells in G. Clonal groups are color-coded. I) Example clone. Cell coordinates (dots) and typical cell diameter (circles). First and second eigenvectors of the second moment matrix, red and blue line, respectively. Clone orientation angle relative to the anterioposterior axis, θ. Anterioposterior and dorsoventral clone spread, dashed lines. J) The density distribution of pD, pI and p3 clone orientation angles is non-uniform (χ2 test p<0.05). K-M) Mean dorsoventral (K), anterioposterior (L) clone spread and ratio of the mean anterioposterior/dorsoventral spread (M). Asterisks, Student’s T-test p<0.05. N) Progenitors in a hemi-section with anterioposterior length=1 cell at t=10h and increasing in 3D as calculated from the clonal and section data (Supplementary Information). A-N) Error bars, SEM. Sample sizes, Table S2.
The proliferation rate is approximately constant before 40hph (~0.08h−1, equivalent to a cell cycle length of ~9h), and decreases at later times (Fig. 3A). In parallel, the duration of the G1 phase of the cell cycle increases relative to the cell cycle length (Fig. 3D-E, fig. S3A, B), but this increase is not spatially uniform. Thus, while the overall cell cycle length is similar between the pMN and pD domains at 60hph, their cell cycle phase distribution is different. This raises the possibility that a homeostatic control mechanism could coordinate G1/S and G2/M progression to maintain uniform proliferation (18).
Since the proliferation rate is spatially uniform, it cannot cause the observed changes in domain proportions. Furthermore, the fraction of apoptotic progenitors is less than 0.5% (Fig. 3B) and cleaved Caspase 3, used to detect apoptosis, can persist for more than 2h in neural tissues (19). Thus the rate of progenitor apoptosis (<0.0025h−1) is negligible compared to the proliferation rate and does not significantly affect the domain growth rates.
To measure the differentiation rate, we quantified the number of Sox2-negative (Sox2—) postmitotic neurons generated from each progenitor domain prior to E11 in transverse sections (Fig. 2E). We defined the differentiation rate as the number of neurons produced per unit time relative to the size of the progenitor domain, which reflects the probability of progenitors to exit the cell cycle. The differentiation rate was not constant over time. For MNs a maximum rate was reached at 60hph (Fig. 3C). For the intermediate and dorsal interneurons peak differentiation occurred at ~100hph (fig. S1G-H). The differentiation rate differed between domains – before 90hph pMNs differentiated at a higher rate (reaching 0.15h−1) than p3 and more dorsal regions (<0.03h−1). The increase in differentiation rate of the pMN domain happens at the same time (40hph) as the size of the domain starts decreasing (Fig. 1G). Thus differential net growth rates resulting from cell-type specific differences in the differentiation rate could be the main factor influencing domain size proportions.
Progenitor domain growth is anisotropic
The measurements of the proliferation and differentiation rates can be used to test whether these are sufficient to account for the temporal changes in domain sizes. If this is the case, cell identity does not switch (i.e. the respecification rate is zero) and the change in number of progenitors is given by:
| equation 1 |
where P is number of progenitors, the temporal change in progenitor number, λ the proliferation rate, and γ the differentiation rate, both of which change over time. If growth explains the dynamics of patterning, the measured net growth rate λ(t) − γ(t) should be equal to the relative increase in progenitor number, .
To measure, in addition to the dorsoventral and apicobasal sizes, it is necessary to measure the change in progenitor number along the anterioposterior axis (Fig 3F). To do this, we used a Tamoxifen-inducible system, whereby CreERT2 knocked into the Sox1 locus (fig. S4A) excises a loxP-flanked STOP sequence resulting in the expression of Enhanced Yellow fluorescent protein (EYFP). With low Tamoxifen doses, EYFP expression was activated in individual progenitors at E9.5 (Fig. 3F, G). 48h later the sizes of the resulting clones were consistent with the measured proliferation and differentiation rate (fig. S4B). The shape of the clones relates the anterioposterior growth rate to the dorsoventral growth rate calculated from Fig. 1G (Supplementary Information). This provides a measurement of the growth of a defined volume of the brachial neural tube (Fig. 3N).
Most labelled cells formed coherent groups, suggesting that cell rearrangements after E9.5 are limited (Fig. 3G, see also (20, 21)). The clones often had irregular shape, to quantify these we used the second moment matrix of cell coordinates (Fig. 3G-I, fig. S4). For all but the pMN domain, clones were elongated along the dorsoventral axis and their orientation was dorsoventrally biased (Fig. 3J-M, fig. S4E). By contrast, pMN clones were on average isotropic. Furthermore, while the dorsoventral spread of clones differed between domains, they had a similar anterioposterior spread (Fig. 3K-L). This implies that mechanical or molecular constraints ensure equivalent anterioposterior growth across the tissue, while the dorsoventral growth rates are position- and cell type-dependent.
