Abstract
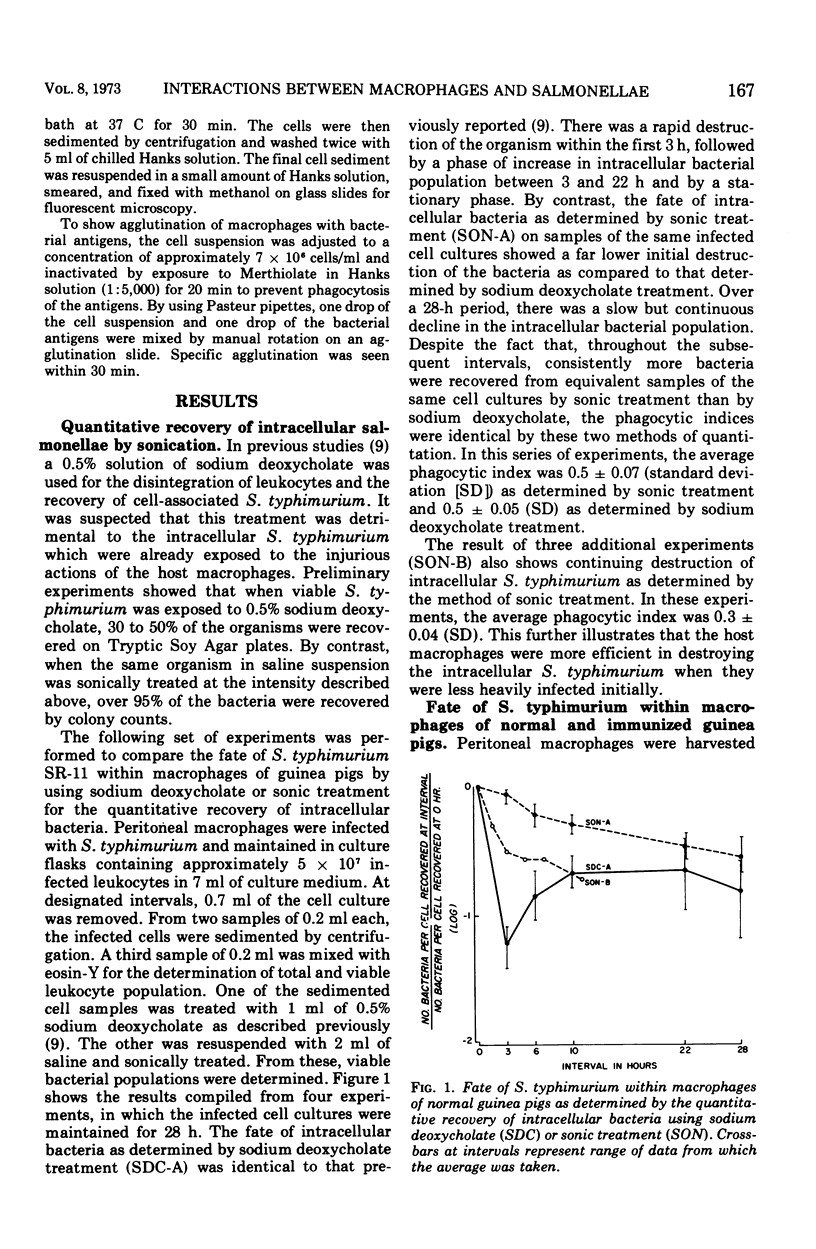
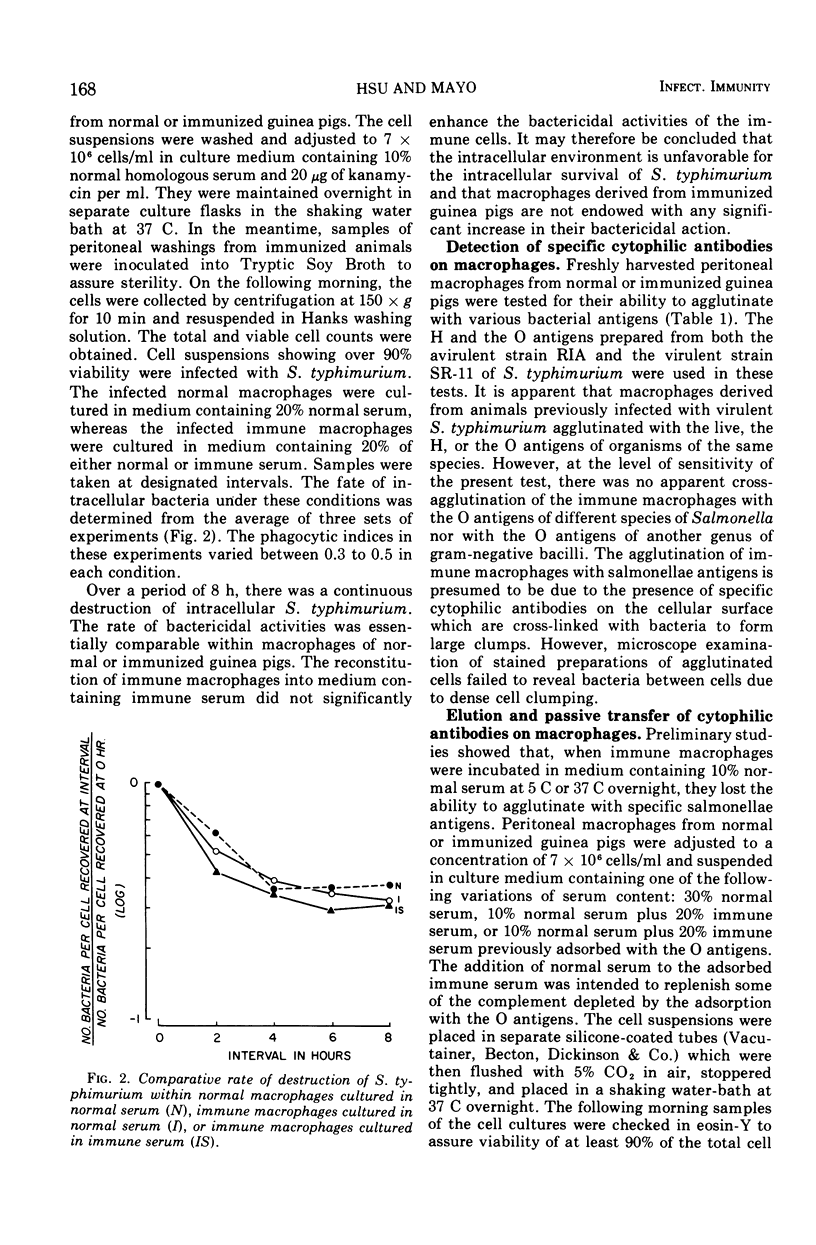
The fate of virulent Salmonella typhimurium within macrophages of guinea pigs was assessed by a suspended cell culture procedure. The present study confirmed that macrophages of normal guinea pigs were capable of inactivating the ingested salmonellae. Macrophages of previously infected guinea pigs were not endowed with any significant increase in their ability to eliminate the ingested pathogen. However, the immune macrophages were observed to clump together tightly when they were exposed to salmonellae. This phenomenon was attributed to the presence of specific cytophilic antibodies on the immune macrophages. When immune macrophages were inactivated with Merthiolate, they agglutinated with both the H and the O antigens of S. typhimurium, but not with the O antigens of other species of Salmonella nor with the O antigens of Escherichia coli. Cytophilic antibodies could be eluted from immune macrophages by incubation in the absence of immune serum. Conversely, cytophilic antibodies could be passively transferred onto normal macrophages by incubation in the presence of immune serum. Furthermore, using immune serum previously adsorbed with the O antigens of S. typhimurium, cytophilic antibodies against the H antigens alone could be transferred onto normal macrophages, or those against the O antigens alone could be eluted from immune macrophages. These data suggest that immune macrophages possess specific cytophilic antibodies against both the H and the O antigens of S. typhimurium. It is proposed that the presence of cytophilic antibodies on immune macrophages represents an expression of antibacterial cellular immunity by enhanced clumping and phagocytic activities of the macrophages.