Highlights
-
•
Comprehensive pre-clinical immunogenicity evaluation of HPV L1 major capsid protein.
-
•
Majority neutralizing antibody response was genotype-specific.
-
•
Reciprocal cross-neutralization between some Alpha-7 and Alpha-9 genotypes.
-
•
Tetravalent formulation (HPV16/18/39/58) induced broadly neutralizing antibodies.
-
•
These data improve our understanding of the antigenic diversity of the L1 protein.
Keywords: Human papillomavirus, Major capsid protein, L1, Antibody, Antigenicity, Diversity
Abstract
Human papillomavirus (HPV) vaccines confer protection against the oncogenic genotypes HPV16 and HPV18 through the generation of type-specific neutralizing antibodies raised against the constituent virus-like particles (VLP) based upon the major capsid proteins (L1) of these genotypes. The vaccines also confer a degree of cross-protection against some genetically related types from the Alpha-9 (HPV16-like: HPV31, HPV33, HPV35, HPV52, HPV58) and Alpha-7 (HPV18-like: HPV39, HPV45, HPV59, HPV68) species groups. The mechanism of cross-protection is unclear but may involve antibodies capable of recognizing shared inter-genotype epitopes. The relationship(s) between the genetic and antigenic diversity of the L1 protein, particularly for non-vaccine genotypes, is poorly understood.
We carried out a comprehensive evaluation of the immunogenicity of L1 VLP derived from genotypes within the Alpha-7 and Alpha-9 species groups in New Zealand White rabbits and used L1L2 pseudoviruses as the target antigens in neutralization assays.
The majority antibody response against L1 VLP was type-specific, as expected, but several instances of robust cross-neutralization were nevertheless observed including between HPV33 and HPV58 within the Alpha-9 species and between HPV39, HPV59 and HPV68 in the Alpha-7 species. Immunization with an experimental tetravalent preparation comprising VLP based upon HPV16, HPV18, HPV39 and HPV58 was capable of generating neutralizing antibodies against all the Alpha-7 and Alpha-9 genotypes. Competition of HPV31 and HPV33 cross-neutralizing antibodies in the tetravalent sera confirmed that these antibodies originated from HPV16 and HPV58 VLP, respectively, and suggested that they represent minority specificities within the antibody repertoire generated by the immunizing antigen. These data improve our understanding of the antigenic diversity of the L1 protein per se and may inform the rational design of a next generation vaccine formulation based upon empirical data.
1. Introduction
Human papillomavirus (HPV) vaccines, Cervarix® and Gardasil®, comprise virus-like particles (VLP) based upon the major capsid protein (L1) of HPV16 and HPV18 and are highly efficacious at preventing persistent infection and more progressive disease associated with these two high risk genotypes in clinical trials [1]. Gardasil® also contains VLP representing HPV6 and HPV11, the principal genotypes associated with genital warts. HPV16 and HPV18 account for ca. 70% of cervical cancers worldwide [2], [3] and recent epidemiological data for Australia [4], the USA [5] and the UK [6], [7] demonstrate reductions in the prevalence of these two genotypes following the introduction of national HPV vaccination programs. Neutralizing antibodies against HPV16 and HPV18 can be detected in the serum and cervicovaginal secretions of vaccinees [8], [9], [10] and passive transfer of immune sera, purified immunoglobulin (IgG) and monoclonal antibodies (MAbs) can protect animals against papillomavirus challenge [11], [12], [13]. These observations have led to the reasonable assumption that vaccine-induced, type-specific protection is mediated by neutralizing antibodies [1], [14].
Cross-protection has also been demonstrated against some genotypes within the Alpha-papillomavirus species groups, Alpha-9 (HPV16-like: HPV31, HPV33, HPV35, HPV52, HPV58) and Alpha-7 (HPV18-like: HPV39, HPV45, HPV59, HPV68) [1], [15], [16] and coincides with the detection of cross-neutralizing antibodies in the serum and cervicovaginal secretions of vaccinees [10], [17], [18], [19], [20]. Such antibodies may be effectors, or their detection may have utility as a correlate or surrogate of vaccine-induced cross-protection [21].
The development of potential next generation vaccines to improve the breadth of genotype coverage [1], [22] is based upon two approaches: improving the immunogenicity of a conserved region of the minor capsid protein (L2) to generate broadly neutralizing antibodies [23], and using a multivalent L1 VLP-based vaccine that induces type-specific antibodies against a wider array of HPV genotypes (HPV6, HPV11, HPV16, HPV18, HPV31, HPV33, HPV45, HPV52, HPV58; V503, Merck Research Laboratories). The latter approach is the most advanced and early clinical trial data show promising immunogenicity and efficacy profiles [24], whereas L2-based candidate vaccines are currently in pre-clinical development [23]. Reduced dosing schedules for the current HPV vaccines are also being investigated with data suggesting non-inferiority of type-specific antibody responses, although there is an impact on the development of cross-neutralizing antibodies [10], [25], [26], [27].
Early pre-clinical immunogenicity [28], [29], [30] and MAb reactivity [17] data suggest a degree of inter-genotype antigenic similarity within the Alpha-7 and Alpha-9 species groups. The extent of this antibody cross-reactivity is unclear as only a limited number of immunogens and target antigens have been used. Some of these data have been generated using L1-based targets [28], rather than pseudovirus targets bearing both the L1 and L2 proteins, with both proteins being necessary for efficient infectivity and the appropriate presentation of L1 conformational epitopes [23], [31], [32].
