Abstract
Dishevelled (DVL) proteins, three of which have been identified in humans, are highly conserved components of canonical and noncanonical Wnt signaling pathways. These multifunctional proteins, originally discovered in the fruit fly, through their different domains mediate complex signal transduction: DIX (dishevelled, axin) and PDZ (postsynaptic density 95, discs large, zonula occludens-1) domains serve for canonical beta-catenin signaling, while PDZ and DEP (dishevelled, Egl-10, pleckstrin) domains serve for non-canonical signaling. In canonical or beta-catenin signaling, DVL forms large molecular supercomplexes at the plasma membrane consisting of Wnt-Fz-LRP5/6-DVL-AXIN. This promotes the disassembly of the beta-catenin destruction machinery, beta-catenin accumulation, and consequent activation of Wnt signaling. Therefore, DVLs are considered to be key regulators that rescue cytoplasmic beta-catenin from degradation. The potential medical importance of DVLs is in both human degenerative disease and cancer. The overexpression of DVL has been shown to potentiate the activation of Wnt signaling and it is now apparent that up-regulation of DVLs is involved in several types of cancer.
Wnt proteins initiate three distinct signaling pathways − the canonical, non-canonical or planar cell polarity (PCP), and Wnt-Ca2+ pathway. The members of the dishevelled (dsh/DVL) protein family are considered to be critical components of Wnt signaling, which are transducing signal into three different cellular routes (1-5). The mechanism how DVL activates distinct downstream pathways has been elucidated recently (6,7), therefore this review attempts to synthesize and explain the current knowledge on DVLs function in Wnt signaling.
Among three Wnt signaling cascades, the canonical Wnt signaling pathway is one of the basic mechanisms of the cell signaling, critical for embryonic development and adult tissue homeostasis, and is widely conserved in the animal kingdom. It is activated by binding of different Wnt ligands (19 were identified in humans) to specific receptors (1). Through several cytoplasmic relay components, the signal is subsequently transduced to beta-catenin. As a consequence, beta-catenin levels raise, and it enters the nucleus to activate transcription of Wnt target genes (2). In the nucleus, beta-catenin finds a partner, a member of the DNA binding transcription factor family LEF/TCF (lymphoid enhancer factor/ T cell factor) (2). Target genes for beta-catenin/LEF/TCF encode c-myc, N-myc, c-jun, and cyclin D1, explaining why constitutive activation of the Wnt pathway can lead to cancer (2,8).
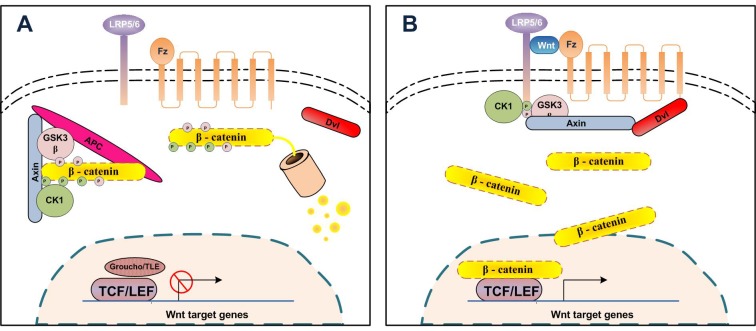
The pathway is inactive when the levels of beta-catenin are kept low. This is achieved by beta-catenin’s degradation in a multiprotein destructive complex consisting of AXIN, APC (adenomatous polyposis coli), CK1 (casein kinase 1), and GSK3β (glycogen synthase kinase 3 beta). This results in beta-catenin phosphorylation, ubiquitination, and finally its degradation in the proteasome (Figure 1). The components of beta-catenin destruction complex represent negative regulators of the pathway. The activation of Wnt signaling pathway also happens in case APC, AXIN, and other components of beta-catenin destruction complex are mutated and non functional (2,8-10).
Figure 1.