Using these data we calculated the relative increase in progenitor number in three dimensions (Supplementary Information) and directly compared it to the net growth rate (equation 1). To validate the measurements, was compared to λ(t) − γ(t) of the whole tissue, because respecification is irrelevant for the total number of progenitors and equation 1 must hold true. This is indeed the case (Fig. 4A), except at 20hph, which corresponds to the time neural crest delaminates from the neural tube (22). Thus decreases over time, indicating that the number of progenitors comprising the brachial spinal cord increases at a decelerating rate, by ~0.08h−1 at 10hph and by ~0.02h−1 at later stages.
Figure 4. Growth accounts for changes in pattern at later times.
A-F) Relative temporal increase in progenitor number in all dimensions (, solid lines) compared to the net growth rate (λ(t) − γ(t)), dashed lines) for each domain. G) Two-phase model of neural tube development. H-I) Comparison of mouse (circles) to chick (diamonds) mean proliferation rate (H) and differentiation rate (I). J-L) Comparison of the mean proliferation rate (J), differentiation rate (K), and fraction of Caspase3+ progenitors (L) between wildtype mouse (solid lines) and Rpl24Bst/+ embryos (dashed lines). A-L) Shaded areas, 95% confidence intervals. Error bars, SEM. Sample sizes, Table S2.
A two-phase model for neural tube pattern formation
We analyzed individual progenitor domains (Fig. 4B-F). Prior to ~40hph there were discrepancies between and λ(t) − γ(t): the pD and pI domains expanded less than predicted by their growth rate (Fig. 4B, C), while ventral domains, which are progressively induced in progenitors that initially had pI identity (Fig. 4D-F, (23)), expanded more. By contrast, after ~40hph, there was a good correspondence between the relative increase in progenitor number of each domain and the net growth rate. Thus after ~40hph the changes in domain size can be accounted for by proliferation and differentiation, suggesting that the net respecification rate is negligible. Consistent with this, 92.1% of the clones in our dataset consisted of a single progenitor type (fig. S4C), indicating that cell identity changes within a lineage are infrequent after E9.5. Because proliferation is spatially uniform (Fig. 3A) the differences in between domains and hence the changes in proportions must be accounted for by cell-type specific differences in the differentiation rates (Fig. 3C).
Together, these observations indicate that neural tube patterning can be viewed as a two-phase process – an early phase (prior to ~40hph), where there is no differentiation and progenitor identity is actively specified, and a late phase, where progenitor identity does not change significantly and the relative domain sizes are controlled by cell type specific differentiation rates (Fig. 3C, 4G).
How are reproducible domain proportions between individuals of different sizes and species achieved during the second phase? In the absence of switches in cell identity and given a spatially uniform proliferation rate, the number of progenitors in any two domains (e.g. the dorsal half Pd, and the ventral half Pv) would each change in accordance to equation 1. Hence, the ratio of progenitors in the two domains would change as:
| equation 2 |
where, γv(t) and γd(t) are the time-dependent differentiation rates in the two domains. Therefore, as long as two individuals have the same differences between the differentiation rates of domains over time, regardless of the absolute tissue size, their progenitor domain proportions would change in the same way. Thus reproducible pattern proportions are achieved if four criteria are satisfied: 1) absence of net cell identity changes, 2) conserved cell-type specific differences in the differentiation rate, 3) conserved temporal dynamics of the differentiation rate in different individuals, and 4) reproducible initial proportions established during the early phase.
Since the chick neural tube shows similar changes in proportions as mouse, we hypothesized that the two-phase model also applies to this species and the differentiation rate dynamics are preserved. To test this hypothesis, we determined the change in progenitor number in chick. Photoconverting Photoswitchable Cyan Fluorescent Protein (PS-CFP) in a stripe of cells in the neural tube of transfected embryos indicated a homogenous anterioposterior growth rate across domains (fig. S5). The proliferation rate, measured from the mitotic index (Fig. 4H, fig. S2F, G, S6) and IdU/BrdU incorporation (fig. S2D-E), was spatially uniform, except in the FP (fig. S2G) and consistent with previous studies (24, 25). At later stages, the proliferation rate in chick was lower than in mouse (Fig. 4H), in agreement with the smaller size of the chick neural tube (fig. S1D-F). The differentiation rate was cell type-dependent, highest in the pMN domain and undergoing similar temporal changes to mouse (Fig. 4I). Finally, comparing λ(t) − γ(t) to (fig. S7) supported the idea that pattern formation in the chick neural tube proceeds in a similar biphasic way as mouse. The similarity in the differentiation dynamics in chick and mouse explains the overall similarity in pattern proportions.
The two-phase model was also consistent with the proportion dynamics observed in Minute mouse embryos. In these embryos the proliferation rate was unaffected (Fig. 4J, (26)), whereas the fraction of apoptotic cells was higher, particularly at later stages, explaining the smaller progenitor number compared to controls (Fig. 4L). The pMN differentiation rate in Rpl24(Bst)/+ embryos was consistent with the observed changes in proportions (Fig. 4K). Together, these data indicate that pattern formation in the Rpl24(Bst)/+ embryos is consistent with the two-phase model and that the preserved differentiation rate dynamics can account for the reproducible pattern of these mice relative to control.