We carried out a comprehensive pre-clinical evaluation of the immunogenicity of L1 VLP derived from multiple HPV genotypes within the Alpha-7 and Alpha-9 species groups and used L1L2 pseudoviruses, representing these same genotypes, as the target antigens in neutralization assays. Such data should improve our understanding of the antigenic diversity of the L1 protein per se and may inform the design of a next generation vaccine formulation that encompasses a limited number of antigens based upon empirical data.
2. Materials and methods
2.1. L1 VLP immunogens
Cervarix® was obtained through the National Vaccine Evaluation Consortium, UK.
L1 VLP representing Alpha-7 and Alpha-9 HPV genotypes and control Bovine Papillomavirus (BPV) were expressed using the Bac-to-Bac® Baculovirus System (Life Technologies), as previously described [33], [34], wherein the L1 genes shared 100% amino acid sequence identity with the L1 genes of the pseudovirus clones [20] used for the neutralization assay (see Section 2.3).
2.2. Immunization protocols
Five week old female BALB/c mice were immunized with saline (naïve) or 1/10th (2 μg each HPV16 and HPV18 VLP) the human dose equivalent of Cervarix® [35] by the intramuscular (IM) or sub-cutaneous (SC) routes. Two schedules were investigated whereby immunizations were carried out at week (W) 0, W3 and W7 or at W0, W4 and W12.
Eight to ten week old female New Zealand White (NZW) rabbits were immunized subcutaneously with saline (naïve) or 1/4th (5 μg each HPV16 and HPV18 VLP) the human dose equivalent of Cervarix® at W0, W4 and W12.
Eight to ten week old female NZW rabbits were immunized subcutaneously with 5 μg each of the indicated in house L1 VLP (or 5 μg each of HPV16, HPV18, HPV39 and HPV58 for the tetravalent preparation). VLP were absorbed onto 3% alhydrogel (250:1 (v/v), Superfos Biosector) for 1–2 h at room temperature under gentle rotation. For the final preparation of the rabbit inoculum, the VLP-alhydrogel mix was diluted in sodium phosphate buffer pH 6.5 (final concentration 2.7 mM NaH2PO4 and 3.3 mM Na2HPO4) with 150 mM NaCl, alhydrogel (250 μg/mL Al3+), Sigma Adjuvant System (25 μg/mL monophosphoryl lipid), and incubated with gentle rotation at room temperature for a minimum of 15 min. Rabbits received additional immunizations at W4 and W12.
In all cases, serum samples were collected prior to the first immunization (pre-immunization) and two weeks following both the second and third doses.
All animal husbandry and regulated procedures were carried out in strict accordance with UK Home Office guidelines and governed by the Animals (Scientific Procedures) Act 1986 which complies with the EC Directive 2010/63/EU and performed under licences PPL 80/2537 and PPL 70/6562-3 granted only after review of all the procedures in the licence by the local Animal Welfare and Ethical Review Bodies.
2.3. Neutralization assay
L1L2 pseudoviruses representing Alpha-7 and Alpha-9 HPV genotypes and BPV, and carrying a luciferase reporter, were expressed from transiently transfected 293TT cells, purified and characterized as previously described [20], [36]. The equivalent of a Tissue Culture Infectious Dose 50% (TCID50) was estimated using the Spearman-Karber equation and a standardized input of 300 TCID50 was used for all pseudoviruses. Serum samples were serially diluted and the 80% reciprocal neutralization titer estimated by interpolation. Heparin (H-4784; Sigma–Aldrich, UK) was included as a positive inhibitor control and as an indicator of inter-assay reproducibility. The median (Inter-quartile range, IQR) inhibitory concentrations (μg/mL) were as follows: HPV16 11.9 (9.5–22.3; n = 7), HPV31 5.1 (3.3–8.1; 6), HPV33 13.1 (7.4–19.4; 6), HPV35 3.1 (2.9–4.9; 6), HPV52 25.2 (13.6–31.9; 6), HPV58 8.2 (3.6–19.4; 6), HPV18 3.9 (3.4–5.0; n = 6) HPV39 5.8 (4.0–7.2; 5), HPV45 3.7 (3.5–3.9; 6), HPV59 13.6 (11.7–16.3; 6), HPV68 7.0 (6.5–12.1; 6) and BPV 73.5 (59.1–75.9; 5).
2.4. Competition of neutralizing antibodies using L1 VLP
Serial dilutions of selected final bleed rabbit sera were pre-incubated for 1hr at room temperature with 2 μg of L1 VLP (HPV16, HPV31, HPV33 or HPV58), followed by addition of 300 TCID50 of L1L2 pseudoviruses representing the same HPV genotypes for 1 h at room temperature, before being transferred to 293TT cells for 72 h at 37 °C. The 80% reciprocal neutralization titers were estimated by interpolation.
2.5. Data analyses
For the comparison of inter-genotype neutralization data a heatmap representation of log10 titers (range 1.0–6.0 log10) was employed with titers below the assay threshold of 20 being censored with a value of 10 (1.0 log10). The phylogenetic relationship between L1 amino acid sequences (neighbor-joining [NJ] tree) and inter-genotype distance matrices (n = 500 bootstrap replicates; heatmap range 0.0–1.0) were created using Mega v4.1 [37].