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Cronly-Dillon S. Comparative efficacy of whole and disintegrated killed vaccines against Salmonella typhimurium in mice. J Med Microbiol. 1972 May;5(2):183–189. doi: 10.1099/00222615-5-2-183. [DOI] [PubMed] [Google Scholar]
- Germanier R. Immunity in experimental salmonellosis. 3. Comparative immunization with viable and heat-inactivated cells of Salmonella typhimurium. Infect Immun. 1972 May;5(5):792–797. doi: 10.1128/iai.5.5.792-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON D. Resistance to reinfection in experimental mouse typhoid. J Hyg (Lond) 1957 Sep;55(3):334–343. doi: 10.1017/s0022172400037244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU H. S. IN VITRO STUDIES ON THE INTERACTIONS BETWEEN MACROPHAGES OF RABBITS AND TUBERCLE BACILLI. II. CELLULAR AND HUMORAL ASPECTS OF ACQUIRED RESISTANCE. Am Rev Respir Dis. 1965 Apr;91:499–509. doi: 10.1164/arrd.1965.91.4.499. [DOI] [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S., Piper V. M. Acquired resistance to and comparative virulence of Salmonella typhimurium demonstrated by cutaneous lesions in guinea pigs. J Reticuloendothel Soc. 1972 Apr;11(4):343–357. [PubMed] [Google Scholar]
- Hsu H. S., Radcliffe A. S. Interactions between macrophages of guinea pigs and Salmonellae. I. Fate of Salmonella typhimurium within macrophages of normal guinea pigs. J Bacteriol. 1968 Jul;96(1):191–197. doi: 10.1128/jb.96.1.191-197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. The binding of the Fc fragment of guinea-pig cytophilic antibody to peritoneal macrophages. Immunology. 