Experimental validation of the two-phase model
The two-phase model for neural tube patterning makes several predictions. First, the rate of respecification of progenitor identity should decrease significantly at late developmental stages. To test this prediction, we measured the rate of Olig2 identity switching at different stages using a Tamoxifen-inducible Olig2KICreER line (27) (Fig. 5A-C). As predicted, the rate of Olig2 identity change decreases from 0.034h−1 to 0.004h−1 the later the Cre activity was induced (Fig. 5C, ANOVA p<0.05). Although this decrease occurs in parallel with the increase of G1 phase length (Fig. 3D), analysis of the cell cycle phase distribution in cells undergoing a Olig2-Nkx2.2 switch, marked by the coexpression of both genes, suggests that respecification can occur at any time of the cell cycle (Fig. 5D, fig. S3C).
Figure 5. Validation of the two-phase model.
A-B) Olig2KICreER induced GFP expression (green) from the indicated times. Olig2 immunostaining, red. C) Quantification of rate of loss of Olig2 identity. Period of Olig2KICreER activity, horizontal grey bars. D) Distribution of cell cycle phases (based on S/G2/M-Fucci expression) in progenitors expressing Olig2, Nkx2.2, or both (Fig. 3E). E-J) Mouse embryo culture at the indicated stages without (E-G) or with (H-J) 5μM Cyclopamine. Sections in H,I are from the same embryo as E,F resp. K) Quantification of the experimental conditions in E-J. The difference in mean boundary position between control and Cyclopamine treatment was significant (Student’s T-test p<0.05) for all boundaries at E8.5 and 3/6 at E9.5-brachial. The different boundaries were measured in independent experiments. Cyclopamine concentration for Nkx6.1 boundary in E8.5 is 3μM. L-M) pSmad and GBS-GFP mean fluorescence intensity vs relative distance from the ventral midline at different stages. N) Profiles in L-M were normalized to the maximum intensity in each time-series and summed to give combined pSmad and GBS-GFP activity. Error bars, SEM. Sample sizes, Table S2.
The decrease in Olig2 respecification rate, despite the continuing changes in Gli activity, raises the possibility that progenitors become less sensitive to Shh signalling. To test this, we reduced Shh signalling using moderate concentrations of the inhibitor cyclopamine at different stages of development in cultured mouse embryos (Fig. 5E-K). The domain boundary positions had little sensitivity to 5μM cyclopamine at E9.5 of development, but shifted significantly at earlier stages (Fig. 5K). Moreover, the time at which gene expression becomes robust to experimental inhibition of Shh signalling coincided with the time at which Olig2 identity changes decreased (Fig. 5C) and the transition between the two phases of development (Fig. 4G).
Examination of the dynamics of Gli activity indicated that the transition between the two phases of pattern formation occurred when Gli activity levels were close to maximal (Fig. 5L, fig. S8, (11)). The BMP signalling levels measured by immunostaining for phosphorylated Smad1/5/8 showed similar temporal dynamics (Fig. 5M, fig. S8). These measurements revealed that the maximum ranges and greatest overlap between the two gradients were prior to 30hph (Fig. 5N). Thus progenitors are exposed to the highest levels of BMP and Shh signaling prior to 30hph. This suggests that the subsequent decrease in BMP and Shh activity permits the stabilization of lineages, in part through the action of the transcriptional network in progenitors (11), and allows for growth-dependent, rather than morphogen-dependent regulation of progenitor domain proportions.
To test direcly that the differentiation rate affects domain proportions, we reasoned that if the spatial differences in the differentiation rate were removed, the pMN domain which normally differentiates at a higher rate than other domains, would increase in relative size. We imposed approximately uniform differentiation rates in chick using in ovo electroporation in two ways: 1) increasing differentiation in all domains by overexpressing Ngn2 or p21 (Fig. 6A, B, fig. S9A, B, (28, 29)); and 2) inhibiting differentiation in all domains by overexpressing YAP, an effector of Hippo signalling (Fig. 6C, D), or the Notch intracellular domain (fig. S9C, D, (30, 31)). These perturbations have an established effect on neuronal differentiation (28-31) and caused a decrease in progenitor number in the first case and an increase in the second, without a significant change in the mitotic index (Fig. 6A, C, fig. S9E). In the case of Ngn2 overexpression the differentiation rate of non-electroporated cells (fig. S9F-G) was also affected and became spatially uniform, suggesting non-autonomous mechanisms, e.g. feedback from postmitotic cells, are involved in differentiation control (32). Importantly, in all conditions the relative size of the pMN domain became larger on the electroporated compared to the contralateral side (Fig. 6B, D) and was similar to the larger pMN relative size at the onset of the experiment (~30h, Fig. 2G). Since these perturbations cause opposite effects on the overall tissue size, this result cannot be explained by changes in the relative range of the signaling gradients and respecification.