3. Results
3.1. Immunization of BALB/c mice and NZW rabbits with Cervarix®
As both HPV vaccines consistently generate HPV31 cross-neutralizing antibodies following immunization, we used this as a benchmark for selecting an appropriate animal model for our pre-clinical immunization studies. BALB/c mice were immunized intra-muscularly with Cervarix® over a 7 week schedule resulting in a median HPV16 neutralizing antibody titer of 10,416 (IQR 7943–16,862; n = 10) (Fig. 1). Cross-neutralization of HPV31, however, was only apparent in one mouse (HPV31 titer of 733) with a very high HPV16 neutralizing titer of 543,122. Cervarix® immunization of BALB/c mice sub-cutaneously or intra-muscularly over a 12 week schedule did not elicit neutralizing antibodies against HPV31 (data not shown). Conversely, immunization of NZW rabbits with Cervarix® over the same 12 week schedule generated a median HPV16 neutralizing antibody titer of 40,792 (IQR 28,214–57,869; n = 8) accompanied by a median HPV31 titer of 152 (IQR 35–346; n = 8). Although differences in dosing levels between mice and rabbits may impact on the antibody responses elicited here, HPV31 neutralizing antibody titers generated in rabbits were similar to the titers found in human vaccinees (Fig. 1) [20], suggesting that NZW rabbits were an appropriate model for examining the generation of cross-neutralizing antibodies following immunization with L1 VLP.
Fig. 1.
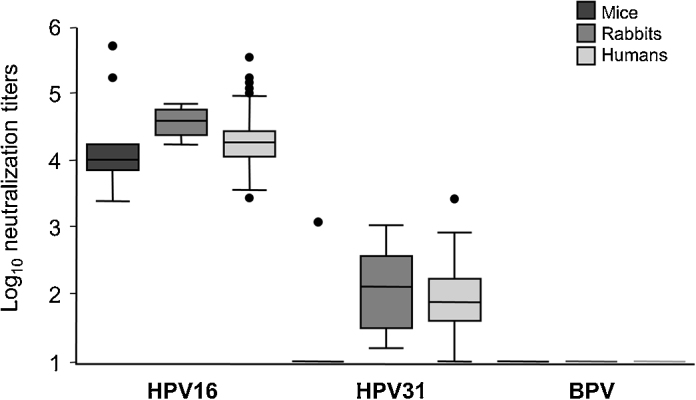
Neutralizing antibody responses following Cervarix® immunization of mice, rabbits and humans. Box and whisker plot of type-specific (HPV16), cross-neutralizing (HPV31) and non-specific control (BPV) responses following immunization of BALB/c mice (3 × 1/10 dose), NZW rabbits (3 × 1/4 dose) and humans (3 × 1 dose; reproduced from [20] for comparison purposes) with indicated dose of Cervarix® HPV vaccine.
3.2. Immunization of NZW rabbits with individual Alpha-7 and Alpha-9 L1 VLP
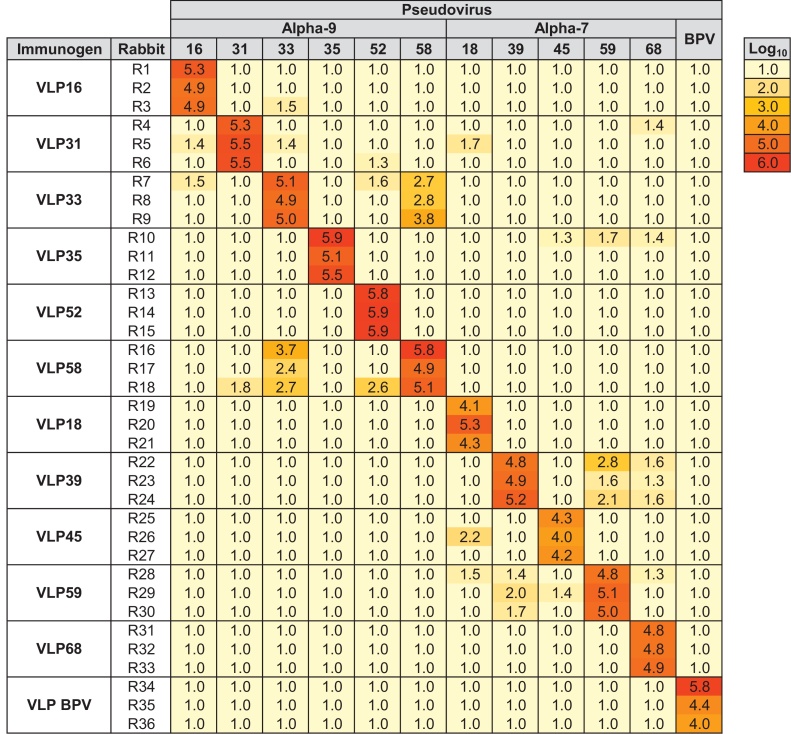
NZW rabbits were immunized with L1 VLP representing individual HPV genotypes from the Alpha-7 and Alpha-9 species groups and the control BPV. As expected, immunization with L1 VLP induced predominantly high titer neutralizing antibodies against the immunizing genotype resulting in a median type-specific titer of 100,287 (IQR 64,478–246,691) (Fig. 2). However, there were several cases wherein L1 VLP elicited antibodies capable of neutralizing pseudoviruses representing other genotypes. Some of these responses were weak and sporadic, while some were of a reasonable titer and consistent between animals in the same group. For example, HPV33 and HPV58 appeared to share common neutralization epitopes resulting in a median reciprocal neutralization titer of 553 (IQR 520–3594). Similarly, although of a lesser magnitude, VLP representing HPV39 and HPV59 also appeared to share common neutralization epitopes.