Wnt signaling pathway and its key components. (A) In the absence of Wnt, beta-catenin destruction complex consisting of AXIN1, adenomatous polyposis coli (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3 beta (GSK3β) is formed. This results in beta-catenin phosphorylation, ubiquitination, and degradation in the proteasome. (B) Binding of Wnt ligands to receptor complex consisting of Frizzled (Fz) and low density lipoprotein receptor-related protein 5 and 6 (LRP5/6) results in recruitment of DVL to the membrane by binding to Fz and AXIN. This disables the formation of beta-catenin destruction complex, allowing beta-catenin to accumulate in the nucleus where it activates Wnt target genes upon binding to lymphoid enhancer factor/ T cell factor (LEF/TCF).
DVL is considered to be a key regulator that rescues cytoplasmic beta-catenin from degradation. Binding of Wnt signaling molecule to membrane receptors activates DVL (2,4). The receptors of the Frizzled (Fz) family are transmembrane seven-pass molecules that work in collaboration with their co-receptors LRP5 and LRP6 (low density lipoprotein receptor-related protein 5 and 6) forming a membrane receptors complex. DVL is recruited to the membrane and it comes in direct contact with the Fz receptor (11). This interaction is essential for phosphorylation of Fz co-receptors LRP5/6 by phosphokinases GSK3β and CK1. Phosphorylation activates LRP5/6 co-receptors, which beside kinases also bind AXIN by the LRP’s cytoplasmic tail. As a consequence, AXIN is recruited to the plasma membrane and it can no longer be a part of beta-catenin destruction complex, so the complex cannot be formed. Besides being able to bind to the co-receptors, AXIN can also bind to DVL. In this way DVL inhibits the activity of AXIN in the destruction complex (8,12). Wnt signal triggers the recruitment of AXIN either to LRP5/6 or to DVL bound to Fz receptors. This leads to the beta-catenin accumulation in the cytoplasm, its consequent nuclear translocation, activation of LEF/TCF transcription factors, and expression of target genes (2-4,6,13-15).
Why is dishevelled in the center of Wnt signaling?
Dishevelled (dsh) is a multifunctional phosphoprotein originally discovered in the fruit fly Drosophila melanogaster. In Drosophila, a single dsh gene is expressed and it is required for proper development. In contrast, three dsh homologue genes (DVL1, DVL2, and DVL3) have been identified in humans showing a high degree of similarity. DVL1 gene is located at 1p36 locus, and its protein is 695 amino acids long; DVL2 gene is located at 17p13.1, and its protein is 736 amino acids long; and DVL3 gene is located at 3q27, and its protein is 716 amino acids long (16-18). The experiments in knockout mice have indicated that each mammalian DVL protein product is able to function cooperatively as well as uniquely (14). DVL2 is the most abundant of the three members (14). Two isoforms of human DVL1 and two of DVL3 are produced by alternative splicing. DVL interacts with more than 50 binding proteins in the cytoplasm and in the nucleus (16).
All dsh/DVL proteins (ranging from nematodes to humans) possess three conserved domains: an aminoterminal DIX (dishevelled, axin), a central PDZ (postsynaptic density 95, discs large, zonula occludens-1), and a carboxyl-terminal DEP (dishevelled, Egl-10, pleckstrin) domain (19). In addition DVL also contains another two regions harboring positively charged amino acid residues. The first is called the basic region and is comprised of conserved serine and threonine residues stretching between the DIX and PDZ domains and the second is the proline-rich region, termed SH3 (src homology 3) binding domain, which is situated downstream of PDZ (16). The proposed peptide structure is implicated to mediate protein-protein interaction, and thus DVL likely serves as an adapter molecule (4,5). Dillman et al (5) have reported that there is a fourth conserved domain in DVL, called DSV or dishevelled domain, but its functional importance is still unclear (Figure 2).
Figure 2.