Figure 6. Perturbing differentiation changes the relative pMN size.
A) Chick electroporation at HH14 of Ngn2+GFP assessed after 48h. B) Number of Sox2+ progenitors per hemisection on the control vs Ngn2 electroporated side for 13 sections (5 embryos) as described in A. C) Electroporation of constitutive YAP+GFP as in A. Progenitors expand both dorsoventrally and apicobasally on the electroporated side. D) Quantification of C for 12 sections (6 embryos). Error bars, SEM.
Discussion
Here we show that progenitor domain proportions in the neural tube continuously change through a conserved sequence that is independent of animal size. Pattern is first established by morphogen driven cell fate specification and then elaborated by cell-type specific regulation of differentiation rates.
How the spatiotemporal changes in the differentiation rate are regulated is a key question. The length of G1, Notch signalling, and proneural genes (e.g. Ascl1, Ngn1/2), which are activated downstream of domain identity regulators such as Olig2, could play a role (28, 33-38). The comparison of mouse to chick and Minute embryos suggests that the temporal changes in the differentiation rate are conserved and independent of embryo size, thus ensuring that domain proportions are comparable between individuals. This constrains the possible molecular mechanisms controlling the differentiation rate and implies that feedback regulation might ensure its robustness (32). The non-autonomous effect following Ngn2 electroporation suggests a possible role for postmitotic neurons in regulating the differentiation rate of progenitors, although other mechanisms cannot be excluded.
The transition from specification to differentiation phase correlates with the dynamics of Shh and BMP signalling. These dynamics depend on transduction cascades, but are also likely to be constrained by the effective ligand diffusion, degradation and the size of the morphogen source (39). Despite the decrease in Shh and BMP signalling, progenitor pattern is maintained during the differentiation phase. This could be explained by bistability produced by the transcriptional network (11). In the floorplate, a transcriptional network downstream of Fgf signaling contributes to these cells becoming refractory to Shh and BMP before E9.5 (40, 41). Our data suggests that neural progenitors in the other domains tolerate a decrease in Gli and pSmad activity, although some level of Shh signalling is clearly required during the differentiation phase (23, 42).
During the specification phase, chick, mouse and Minute embryos are of similar initial size. This might result from the early period of regulative growth apparent from surgically manipulated embryos (43, 44). Thus the timing of the transition from specification to differentiation could be connected to an optimal tissue size for the formation and activity of morphogen gradients. Further quantitative analysis will be needed to understand both morphogen-dependent and independent scaling mechanisms in other tissues and species.
Materials and Methods
Mouse strains
To generate Sox1CreER the Sox1 reading frame was replaced with CreERT2 (fig. S4A). The following strains were previously described: Tg(GBS-GFP) (11), Olig2KICreER (27), CAG-CAT-GFP (45), Fucci-S/G2/M #504 (46), Rpl24(Bst) (15, 47). Strains were maintained on a Parkes background to maximize litter size.
Embryo staging
Embryos were staged according to the number of somites, where one somite is generated every 2h in mouse and 1.5h in chick (48). Hamburger and Hamilton staging criteria were used for late stages of chick development (49). The chick dataset was registered to mouse, so that the brachial level somite at which the measurements were made was generated at equivalent Corresponding time (Table S1).
For GBS-GFP and pSmad measurements embryos were initially staged by somite number. The dorsoventral lengths of the neural tubes were fitted to DVlength = aebt, where a and b are fit parameters, t is time (Fig. S8F), and then restaged based on the fit.
Immunohistochemistry and imaging
Transverse sections were processed as described (11). Idu/BrdU antigens were exposed by 40min DNAseI treatment at 37 °C, except mouse E8.5 where 2N HCl was used.
Flat mount preparation: the neural tube was cut at the roofplate, fixed in 4% PFA and subsequently methanol. Antibody incubations and washes were 24h each. The left/right neural tube halves were split, then mounted with grease spacers between slide and coverslip.
Sections were imaged with 40×/1.25NAOil objective, flat mounts with 20×/0.7NADry on a Leica TCS-SP5-MP. Single optical sections were taken, except for GBS-GFP and pSmad analysis, where a maximum projection of 3 z-slices 1 μm apart were used. For flat mounts, the entire apicobasal depth of the progenitor layer was imaged with z-slices 1.5 μm apart.
Antibodies used were: goat anti-Sox2 (R&D, 1:100), rat anti-pH3 (Novus Biologicals, 1:2000), rabbit anti-Olig2 (Millipore, 1:1000), rabbit anti-Arx (50) (from J. Chelly, 1:1000), mouse anti-Pax3(c) (DSHB, 1:100), rabbit anti-Islet1 (51) (from T. Jessell, 1:3000), sheep anti-GFP (Biogenesis, 1:1000), mouse anti-Nkx2.2 (DSHB, 1:25), rabbit anti-mAzami Green (MBL, 1:100), rabbit anti-cleaved Caspase3 (Cell Signaling, 1:500); rabbit anti-pSmad1/5/8 (from Ed Laufer), mouse anti-BrdU/IdU (1:80, BD clone B44), rat anti-BrdU (1:80, Abcam, clone BU1/75).