Fig. 2.
Heatmap of type-specific and cross-neutralizing antibody responses against Alpha-7 and Alpha-9 pseudoviruses by serum from NZW rabbits immunized with individual VLP. Log10 neutralization titers of sera from NZW rabbits (n = 3) following immunization with three doses of indicated VLP and tested against indicated Alpha-7, Alpha-9 or BPV pseudoviruses. Key indicates log10 heatmap gradient.
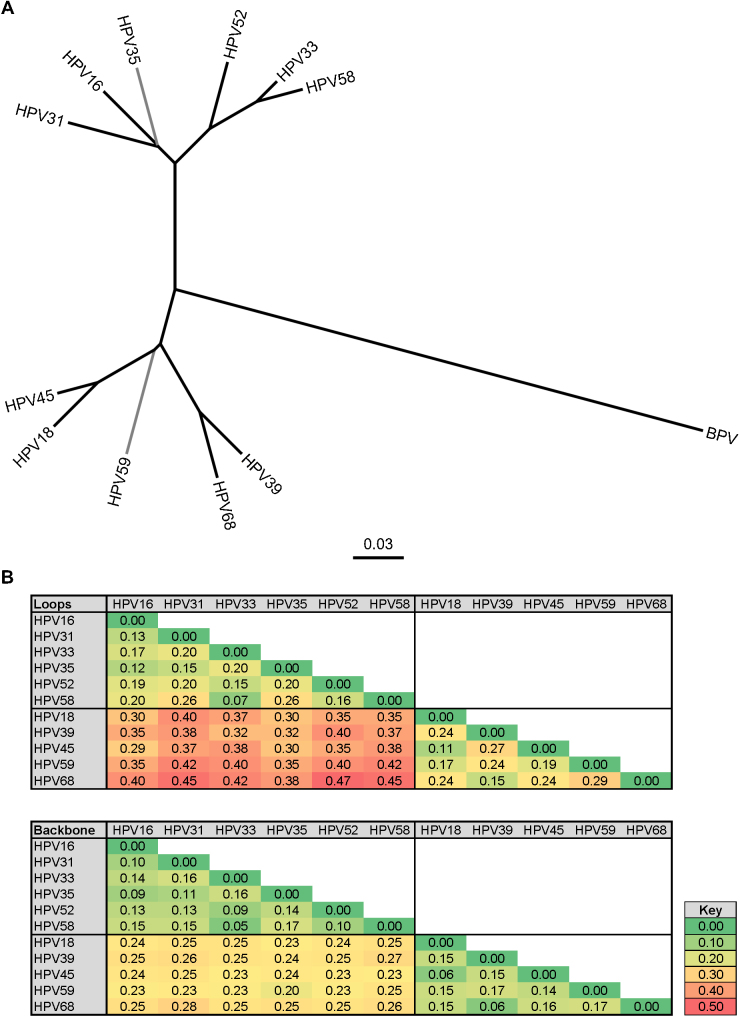
A phylogenetic representation of the amino acid sequences used for the Alpha-7 and Alpha-9 VLP and pseudovirus L1 proteins demonstrates the close relationship between certain genotypes within each of these two species groups (Fig. 3A). The amino acid sequences of the surface-exposed loops [38], [39] are likely to contribute to the antigenic similarity and distinction between HPV genotypes (Fig. 3B). The median intra-species group surface-exposed loop genetic distances for these Alpha-7 and Alpha-9 L1 sequences were similar at 0.19 (IQR 0.15–0.20) and 0.24 (0.18–0.24), respectively (p = 0.146), and substantially lower than the median inter-species genetic distance of 0.37 (0.35–0.40; p < 0.001). Within the Alpha-9 species group, the antigenic similarity between HPV33 and HPV58 is perhaps reflected in the low genetic distance between these genotypes. The apparent antigenic relationship between HPV39 and HPV59 within the Alpha-7 species group, however, is not similarly reflected by low genetic distances.
Fig. 3.
Phylogenetic relationship between the major capsid proteins of the Alpha-7 and Alpha-9 genotypes. (A) Amino acid sequences of the L1 major capsid proteins representing both VLP and pseudoviruses of the Alpha-7 (HPV18, HPV39, HPV45, HPV59, HPV68) and Alpha-9 (HPV16, HPV31, HPV33, HPV35, HPV52, HPV58) genotypes and the control BPV [20]. Radial representation of NJ tree with branches having less than 80% bootstrap (n = 500 replicates) support (HPV35 and HPV59) indicated in gray. (B) Heatmap representation of inter-genotype genetic distances based upon external surface loops only or remaining (non-loop) backbone for the L1 proteins of the indicated Alpha-7 and Alpha-9 genotypes. Key indicates heatmap gradient.
There were other sporadic instances of weaker cross-neutralization, for example between HPV16, HPV31 and HPV33. Interpretation of these weaker responses, however, has to be tempered by the observation that three of the thirty-six rabbits generated weak inter-species responses: two animals immunized with HPV31 VLP (one with cross-reactivity against HPV18 and one against HPV68) and one animal immunized with HPV35 VLP (cross-reactivity against HPV45, HPV59 and HPV68). Weak intra-species group responses are intuitively likely to be genuine, but given the inter-species genetic distances in the surface-exposed loops (Fig. 3B) weak inter-species responses should be interpreted with some caution.