1971 May;20(5):817–829. [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., SATO I., TANAKA T. Experimental salmonellosis. Intracellular growth of Salmonella enteritidis ingested in mononuclear phagocytes of mice, and cellular basis of immunity. J Bacteriol. 1961 Jun;81:863–868. doi: 10.1128/jb.81.6.863-868.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- SATO I., TANAKA T., SAITO K., MITSUHASHI S. Inhibition of Salmonella enteritidis ingested in mononuclear phagocytes from liver and subcutaneous tissue of mice immunized with live vaccine. J Bacteriol. 1962 Jun;83:1306–1312. doi: 10.1128/jb.83.6.1306-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., RAMSEIAR H. CELLULAR REACTIONS IN INFECTION. Adv Immunol. 1964;27:117–173. doi: 10.1016/s0065-2776(08)60707-5. [DOI] [PubMed] [Google Scholar]
- Ushiba D., Nakae T., Akiyama T., Kishimoto Y. Characterization of "clearance" factor and "cell-bound" antibody in experimental typhoid. J Bacteriol. 1966 May;91(5):1705–1712. doi: 10.1128/jb.91.5.1705-1712.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Serum-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):374–380. doi: 10.1128/iai.4.4.374-380.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Grunspan R., Ganguly R. Oral immunization of mice with killed Salmonella typhimurium vaccine. Infect Immun. 1972 Jul;6(1):58–61. doi: 10.1128/iai.6.1.58-61.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells P. S., Hsu H. S. Interactions Between Macrophages of Guinea Pigs and Salmonellae II. Phagocytosis of Salmonella typhimurium by Macrophages of Normal Guinea Pigs. Infect Immun. 1970 Aug;2(2):145–149. doi: 10.1128/iai.2.2.145-149.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