Dishevelled protein structure with conserved domains and regions: DIX (dishevelled, axin) domain, DSV (dishevelled) domain, the basic region, PDZ (postsynaptic density 95, discs large, zonula occludens-1) domain, proline-rich region, and DEP (dishevelled, Egl-10, pleckstrin) domain.
The N-terminal DIX domain extends in humans for 85 amino acids for DVL1, 83 for DVL2, and 82 for DVL3, and is also present in the AXIN protein, which seems to be a scaffolding factor for Wnt signaling (15,16). DIX domain is necessary but not sufficient for the DVL-AXIN interaction and some other sequences located near to the DIX domain may be requisite. The domains do not interact directly with each other (4). As mentioned previously, DVL can bind AXIN and inhibit its activity (3), thus dissociating the axin-assembled beta-catenin destruction complex by displacing AXIN, or by recruiting another protein called FRAT (frequently rearranged in advanced T-cell lymphomas). Phosphorylated DVL has a high affinity for FRAT, and this binding also induces the disintegration of beta-catenin destruction complex and the activation of the pathway (20). Possibly the interaction of DVL1 with FRAT will cause a conformational change of the degradation complex that phosphorylates beta-catenin.
The central PDZ domain is 73 amino acids long in all three human homologs and it provides a docking site for protein kinases, phosphatases, and adaptor proteins. The proteins that bind to the PDZ domain are best known for their roles in submembranous receptor assembly, where they integrate signaling molecules into larger complexes with subsequent signal transduction (4). The direct interaction of PDZ domain with the Fz through the conserved C-terminal cytoplasmic Fz sequence is essential in transduction of the signal from Fz to the downstream components of the Wnt pathway. It is known that Fz-Dvl and Dvl-Axin protein interactions are relatively weak but dynamic (11).
DEP domain located between PDZ domain and C-terminal region of DVL protein consists of 75 amino acids in all three human homologs. The same polypeptide motif can also be found in signaling factors such as the regulator of G-protein signaling (RGS) protein family, which harbors conserved, catalytic RGS domains as well as DEP domains in their N termini (1). It has been shown that DEP domain enables protein-protein interaction between DVL and DAAM1 (dishevelled associated activator of morphogenesis 1), a formin-homology protein involved in actin polymerization. By mediation of basic residues in DEP domain, DVL binds to membrane lipids during planar epithelial polarization (21).
Considering its hub position in Wnt signaling (4), it is not surprising that domains of DVL proteins contain binding sites for a large number of different proteins, including several kinases. Simply speaking, DVL proteins use different domains through which they mediate complex signal transduction: DIX and PDZ domains are crucial for cannonical beta-catenin signaling, while PDZ and DEP domains are critical for PCP signaling by mediation of the cytoplasmic-to-membrane translocation (1). Recent studies (22) have given more weight to the claim that DIX domain can also be included in non-canonical PCP signaling and DEP domain can affect beta-catenin signaling (22,23). The cellular pool of available DVL is limited, meaning that activation of one pathway makes DVL unavailable in other locations to activate the other pathways. This means that activation of the canonical Wnt pathway could down-regulate non-canonical Wnt signaling, and vice versa (24).
Dishevelled nuclear shuttling
The subcellular localization of DVL is proposed to be causative of the choice of different Wnt pathway routes. Literature data suggest that there are two cellular pools of DVL: one translocates to the nucleus to mediate the canonical signaling while the other remains in the cytoplasm or goes to the plasma membrane and mediates both canonical and non-canonical signaling (1,3,4). Its nuclear localization, which is required for the canonical Wnt beta-catenin signaling, also suggests that the involvement of DVL in Wnt signaling is more complex than previously thought. Regulation of protein shuttling into and out of the nucleus is influenced by the activity of nuclear localization signals (NLS) and nuclear export signals (NES) (25). Wnt signaling utilizes DIX and PDZ domains of DVL to induce the stabilization of cytosolic beta-catenin. When DVL is in the nucleus, it interacts with phosphorylated c-jun and nuclear beta-catenin and mediates the formation of a functional complex consisting of DVL-c-jun-beta-catenin-TCF. Through TCFs sequence-specific DNA binding domain (HMG box), newly formed complex binds on the promoter of Wnt target genes and regulates gene transcriptional activity. Formation of this quaternary functional complex was proposed by Gan et al (26) and it may suggest the transcriptional function of DVL in the nucleus. How DVL’s voyage to the nucleus is regulated remains not completely clear (4,27). In contrast, DVL in the cytoplasm moves to the plasma membrane where it forms large molecular supercomplexes (ie, signalosomes) consisting of Wnt-Fz-LRP5/6-DVL-AXIN necessary for the transmission of signals from the receptor to downstream effectors (22,28). Beside beta-catenin and DVL, many components of the canonical signaling pathway such as APC, AXIN1, and GSK3β appear to traffic between the cytoplasm and the nucleus (27).