Pre-immune sera were negative for neutralizing antibodies against all Alpha-7 and Alpha-9 HPV pseudoviruses and the control BPV (data not shown).
3.3. Immunization with tetravalent immunogen
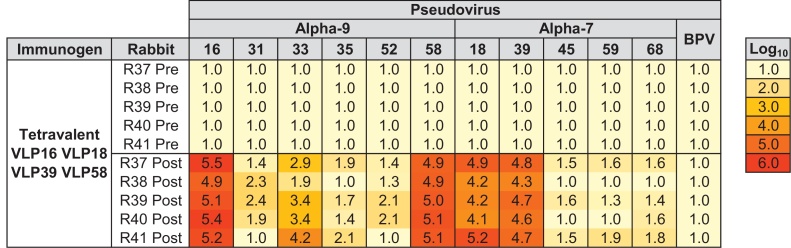
A tetravalent preparation containing HPV16, HPV18, HPV39 and HPV58 VLP was used to immunize a group of five NZW rabbits following the same schedule as that for the individual immunizations (Fig. 4). All five rabbits generated high titer neutralizing antibodies against the immunizing genotypes HPV16, HPV18, HPV39 and HPV58 and the titers were similar to those obtained when used as individual immunogens with median individual and tetravalent type-specific neutralization titers for HPV16 (80,813 vs. 161,025), HPV18 (21,941 vs. 17,637), HPV39 (86,678 vs. 53,612) and HPV58 (140,129 vs. 105,258) as indicated (Fig. 2, Fig. 4). Conversely, the breadth of cross-neutralization seen against the Alpha-7 and particularly the Alpha-9 pseudoviruses was greater than when VLP were used individually: all five rabbits generated neutralizing antibodies against HPV33 and three to four of five rabbits also generated neutralizing antibodies against HPV31, HPV35, HPV45, HPV52, HPV59 and HPV68. None of the five rabbits generated antibodies capable of neutralizing BPV and pre-immune sera were negative for neutralizing antibodies against all Alpha-7 and Alpha-9 HPV pseudoviruses.
Fig. 4.
Heatmap of type-specific and cross-neutralizing antibody responses against Alpha-7 and Alpha-9 pseudoviruses by serum from NZW rabbits immunized with tetravalent VLP. Log10 neutralization titers of sera against indicated Alpha-7, Alpha-9 or BPV pseudoviruses taken from NZW rabbits (n = 5) prior to (Pre) and following (Post) immunization with three doses of tetravalent VLP formulation. Key indicates log10 heatmap gradient.
3.4. Specificity of cross-reactive antibodies
To establish which of the HPV16 and/or HPV58 VLP immunogen(s) were responsible for the generation of the cross-neutralizing antibody responses against HPV31 and HPV33 we used VLP as competing antigens in neutralization tests (Table 1 and Supplemental Fig. S1). For this purpose, serum from animals R38, R39 and R40 were selected based upon their high HPV31 and HPV33 neutralizing antibody titers.
Table 1.
Specificity of rabbit neutralizing antibodies elicited to tetravalent immunogen.a
| Rabbit | Competing antigen | Fold reduction in neutralizing titer to indicated pseudovirus (PSV) by addition of VLP compared to no VLP controlb |
|||
|---|---|---|---|---|---|
| PSV16 | PSV31 | PSV33 | PSV58 | ||
| R38 | VLP16 | 169 | 19 | ≤1 | ≤1 |
| VLP31 | ≤1 | 19 | ≤1 | ≤1 | |
| VLP33 | ≤1 | ≤1 | ≤1 | ≤1 | |
| VLP58 | ≤1 | ≤1 | ≤1 | 455 | |
| R39 | VLP16 | 1052 | 26 | ≤1 | ≤1 |
| VLP31 | ≤1 | 10 | ≤1 | ≤1 | |
| VLP33 | ≤1 | ≤1 | 39 | ≤1 | |
| VLP58 | ≤1 | ≤1 | 76 | 579 | |
| R40 | VLP16 | 304 | ≤1 | ≤1 | ≤1 |
| VLP31 | ≤1 | 2 | ≤1 | ≤1 | |
| VLP33 | ≤1 | ≤1 | 17 | ≤1 | |
| VLP58 | ≤1 | ≤1 | 96 | 398 | |
Tetravalent immunogen comprised VLP derived from HPV16, HPV18, HPV39 and HPV58.
Fold reduction in neutralizing titer of indicated serum (R38, R39 or R40) against indicated pseudovirus target following pre-incubation with indicated competing antigen compared to control. Reductions of ≥10 fold are indicated in bold type.
Supplementary Fig. S1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2014.07.116.
Type-specific and cross-neutralizing antibody specificity. Neutralizing antibody capacity of tetravalent rabbit sera following pre-incubation (competition) with indicated VLP (red bars) compared to no VLP control (blue bars) against indicated pseudovirus (PsV) target.