Chick electroporation
Chick electroporation was performed in ovo at HH14 and analysed 48h later. Plasmids used: pMIW-YAP (from Xinwei Cao), pCAGGS-NICD (from Olivier Pourquie), pCAGGS-p21 (from Cheryll Tickle), pCAGGS-Ngn2-IRES-GFP (from Francois Guillemot), pPS-CFP2-N (Evrogen), pCI-H2B-EGFP (from Tatjana Sauka-Spengler). The first three were co-electoporated with pCAGGS-NLS-GFP to mark transfected cells. Final concentrations: 0.5 μg/μl for pMIW-YAP and pPS-CFP2-N, 1 μg/μl for all other plasmids.
Mouse embryo culture
Mouse embryo culture was performed as previously described (11).
IdU/BrdU incorporation
Chick: 0.5mg/ml IdU or 3.3mg/ml BrdU (Sigma) in PB, 10% sucrose, 0.5% Fast Green, were injected into the neural tube, and 50μl was added on top of the embryo. Embryos were treated with IdU for 1.5h, then BrdU for 50min.
Mouse: Pregnant mice were intraperitoneally injected with 0.6mg IdU and after 1.5h with 1mg BrdU. Embryos were harvested after 30 min.
Chick live imaging
Membrane-anchored GFP transgenic chicken eggs were obtained from the Roslin Institute, Edinburgh. Embryos were electroporated with pCI-H2B-EGFP 8-15h prior to imaging.
For live imaging we established a sagittal slice culture protocol, based on a “clot” method (52, 53). The brachial and anterior thoracic region was dissected in L15 medium, then transferred to a glass-bottom dish (MatTek) in a small drop of culture medium (DMEM-F12 1:1, supplemented with N2, B27 (Life Technologies)) containing 10mg/ml fibrinogen (Calbiochem). Thrombin (0.5U/μl, Amersham) was added and a fibrin gel was allowed to form for a few minutes. The slice was covered with ~0.5ml culture medium, the dish was humidified. Z-stacks were collected for 3h on an inverted Leica-SP5 confocal at 37°C.
Photoconversion in chick embryos
Stage HH16 chick embryos electroporated with pPS-CFP2-N were cultured using the EC culture method (54), dorsal side up, and immediately photoconverted along the entire DV length using Leica MP-SP5 microscope, 10X/0.7NADry lens, 30% UV laser power, ~25s scan time. 15h or 0h after photoconversion the neural tube was flat mounted in PBS and immediately imaged.
DV boundary positions and pSmad and Gli activity profiles
Image analysis was performed in Fiji (http://fiji.sc/Fiji) and data analysis in MATLAB (Mathworks, MA, USA).
The mean fluorescence intensity in immunostained sections was quantified across a 10μm region adjacent to the apical lumen. The data was background-subtracted and smoothed with 5μm moving average. Boundaries were defined as the positions where the intensity increased above 10% of maximum.
pSmad and GBS-GFP intensity profiles were normalized to the mean profile for each time point, similarly to a described procedure (55), by normalized intensity = a * (raw intensity), where a is a fit parameter. Profiles that correlated poorly with the mean (R2<0.5) were discarded. These were usually sections damaged during dissection and represented <5% of the data.
Seven independent time-courses of pSmad and GBS-GFP were collected. Sections in each time-course were stained and imaged together to minimize technical variability. The 7 datasets were pooled by normalizing to the median value of Φ for each dataset, where Φ is the 90th percentile of the fluorescence intensity of each profile
Progenitor and neuron numbers and differentiation rate
The number of progenitors per section was inferred from the Sox2+ domain area (Fig. 1B) and the average density of Sox2+ nuclei for each stage (Fig. S1A, B) determined from a subset of sections. The number of neurons was determined by counting Dapi+, Sox2− nuclei. Islet1+ and interspersed Islet1− nuclei (which are HB9/MNR2+ (not shown)) in the ventral horn were considered MNs. The Pax3 boundary was used to separate V2-V0 and dorsal interneurons.
Neurons (N) are produced from progenitors (P) with a rate of differentiation γ, i.e. dN/dt = γP. We obtained an estimate of γ using , where Δt was the time interval between two time points, ΔN was the difference in neuron number between the end and the beginning of the time interval and Pi is the progenitor number in the middle of the interval. The error of γ was calculated by propagation of the standard deviations of all participating variables, assuming that the errors are independent and uncorrelated. The 95% confidence intervals, calculated from the mean, standard deviation, sample size and Student’s T-value, were linearly interpolated between time points.