Regulation of DVL activity
To understand the function of DVL's domains and regions several studies have been reported in experimental models (2,3). In spite of a multitude of protein interactions, DVL has no known enzymatic activity. Positive regulation of DVL activity is achieved by phosphorylation of the protein (4). Upon binding of Wnt molecule to receptors, extensive phosphorylation of DVL is induced. Kinases and other factors involved in this hyperphosphorylation event include casein kinase 1 (CK1), casein kinase 2 (CK2), PAR1, and β-arrestin (29,30). The phospho-residues are located along the DVL protein, including the conserved domains.
Negative regulation of DVL activity is accomplished through polyubiquitination by DVL-interacting proteins such as KLHL12, NEDL1, Dapper1, Prickle1, and inversin. Protein phosphatase 2A (PP2A) is also involved in the regulation of DVL activity. It can have a positive or negative influence, depending on which regulatory subunit realizes the binding to DVL (1,4,7,8). Even deubiquitination of DVL by deubiquitinating enzyme (DUB) Usp14 is required for Wnt signaling (7) and these events can switch DVL between canonical and non-canonical pathways (8).
DVL and development
Insights into the mechanisms of Wnt action have emerged from several fields of research: genetics in Drosophila and Caenorhabditis elegans; biochemistry in cell culture; and ectopic gene expression in Xenopus embryos. Wnt signaling is essential for mammalian embryogenesis (9,31) as well. Many Wnt genes in the mouse have been mutated, leading to specific developmental defects. The Wnt pathway acts as a regulator of cell patterning, proliferation, differentiation, cell-to-cell communication, adhesion and migration, cell survival, and apoptosis. It is required for normal development of some organs and organic systems, in particular the central nervous system through synaptic rearrangements (32). The Wnt pathway regulates the normal development of the neural plate, neural tube, brain, spinal cord, and sensory and motor neurons (9,33,34). In addition to neural tissues, Wnt pathway is critical for vascular and cardiac systems development and also modulates osteoblast physiology (35,36).
Dsh gene family has a significant role in the broad spectrum of developmental processes (3,37). Dsh alleles were first discovered in Drosophila mutants whose marginal wing bristles were “deranged and sparse” (38), and hence the name dishevelled. The allelic locus was rediscovered later on when it was shown that dsh was an important segment polarity gene in the early Drosophila embryo. Drosophila gene dsh was cloned in 1994 (39,40) and the first Xenopus homologue dvl in 1995 (41). The three murine homologues were cloned soon after (42-44), followed by cloning of three human DVL genes (3,17,18,43). The function of dvl homologues seems to have diverged among the vertebrates. The evolution of dvl homologues with their functional specificities is supported by the findings that in Xenopus, dvl1 and dvl2 homologues, but not dvl3, are necessary to mediate the Wnt-dependent signals that control neural crest specification. On the other hand, in rodents Dvl2 and Dvl3 are involved in neural crest development, but not Dvl1 (5). The lack of Dvl3 in mice affects the formation of the neural tube, heart, and inner ear (37). It is noteworthy that defects of these organs are much more severe when the mice are deficient in more than one Dvl family member. The role of Dvl1 and Dvl2 in somite segregation has also been investigated revealing that lack of these genes causes skeletal malformations in mice (45). Since the protein expression patterns during mouse development overlap, it seems that there are several developmental processes in which all three Dvls are functionally redundant. It is still not clear whether Dvl´s role in developmental processes is regulated through the canonical pathway, noncanonical pathway, or both (37).