Pre-incubation with HPV16 and HPV58 VLP reduced neutralizing antibody titers against their respective pseudoviruses by a median 427-fold (or 2.6 log10). For the two animals, R38 and R39, that had the highest levels of HPV31 neutralizing antibodies (Fig. 4), competition with HPV16 or HPV31 VLP, but not HPV33 or HPV58 VLP, reduced neutralizing antibody titers against HPV31 pseudovirus. Similarly, for animals R39 and R40 only competition with HPV33 or HPV58 VLP reduced the HPV33 neutralizing antibody titer. These data corroborate the source of the cross-neutralizing antibodies, as expected (Fig. 2), and appear to discount any potential additive effect within the context of a tetravalent immunogen. In addition, competition for HPV31 and HPV33 neutralizing antibodies with HPV31 and HPV33 VLP, respectively, did not impact on the pseudovirus neutralization of the archetypal HPV16 and HPV58 pseudoviruses, respectively.
4. Discussion
We undertook a comprehensive evaluation of the antigenic and immunogenic properties of the major capsid proteins derived from HPV genotypes within the Alpha-7 and Alpha-9 species groups.
We immunized BALB/c mice and NZW rabbits with Cervarix® and compared the resulting HPV16, HPV31 and BPV neutralization titers to those generated in humans [20]. The virtual absence of HPV31 cross-neutralizing antibodies in mice sera, compared to the similar HPV31 neutralizing antibody titers generated in rabbits and humans, led us to select NZW rabbits as the host species for the remainder of the study.
The neutralization checkerboard derived using single VLP immunogens and pseudovirus target antigens corroborates and extends previous observations on the largely type-specific nature of VLP-derived neutralizing antibodies. However, we did observe reciprocal cross-neutralization between HPV33 and HPV58 and, to a lesser extent, between HPV39 and HPV59 suggesting some antigenic similarity between these genotypes. A genetic distance matrix of the amino acid sequences of the surface-exposed loops further clarified the relationships between these Alpha-7 and Alpha-9 genotypes [39], [40], [41] and suggested that the observed antigenic proximity of HPV33 and HPV58 may be reflected in the L1 amino acid sequence similarity of these two types, although the apparent reciprocal recognition between HPV39 and HPV59 is less obvious from the phylogenetic relationship between these two types.
A shortcoming to this study was that we did not observe consistent cross-neutralization of HPV31 and HPV45 following immunization with individual VLP representing HPV16 and HPV18, respectively, as expected [10], [17], [18], [19], [20]. This contrasts with the generation of HPV31 antibodies in NZW rabbits following immunization with Cervarix® and immunization with the tetravalent preparation that generated a broad response, including cross-neutralization of HPV31 and HPV45 pseudoviruses. There are possible reasons for these discrepancies, including potential differences in the exact VLP and adjuvant formulations between the individual and tetravalent preparations, the potential sub-optimal immunostimulatory capacity of commercial adjuvants and in house formulation, the variability inherent in using small groups of animals and the possibility of differential immunogenicity when certain VLP are used in combination, not apparent when used individually. The type-specific neutralization titers against HPV16, HPV18, HPV39 and HPV58 were similar in the individual and tetravalent preparations, suggesting that any formulation differences were quite subtle. These data also suggest that the type-specific responses did not suffer from immune interference, as has been reported from the use of other multivalent preparations containing HPV58 VLP [42]. We did not test other multivalent formulations using other combinations of antigens which may have been informative.
Few MAbs have been generated against VLP from genotypes other than HPV6, HPV11, HPV16 and HPV18 [40], [43], [44], therefore data on the antigenicity of the L1 protein is largely limited to these genotypes. MAbs capable of binding L1 proteins representing multiple genotypes from the same species group can be found [40], [44]. However, apart from cross-neutralization between HPV18 and HPV45 which appears to be replicated by available MAbs [17], [40], no other inter-genotype cross-neutralizing MAbs have been identified. Little is known about the specificity of antibodies elicited by the current HPV vaccines except that cross-reactive antibodies are derived from the immunizing HPV16 and HPV18 VLP [45], as expected, and that cross-neutralizing antibodies against genotypes in the Alpha-9 species group appear to be a minority population [33]. In the present study, competition of HPV31 and HPV33 neutralizing antibodies by addition of homologous VLP and the lack of an impact on the archetypal HPV16 and HPV58 pseudovirus neutralization titers, respectively, appear to corroborate observations [33] that cross-neutralizing antibodies comprise minor specificities within the antibody repertoire elicited following VLP immunization. However, differential affinities for the immunizing and target antigens cannot be ruled out by this approach.
Cross-neutralizing antibody titers generated by HPV33 or HPV58 in the individual preparations (or by HPV58 in the tetravalent preparation) were an order of magnitude higher than those elicited by HPV16 VLP against HPV31 pseudovirus in the tetravalent preparation. If cross-neutralizing antibodies against HPV31 detected in the serum and genital secretions of vaccinees [10], [18], [19], [20] are involved in mediating cross-protection [1], [15], [16] then one could postulate that the higher levels of cross-neutralizing antibodies elicited by immunization with HPV33 (or HPV58) would be at least equally protective. A similar judgment could be leveled at HPV39 VLP which generated neutralizing antibodies against HPV59 and HPV68. These data suggest that a multivalent next generation vaccine could perhaps be optimized to generate antibodies capable of recognizing a wide array of Alpha-7 and Alpha-9 HPV genotypes with a limited number of L1 VLP immunogens.