Proliferation rate by IdU/BrdU incorporation
Three cell populations were distinguished by anti-IdU/BrdU, anti-BrdU and anti-Sox2 immunostaining: 1) Sox2+, IdU+, BrdU− (denoted IduL) are progenitors labelled by IdU during the 1.5h pulse, which have exited S phase before BrdU addition; 2) Sox2+, IdU+, BrdU+; 3) Sox2+, Idu−, BrdU−.
The IdU labelling index IduLI is defined as the ratio of IduL over the total number of Sox2+ progenitors. It is related to the pulse duration θ(Idul) = 1.5h, and the total cell cycle length θ(tot) as:
| equation 3 |
In turn, the proliferation rate λ is related to θ(tot) and IduLI as:
| equation 4 |
Two assumptions are made: 1) cells divide asynchronously, 2) the length of the pulse is shorter than the G2 phase. Theis supported by the random appearance of mitotic figures in flat mounts and no IduL cells were found in telophase, indicating that they are not completing mitosis within the experiment.
Mitotic index
The relationship between the duration of mitosis, the total cell cycle length and the fraction of cells in mitosis depends on the exact growth characteristics of the tissue (56). The mitotic index, MI, for an exponentially growing tissue is defined as:
| equation 5 |
where θ(pH3) is the duration of the pH3-positive phase of mitosis and θ(tot) is the cell cycle length. The proliferation rate λ is related to θ(tot) and MI by:
| equation 6 |
Thus, to calculate λ, it is enough to know MI and the duration of mitosis. MI was directly measured from sections. To determine the duration of mitosis, we used the fact that λ is related to both MI (equation 6) and IduLI (equation 4). Hence,
| equation 7 |
The average values of MI and IduLI from all stages that were identical between the two types of experiments were used for calculating θ(pH3). The resulting mitosis duration is 24.6±7.0min for chick and 27.7±11.3min for mouse. These are similar to published values (24, 25).
MI in flat mounts (Fig. S2C, F) was estimated from the number of pH3+ cells per apical area, normalized to the average apicobasal length for the respective stage and domain measured in sections (Fig. 1F, S1C).
Cell cycle phase distribution
Mitotic cells were identified using Dapi; S+G2 cells using the Fucci-S/G2/M-Green fluorescence intensity. The fluorescence intensities of Olig2, Nkx2.2 and Fucci-S/G2/M-Green were measured in individual nuclei. The Olig2 and Nkx2.2 levels were background subtracted and normalized to the average intensity in the respective domain. Cells with Olig2/Nkx2.2 ratio between 0.1 and 10 were defined as coexpressing.
Coexpressing cells are assumed to undergo a transition from an Olig2 to an Nkx2.2 stable state or, with a smaller probability, vice versa (11). If the transition occurs preferentially in G1 or S+G2 phase, the cell cycle phase distribution in cells that are at the onset of the transition could appear different than in non-coexpressing populations. However, the distribution of both G1 and S+G2 phases across the Olig2-Nkx2.2 level difference was normal (fig. S3C) suggesting that the transition may occur at any time of the cell cycle.
Olig2 lineage tracing
Olig2KICreER mice (27) were crossed to CAG-LoxP-CAT-LoxP-EGFP (45). Cre expression was induced in Olig2+ progenitors by injecting 3mg of Tamoxifen. As a result of the recombination, EGFP was induced with a delay of 12h (Fig. S10), allowing the tracking of cells after they lose Olig2 expression. Embryos were harvested 48h after injection, hence the effective time interval of EGFP labelling was ~36h.
The change in the number of labelled progenitors P, regardless of their type, during the time course of the experiment Δt = t2 − t1 (t1 - time at the beginning, t2 − end time) is:
| equation 8 |
Here, P(t2) are all labelled progenitors at t2 and ΔN are all labelled neurons. Δt=36h and the proliferation rate, λ, is known (the values in Fig. 3A were used), hence the only unknown is P(t1). Since activation at t1 occurs only in Olig2+ cells, P(t1) = POlig2(t1). Hence, the change in the Olig2+ progenitors alone is:
| equation 9 |
where POlig2(t2) are the labelled Olig2 progenitors at t2, ΔMN the number of labelled motor neurons, and ψ, which remains the only unknown, is the rate of loss of Olig2 identity at the expense of other progenitor types. Because Δt is large, the reported values of ψ (Fig. 5C) are approximate.
Clonal analysis
Sox1CreER/+ mice were crossed to Gt(Rosa)26-FloxSTOP-Flox-EYFP homozygotes. Pregnant mice were intraperitoneally injected with 0.3mg Tamoxifen at E9.5 and sacrificed 48h later. The labelled cells formed clusters which were usually >10 cell diameters away from each other, while within a cluster cells were rarely separated by more than 3 unlabelled cells. Therefore, labelled cells were grouped into clones by scanning a 29μm radius (≈6 cell diameters) around each cell. For most images changing the radius by ±5μm preserved clone grouping. Occasional too densely labelled images were discarded.