In human developmental disorders, DVLs are reported only as candidate genes involved in certain syndromes, for instance the Schwartz-Jampel syndrome mapped to chromosome 1p36-p34 (17) and Charcot-Marie-Tooth disease type 2A mapped to 1p36-1p35 (17). It is also speculated that DVL1 may have a role as a neural differentiation factor, which makes it a candidate gene for neuroblastomatous transformation. Bedell et al (46) suggested that DVL gene may play a role in the pathogenesis of the 1p36 deletion syndrome.
DVL and cancer
The potential medical importance of Wnt signaling pathway has long been recognized in both human degenerative diseases and cancer. Many tumor types show high levels of beta-catenin and it is known that beta-catenin’s translocations to the nucleus indicate its acquisition of oncogenic activity. The mutations attributed to APC, axin, and beta-catenin, which encode components of the beta-catenin destruction complex are very common in a variety of investigated tumors (2,8). The constitutive activation of the Wnt pathway can lead to cancer (10) and beta-catenin can be proclaimed an oncogene. Since DVL protein is known as the central mediator of Wnt signaling, its inclusion in tumor formation has been under intensive investigation. DVLs are overexpressed in various tumor types, including lung cancer, prostate cancer, breast cancer, cervical squamous cell carcinoma, and gliomas (47-51). However, the importance of individual DVLs in tumor prognosis is in general poorly defined.
The functional consequences of the DVL family protein expression in tumor etiology are still not clear and the data reported are controversial. The majority of reports (49,50) indicate DVL overexpression and amplification, but there are also reports (50) on gross deletions of DVL loci. In primary lung cancer DVL3 is overexpressed in non small cell lung cancer, implying that these events upstream of beta-catenin are critical for activation of Wnt signaling (50). Surprisingly, overexpression of DVL1 or DVL2 was not detected. DVL3 mRNA is significantly higher in pleural effusions from patients with adenocarcinoma, suggesting that it might be used as a marker of pleural micrometastasis (49).
DVL family proteins are overexpressed in primary lung cancers (52) and the expression levels of DVLs were significantly higher in adenocarcinomas than in squamous cell carcinomas (48,53). The positive expression rates of DVLs are higher in stages III and IV tumors. Nodal metastases show higher expression levels of DVL1 and DVL3 than primary growths. However, the correlation with tumor prognosis has also not been established yet. Our investigations of DVL1 and DVL3 protein levels showed overexpression in brain metastasis of lung cancers (54) (Figure 3). Although reports indicate DVLs location in the nucleus to be only occasional (53), our study on brain metastases showed nuclear staining of both DVL1 and DVL3 proteins (54).
Figure 3.
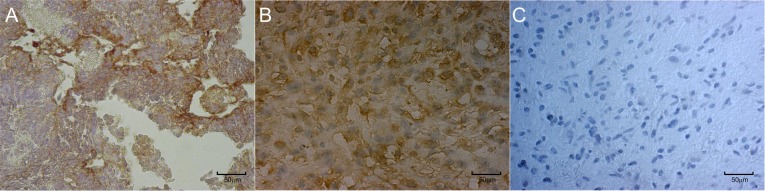
(A) Brain metastasis immunohistochemically stained for detection of dishevelled 3 (DVL3) protein (monoclonal mouse anti-human DVL3, Santa Cruz Biotehnology, Dallas, TX, USA), showing membranous staining. (B) Glioblastoma sample immunohistochemically stained for detection of dishevelled 1 (DVL1) protein (monoclonal mouse anti-human DVL1, Santa Cruz Biotehnology). (C) Control staining.