Alternatively, these data could also be used to support the approach of a multivalent next generation vaccine that wholly relies on the generation of high titer type-specific antibodies. A next generation HPV vaccine comprising multiple VLP, such as the V503 vaccine candidate [24], is likely to provide greater coverage than the current bivalent (Cervarix®) and quadrivalent (Gardasil®) HPV vaccines [46]. Two other next generation VLP-based vaccine candidates may also be in the pipeline: one containing HPV16, HPV18, HPV31 and HPV45 VLP and another comprising HPV16, HPV18, HPV33 and HPV58 VLP [47]. There are significant cost implications for such vaccines though these may be mitigated by observations that type-specific antibody titers following reduced dosing schedules of the current HPV vaccines were non-inferior to those generated under the standard three dose schedule [25], [26], [27]. Fewer than three vaccine doses, however, may impact on the generation of cross-neutralizing antibodies [10], [25] due to their reduced kinetics and the low levels found in the serum and genital secretions of vaccinees compared to vaccine type antibodies [10], [18], [19], [33], [48]. Given the low and possibly transient levels of cross-neutralizing antibodies generated by immunization with VLP, a single dose of a multivalent vaccine may be sufficient to elicit appropriate high titer, type-specific antibodies against a range of incorporated genotypes.
In summary, these data clarify the extent of antigenic diversity of the major capsid proteins of HPV genotypes that segregate into the Alpha-7 and Alpha-9 species groups, have implications for the optimized composition of next generation HPV vaccines based upon L1 VLP and contribute to our understanding of the immunogenicity of the major capsid protein of HPV.
Acknowledgments
This work was supported by the UK Medical Research Council (grant number G0701217). We are indebted to Prof. John T. Schiller and Dr. Chris Buck (National Cancer Institute, Bethesda, U.S.A.) and Dr. H Faust and Prof. J. Dillner (Malmö University Hospital, Malmö, Sweden) for access to the majority of the pseudovirus clones used in this study. We thank GlaxoSmithKline Biologicals SA for the donation of VLP and AS04 for use in pilot formulation studies of the in house VLP preparations for the rabbit immunizations. We thank Professor E. Miller from the National Vaccine Evaluation Consortium (NVEC) for the HPV vaccine Cervarix® used in this study, Professor J.V. Parry (PHE) for helpful discussion and Nicky Jones and Kate Breed (NIBSC) for technical support.
Conflict of interest statement: The authors declare no conflicts of interest.
References
- 1.Schiller J.T., Castellsague X., Garland S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl. 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 3.Li N., Franceschi S., Howell-Jones R., Snijders P.J., Clifford G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2010;128:927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 4.Tabrizi S.N., Brotherton J.M., Kaldor J.M., Skinner S.R., Cummins E., Liu B. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–1651. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz L.E., Hariri S., Lin C., Dunne E.F., Steinau M., McQuillan G. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 6.Mesher D., Soldan K., Howell-Jones R., Panwar K., Manyenga P., Jit M. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32:26–32. doi: 10.1016/j.vaccine.2013.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenberg P., Clifton S., Beddows S., Field N., Soldan K., Tanton C. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal) Lancet. 2013;382:1795–1806. doi: 10.1016/S0140-6736(13)61947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Edwards R.P., Zepp F. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccines. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 9.Kemp T.J., Garcia-Pineres A., Falk R.T., Poncelet S., Dessy F., Giannini S.L. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26:3608–3616. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper E., Bissett S.L., Howell-Jones R., Waight P., Soldan K., Jit M. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12-15 year old girls. PLOS ONE. 2013;8:e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzich J.A., Ghim S.J., Palmer-Hill F.J., White W.I., Tamura J.K., Bell J.A. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitburd F., Kirnbauer R., Hubbert N.L., Nonnenmacher B., Trin-Dinh-Desmarquet C., Orth G. Immunization with virus like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longet S., Schiller J.T., Bobst M., Jichlinski P., Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85:13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley M. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol. 2010;118:S2–S7. doi: 10.1016/j.ygyno.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler C.M., Castellsague X., Garland S.M., Szarewski A., Paavonen J., Naud P. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 16.Brown D.R., Kjaer S.K., Sigurdsson K., Iversen O.E., Hernandez-Avila M., Wheeler C.M. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.F., Brownlow M., Brown M., Kowalski R., Esser M.T., Ruiz W. Antibodies from women immunized with gardasil ((R)) cross-neutralize HPV 45 pseudovirions. Hum Vaccines. 2007;3:109–116. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 18.Kemp T.J., Hildesheim A., Safaeian M., Dauner J.G., Pan Y., Porras C. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29:2011–2014. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Fox B., Scholar S. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccines. 2011;7:1359–1373. doi: 10.4161/hv.7.12.18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Draper E., Bissett S.L., Howell-Jones R., Edwards D., Munslow G., Soldan K. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine. 2011;29:8585–8590. doi: 10.1016/j.vaccine.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plotkin S.A. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 22.Stanley M. Prospects for new human papillomavirus vaccines. Curr Opin Infect Dis. 2010;23:70–75. doi: 10.1097/QCO.0b013e328334c0e1. [DOI] [PubMed] [Google Scholar]