Clone shape descriptors (Table S2) were derived from the second moment matrix of the cell coordinates of each clone, in which the unit eigenvectors represent two orthogonal axes, and the eigenvalues the variance along these axes. The first eigenvector represents the orientation of the clone with respect to the anterioposterior axis. The anterioposterior and dorsoventral spread of a clone were defined as the distance within ±2 standard deviations from its centre of mass.
The distribution of clone orientation angles was determined using a kernel density estimation method and was different in the pMN domain compared to pD, pI and p3 (two-sample Kolmogorov-Smirnov test p<0.05).
The density of clonal nuclei (estimated by fitting an ellipse around large clones) corresponded to 19±14μm2/cell. The density of non-clonal nuclei, determined in the Pax3 domain in small volumes (~120 cells) was 2.7±0.1μm2/cell, suggesting that clonal nuclei are ~7 times more dispersed than non-clonal nuclei.
Supplementary Material
Acknowledgements
We thank: B. Simons, C. Tabin, A. Ugur, M. González-Gaitán, N. Bushati and M. Cohen for comments on the manuscript; B. Simons and G. Zhang for discussions and help with the clonal analysis; R. Pérez and S. Loubéry for discussions; S. Booth, C. Scott, C. Galichet, S. Malas, K. L. Lee, and A. Ekonomou for generating the Sox1CreER strain; X. Cao, T. Sauka-Spengler, E. Laufer, J. Chelly, T. Jessell, C. Tickle, O. Pourquie, M. Götz for providing reagents; N. Mousavy for technical assistance. This work was funded by the MRC (U117560541) and Wellcome Trust (WT098326MA). A.K. was supported by FEBS, Marie-Curie and MRC fellowships. Supplement contains additional data.
References
- 1.Kicheva A, Cohen M, Briscoe J. Developmental Pattern Formation: Insights from Physics and Biology. Science. 2012;338:210–212. doi: 10.1126/science.1225182. [DOI] [PubMed] [Google Scholar]
- 2.Davidson EH. Emerging properties of animal gene regulatory networks. Nature. 2010;468:911–920. doi: 10.1038/nature09645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaeger J, Irons D, Monk N. Regulative feedback in pattern formation: towards a general relativistic theory of positional information. Development. 2008;3183:3175–3183. doi: 10.1242/dev.018697. [DOI] [PubMed] [Google Scholar]
- 4.Hamaratoglu F, de Lachapelle AM, Pyrowolakis G, Bergmann S, Affolter M. Dpp signaling activity requires Pentagone to scale with tissue size in the growing Drosophila wing imaginal disc. PLoS Biol. 2011;9:e1001182. doi: 10.1371/journal.pbio.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregor T, Bialek W, de Ruyter van Steveninck RR, Tank DW, Wieschaus EF. Diffusion and scaling during early embryonic pattern formation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18403–7. doi: 10.1073/pnas.0509483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung D, Miles C, Kreitman M, Ma J. Adaptation of the length scale and amplitude of the Bicoid gradient profile to achieve robust patterning in abnormally large Drosophila melanogaster embryos. Development. 2014;141:1–12. doi: 10.1242/dev.098640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inomata H, Shibata T, Haraguchi T, Sasai Y. Scaling of dorsal-ventral patterning by embryo size-dependent degradation of Spemann’s organizer signals. Cell. 2013;153:1296–311. doi: 10.1016/j.cell.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Ben-zvi D, Shilo B, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–11. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 9.Alaynick WA, Jessell TM, Pfaff SL. SnapShot: Spinal Cord Development. Cell. 2011;146:178. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dessaud E, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–20. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 11.Balaskas N, et al. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell. 2012;148:273–84. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das RM, Storey KG. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science. 2014;343:200. doi: 10.1126/science.1247521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deneen B, et al. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–68. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development. 2005;132:143–54. doi: 10.1242/dev.01566. [DOI] [PubMed] [Google Scholar]
- 15.Oliver ER, Saunders TL, a Tarlé S, Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–20. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 2005;283:113–27. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Boehm B, et al. The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol. 2010;8:e1000420. doi: 10.1371/journal.pbio.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis T, aEdgar B. Negative regulation of dE2F1 by cyclin-dependent kinases controls cell cycle timing. Cell. 2004;117:253–64. doi: 10.1016/s0092-8674(04)00247-8. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, et al. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J. Cell Biol. 2011;195:1047–60. doi: 10.1083/jcb.201104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leber M, Sanes J. Migratory Cord Paths of Neurons and Glia in the Embryonic Chick Spinal. J. Neurosci. 1995;15:1236–1248. doi: 10.1523/JNEUROSCI.15-02-01236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erskine L, Patel K, Clarke JDW. Progenitor Dispersal and the Origin of Early Neuronal Phenotypes in the Chick Embryo Spinal Cord. Dev. Biol. 1998;41:26–41. doi: 10.1006/dbio.1998.8912. [DOI] [PubMed] [Google Scholar]