Breast cancers show aberrant expression of the DVL1 gene (47). Amplification and up-regulation of DVL1 gene are involved in breast carcinogenesis, especially in the acceleration of tumor growth. The involvement of DVL in invasive ductal carcinoma of the breast was also reported by Prasad et al (55). Mizutani et al (56) got similar results in prostate cancer. Their sample of 20 primary prostate cancer showed significant overexpression of DVL1 (65%). Correlation between DVL1 expression and beta-catenin expression was also confirmed. DVL2 is overexpressed in human high-grade gliomas, suggesting a role for active Wnt signaling in regulating the biology of these tumors (57). DVL1 is overexpressed in over two thirds of primary cervical squamous cell cancers when compared to corresponding non-cancerous uterine squamous cell tissues (58). Subsequently, amplification and increased expression of DVL genes may play an important role in the development of a portion of human cancers through derangement of the Wnt signaling pathway.
DVL is very much involved in invasion and metastasis of tumors – the so called epithelial-to-mesenchymal transition (EMT). The occurrence of EMT during tumor progression resembles the developmental scenario and sheds light on important mechanisms governing metastasis, where noninvasive tumor cells acquire motility and ultimately disseminate to places distant from the primary site. Wnt signaling pathway has a particularly tight link with EMT. Moreover, the stabilization and nuclear accumulation of beta-catenin can induce EMT (59,60) by activating the transcriptional repressors Snail and Slug that suppress E-cadherin expression thus inducing EMT (61). Moreover LEF 1 when overexpressed leads to enhanced tumor invasiveness and induces EMT (62-64). Subsequently DVLs too are involved in tumor metastasis (60,65).
Conclusion and future perspectives
Ever since the first discovery of the Dsh allele in Drosophila mutants, dishevelled genes and proteins have been assigned the central role in the mediation of Wnt signaling. The Wnt signal utilizes DIX and PDZ or PDZ and DEP domains of DVL proteins to channel signal into canonical or non-canonical downstream pathways.
Recent data suggest that in the canonical pathway DVL is responsible for the disassembly of the beta-catenin destruction complex and recruitment of AXIN to the membrane, where DVL forms large molecular supercomplexes. DVL is considered to be a key regulator that rescues cytoplasmic beta-catenin from degradation.
DVL activity is dynamically regulated by phosphorylation, ubiquitination, and degradation and it seems to be dependable on the cellular context as well. Constitutive activation of the Wnt pathway can lead to cancer. The mutations attributed to APC, axin, and beta-catenin, which encode components of the beta-catenin destruction complex are very common in a variety of tumors.
Therefore, molecular components of Wnt pathway (like beta-catenin for instance) are relevant biomarkers helpful in better diagnosis and treatment. DVLs are overexpressed in various tumor types, including lung cancer, prostate cancer, breast cancer, cervical squamous cell carcinoma, and gliomas. However, the importance of individual DVLs in tumor prognosis still needs elucidation. The approaches to decrease DVL expression, as well as agents blocking selected specific DVL interactions, may be of particular interest as potential targets for therapeutic interventions.
Acknowledgments
Funding This work was supported by grants No. 6625 from the Croatian Science Foundation and No.1.2.1.19. from the University of Zagreb, Republic of Croatia.
Ethical approval was received from the Medical School University of Zagreb and Sestre Milosrdnice University Hospital.