- 23.Wang J.W., Roden R.B. L2, the minor capsid protein of papillomavirus. Virology. 2013;445:175–186. doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joura E. Eurogin; Florence, Italy: 2013. Efficacy and immunogenicity of a novel 9-valent HPV L1 virus-like particle vaccine in 16 to 26 year old women. SS 8-4. [Google Scholar]
- 25.Safaeian M., Porras C., Pan Y., Kreimer A., Schiller J.T., Gonzalez P. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6:1242–1250. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobson S.R., McNeil S., Dionne M., Dawar M., Ogilvie G., Krajden M. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 27.Kreimer A.R., Rodriguez A.C., Hildesheim A., Herrero R., Porras C., Schiffman M. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combita A.L., Touze A., Bousarghin L., Christensen N.D., Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol. 2002;76:6480–6486. doi: 10.1128/JVI.76.13.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giroglou T., Sapp M., Lane C., Fligge C., Christensen N.D., Streeck R.E. Immunological analyses of human papillomavirus capsids. Vaccine. 2001;19:1783–1793. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- 30.Ochi H., Kondo K., Matsumoto K., Oki A., Yasugi T., Furuta R. Neutralizing antibodies against human papillomavirus types 16, 18, 31, 52, and 58 in serum samples from women in Japan with low-grade cervical intraepithelial neoplasia. Clin Vaccine Immunol. 2008;15:1536–1540. doi: 10.1128/CVI.00197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buck C.B., Day P.M., Trus B.L. The papillomavirus major capsid protein L1. Virology. 2013;445:169–174. doi: 10.1016/j.virol.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culp T.D., Spatz C.M., Reed C.A., Christensen N.D. Binding and neutralization efficiencies of monoclonal antibodies, Fab fragments, and scFv specific for L1 epitopes on the capsid of infectious HPV particles. Virology. 2007;361:435–446. doi: 10.1016/j.virol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bissett S.L., Draper E., Myers E.R., Godi A., Beddows S. Cross-neutralizing antibodies elicited by the Cervarix® human papillomavirus vaccine display a range of Alpha-9 inter-type specificities. Vaccine. 2014;32:1139–1146. doi: 10.1016/j.vaccine.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo Z., Bissett S.L., Giemza R., Beddows S., Oeser C., Lewis D.J. Systemic and mucosal immune responses to sublingual or intramuscular human papilloma virus antigens in healthy female volunteers. PLOS ONE. 2012;7:e33736. doi: 10.1371/journal.pone.0033736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannini S.L., Hanon E., Moris P., Van Mechelen M., Morel S., Dessy F. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Pastrana D.V., Buck C.B., Pang Y.Y., Thompson C.D., Castle P.E., FitzGerald P.C. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 38.Bishop B., Dasgupta J., Klein M., Garcea R.L., Christensen N.D., Zhao R. Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies. J Biol Chem. 2007;282:31803–31811. doi: 10.1074/jbc.M706380200. [DOI] [PubMed] [Google Scholar]
- 39.Chen X.S., Garcea R.L., Goldberg I., Casini G., Harrison S.C. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 40.Brown M.J., Seitz H., Towne V., Muller M., Finnefrock A.C. Development of neutralizing monoclonal antibodies for oncogenic HPV types 31, 33, 45, 52, and 58. Clin Vaccine Immunol. 2014;21:587–593. doi: 10.1128/CVI.00773-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter J.J., Wipf G.C., Benki S.F., Christensen N.D., Galloway D.A. Identification of a human papillomavirus type 16-specific epitope on the C-terminal arm of the major capsid protein L1. J Virol. 2003;77:11625–11632. doi: 10.1128/JVI.77.21.11625-11632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T., Xu Y., Qiao L., Wang Y., Wu X., Fan D. Trivalent Human Papillomavirus (HPV) VLP vaccine covering HPV type 58 can elicit high level of humoral immunity but also induce immune interference among component types. Vaccine. 2010;28:3479–3487. doi: 10.1016/j.vaccine.2010.02.057. [DOI] [PubMed] [Google Scholar]
- 43.Brendle S.A., Culp T.D., Broutian T.R., Christensen N.D. Binding and neutralization characteristics of a panel of monoclonal antibodies to human papillomavirus 58. J Gen Virol. 2010;91:1834–1839. doi: 10.1099/vir.0.017228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizk R.Z., Christensen N.D., Michael K.M., Muller M., Sehr P., Waterboer T. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J Gen Virol. 2008;89:117–129. doi: 10.1099/vir.0.83145-0. [DOI] [PubMed] [Google Scholar]
- 45.Scherpenisse M., Schepp R.M., Mollers M., Meijer C.J., Berbers G.A., van der Klis F.R. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLOS ONE. 2013;8:e74797. doi: 10.1371/journal.pone.0074797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van de Velde N., Boily M.C., Drolet M., Franco E.L., Mayrand M.H., Kliewer E.V. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104:1712–1723. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 47.Van Damme P., Leroux-Roels G., Simon P., Foidart J.M., Donders G., Hoppenbrouwers K. Effects of varying antigens and adjuvant systems on the immunogenicity and safety of investigational tetravalent human oncogenic papillomavirus vaccines: results from two randomized trials. Vaccine. 2014;32:3694–3705. doi: 10.1016/j.vaccine.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 48.Kemp T.J., Safaeian M., Hildesheim A., Pan Y., Penrose K.J., Porras C. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix((R)) Vaccine. 2012;31:165–170. doi: 10.1016/j.vaccine.2012.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Type-specific and cross-neutralizing antibody specificity. Neutralizing antibody capacity of tetravalent rabbit sera following pre-incubation (competition) with indicated VLP (red bars) compared to no VLP control (blue bars) against indicated pseudovirus (PsV) target.