- 22.Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev. Biol. 2012;366:34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Dessaud E, et al. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010;8:e1000382. doi: 10.1371/journal.pbio.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Megason SG, Mcmahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 25.Wilcock AC, Swedlow JR, Storey KG. Mitotic spindle orientation distinguishes stem cell and terminal modes of neuron production in the early spinal cord. Development. 2007;134:1943–54. doi: 10.1242/dev.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkić M, et al. The p53 tumor suppressor causes congenital malformations in Rpl24-deficient mice and promotes their survival. Mol. Cell. Biol. 2009;29:2489–504. doi: 10.1128/MCB.01588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takebayashi H, et al. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002;12:1157–63. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- 28.Novitch BG, Chen AI, Jessell TM, York N. Coordinate Regulation of Motor Neuron Subtype Identity and Pan-Neuronal Properties by the bHLH Repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 29.Erhardt J, Pittman RN. Ectopic p21WAF1 Expression Induces Differentiation-specific Cell Cycle Changes in PC12 Cells Characteristic of Nerve Growth Factor Treatment. J. Biol. Chem. 1998;273:23517–23523. doi: 10.1074/jbc.273.36.23517. [DOI] [PubMed] [Google Scholar]
- 30.Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–34. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang P, Xiong F, Megason SG, Schier AF. Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord. PLoS Genet. 2012;8:e1002762. doi: 10.1371/journal.pgen.1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lander AD, Gokoffski KK, Wan FYM, Nie Q, Calof AL. Cell lineages and the logic of proliferative control. PLoS Biol. 2009;7:e15. doi: 10.1371/journal.pbio.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martynoga B, Drechsel D, Guillemot F. Molecular control of neurogenesis: a view from the mammalian cerebral cortex. Cold Spring Harb. Persp Biol. 2012;4:a008359. doi: 10.1101/cshperspect.a008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 2003;116:4947–55. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- 35.Marklund U, et al. Domain-specific control of neurogenesis achieved through patterned regulation of Notch ligand expression. Development. 2010;137:437–45. doi: 10.1242/dev.036806. [DOI] [PubMed] [Google Scholar]
- 36.Lee S-K, Lee B, Ruiz EC, Pfaff SL. Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev. 2005;19:282–94. doi: 10.1101/gad.1257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saade M, et al. Sonic hedgehog signaling switches the mode of division in the developing nervous system. Cell Rep. 2013;4:492–503. doi: 10.1016/j.celrep.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 38.Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Gen Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 39.Wartlick O, Kicheva A, González-Gaitán M. Morphogen gradient formation. Cold Spring Harb Perspect Biol. 2009;1:a001255. doi: 10.1101/cshperspect.a001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribes V, et al. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010;24:1186–200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasai N, Kutejova E, Briscoe J. Integration of Signals along Orthogonal Axes of the Vertebrate Neural Tube Controls Progenitor Competence and Increases Cell Diversity Noriaki. PLoS Biol. 2014 doi: 10.1371/journal.pbio.1001907. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ericson J, Morton S, Kawakami a, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–73. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 43.Lewis NE, Rossant J. Mechanism of size regulation in mouse embryo aggregates. J. Embryol. Exp. Morphol. 1982;72:169–181. [PubMed] [Google Scholar]
- 44.Rands GF. Size regulation in the mouse embryo II. The development of half embryos. J. Embryol. Exp. Morphol. 1986;98:209–217. [PubMed] [Google Scholar]
- 45.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 2006;98:1547–54. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 46.Sakaue-Sawano A, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–98. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 47.Southard J, Eicher E. Belly spot and tail (Bst) Mouse. News Lett. 1977;56:40. [Google Scholar]
- 48.Gomez C, et al. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- 49.Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88 [PubMed] [Google Scholar]
- 50.Poirier K, et al. Neuroanatomical distribution of ARX in brain and its localisation in GABAergic neurons. Brain Res Mol Brain Res. 2004;122:35–46. doi: 10.1016/j.molbrainres.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Tsuchida T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–70. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 52.Forer A, Pickett-Heaps JD. Cytochalasin D and latrunculin affect chromosome behaviour during meiosis in crane-fly spermatocytes. Chromosom. Res. 1998;6:533–49. doi: 10.1023/a:1009224322399. [DOI] [PubMed] [Google Scholar]
- 53.Januschke J, Gonzalez C. The interphase microtubule aster is a determinant of asymmetric division orientation in Drosophila neuroblasts. J. Cell Biol. 2010;188:693–706. doi: 10.1083/jcb.200905024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman SC, Collingon J, Schoenwolf GC, Lumsden A. Improved Method for Chick Whole-Embryo Culture Using a Filter Paper Carrier. Dev. Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 55.Gregor T, Tank DW, Wieschaus EF, Bialek W. Probing the limits to positional information. Cell. 2007;130:153–64. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith CL, Dendy PP. Relation between mitotic index, duration of mitosis, generation time and fraction of dividing cells in a cell population. Nature. 1962;193:555–6. doi: 10.1038/193555a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.