Declaration of authorship AK contributed to the idea, wrote the manuscript, revised it for important intellectual content, and approved the final version of the manuscript. SBK contributed to the data interpretation, manuscript editing, revised the manuscript for important intellectual content, and approved the final version. NPŠ produced the idea, designed the study, contributed to data interpretation, wrote the manuscript, revised it for important intellectual content, and approved the final version of the manuscript.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Wharton KA. Runnin’ with the Dvl: Proteins that associate with Dsh/Dvl and their significance to wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–36. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 4.Gao C, Chen YG. Dishevelled: The hub of wnt signaling. Cell Signal. 2010;22:717–27. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Dillman AR, Minor PJ, Sternberg PW. Origin and evolution of dishevelled. G3 (Bethesda) 2013;3:251–62. doi: 10.1534/g3.112.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanfeng WA, Berhane H, Mola M, Singh J, Jenny A, Mlodzik M. Functional dissection of phosphorylation of Dishevelled in Drosophila. Dev Biol. 2011;360:132–42. doi: 10.1016/j.ydbio.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung H, Kim BG, Han WH, Lee JH, Cho J-Y, Park WS, et al. Deubiquitination of dishevelled by Usp14 is required for Wnt signaling. Oncogenesis. 2013;2:e64. doi: 10.1038/oncsis.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21:103–24. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–60. doi: 10.1016/S1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh K, Antipova A, Ratcliffe MJ, Sokol S. Interaction of Dishevelled and Xenopus Axin related protein is required for Wnt signal transduction. Mol Cell Biol. 2000;20:2228–38. doi: 10.1128/MCB.20.6.2228-2238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–84. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y-N, Gao Y, Wang H-y. Differential mediation of the wnt canonical pathway by mammalian Dishevelleds-1, -2, and 3. Cell Signal. 2008;20:443–52. doi: 10.1016/j.cellsig.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pecina-Slaus N, Nikuseva Martic T, Kokotovic T.AXIN1 (axin 1). Atlas Genet Cytogenet Oncol Haematol. 201115933–7.Available fromhttp://AtlasGeneticsOncology.org/Genes/AXIN1ID379ch16p13. htmlAccessed: October 30, 2014 [Google Scholar]
- 16.Protein Knowledgebase (UniProtKB). Available from: http://www.uniprot.org/uniprot/O14640. Accessed: October 20, 2014.
- 17.Pizzuti A, Amati F, Calabrese G, Mari A, Colosimo A, Silani V, et al. cDNA characterization and chromosomal mapping of two human homologs of the Drosophila dishevelled polarity gene. Hum Mol Genet. 1996;5:953–8. doi: 10.1093/hmg/5.7.953. [DOI] [PubMed] [Google Scholar]
- 18.Semënov MV, Snyder M. Human dishevelled genes constitute a DHR-containing multigene family. Genomics. 1997;42:302–10. doi: 10.1006/geno.1997.4713. [DOI] [PubMed] [Google Scholar]
- 19.The Wnt Homepage. Available from: http://www.stanford.edu/group/nusselab/cgi-bin/wnt/. Accessed: October 20, 2014.
- 20.Hino S, Michiue T, Asashima M, Kikuchi A. Casein kinase I epsilon enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of beta-catenin. J Biol Chem. 2003;278:14066–73. doi: 10.1074/jbc.M213265200. [DOI] [PubMed] [Google Scholar]
- 21.Ganner A, Lienkamp S, Schäfer T, Romaker D, Wegierski T, Park TJ, et al. Regulation of ciliary polarity by the APC/C. Proc Natl Acad Sci U S A. 2009;106:17799–804. doi: 10.1073/pnas.0909465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, He X. DEEP insights through the DEP domain. Structure. 2010;18:1223–5. doi: 10.1016/j.str.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consonni SV, Maurice MM, Boss JL. DEP domains: structurally similar but functionally different. Nat Rev Mol Cell Biol. 2014;15:357–62. doi: 10.1038/nrm3791. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Klein TJ, Mlodzik M. Subcellular localization of frizzeled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:E158. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh K, Brott BK, Bae G, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function inWnt/β-catenin signaling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gan XQ, Wang JY, Ying X, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, β-catenin, and TCF form a complex leading to stabilization of β-catenin-TCF interaction. J Cell Biol. 2008;180:1087–100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol. 2005;4:2. doi: 10.1186/jbiol22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Wang H. Dvl3 translocates IPMK to the cell membrane in response to Wnt. Cell Signal. 2012;24:2389–95. doi: 10.1016/j.cellsig.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernatík O, Sedová K, Schille C, Ganji RS, Cervenka I, Trantírek L, et al. Functional analysis of dishevelled-3 phosphorylation identifies distinct mechanisms driven by casein kinase 1ϵ and frizzled5. J Biol Chem. 2014;289:23520–33. doi: 10.1074/jbc.M114.590638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu N, Ishitani S, Sato A, Shibuya H, Ishitani T. Hipk2 and PP1c Cooperate to Maintain Dvl Protein Levels Required for Wnt Signal Transduction. Cell Reports. 2014;8:1391–404. doi: 10.1016/j.celrep.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Pecina-Slaus N. Wnt signal transduction pathway and apoptosis: a review. Cancer Cell Int. 2010;10:22. doi: 10.1186/1475-2867-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patapoutian A, Reichardt LF. Roles of wnt proteins in neural development maintenance. Curr Opin Neurobiol. 2000;10:392–9. doi: 10.1016/S0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, et al. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159:867–80. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastidas F, De Calisto J, Mayor R. Identification of neural crest competence territory: role of Wnt signaling. Dev Dyn. 2004;229:109–17. doi: 10.1002/dvdy.10486. [DOI] [PubMed] [Google Scholar]
- 35.Bodine PVN. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–53. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Shang H, Zhang C, Liu Y, Zhao Y, Shuang F, et al. The E3 ligase RNF185 negatively regulates osteogenic differentiation by targeting Dvl2 for degradation. Biochem Biophys Res Commun. 2014;447:431–6. doi: 10.1016/j.bbrc.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahmy OG, Fahmy MJ. Interallelic complementation in the r locus of D. melanogaster. New mutant report. Drosoph Inf Serv. 1959;33:85. [Google Scholar]
- 39.Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–30. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 40.Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. Dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–60. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- 41.Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:3487. doi: 10.1242/dev.121.10.3487. [DOI] [PubMed] [Google Scholar]
- 42.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 43.Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/S0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- 44.Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Dev Dyn. 1996;207:253–62. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 46.Bedell JA, Wagner-McPherson CB, Bengtsson U, Handa K, Dumars KW, Marsh JL, et al. A 1p deletion syndrome patient is hemizygous for a human homologue of the Drosophila dishevelled gene. Am J Hum Genet. 1996;59(4) Suppl:A298. [Google Scholar]
- 47.Nagahata T, Shimada T, Harada A, Nagai H, Onda M, Yokoyama S, et al. Amplification, up-regulation and over-expression of DVL-1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci. 2003;94:515–18. doi: 10.1111/j.1349-7006.2003.tb01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y, Yang Z-Q, Wang Y, Miao Y, Liu Y, Dai S-D, et al. Dishevelled-1 and Dishevelled-3 affect cell invasion mainly through canonical and noncanonical Wnt pathway, respectively, and associate with poor prognosis in nonsmall cell lung cancer. Mol Carcinog. 2010;49:760–70. doi: 10.1002/mc.20651. [DOI] [PubMed] [Google Scholar]
- 49.Li X-Y, Liu S-L, Cha N, Zhao Y-J, Wang S-C, Li W-N, et al. Transcription expression and clinical significance of Dishevelled-3 mRNA and δ-catenin mRNA in pleural effusions from patients with lung cancer. Clin Dev Immunol. 2012;2012:904946. doi: 10.1155/2012/904946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uematsu K, He B, You L, Xu ZBM, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer:evidence of disheveled overexpression. Oncogene. 2003;22:7218–21. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 51.Uematsu K, Kanazawa S, You L, He B, Xu Z, Li K, et al. Wnt pathway activation in Mesothelioma: Evidence of Dishevelled overexpression and transcriptional activity of β-catenin. Cancer Res. 2003;63:4547–51. [PubMed] [Google Scholar]
- 52.Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:dju 356. doi: 10.1093/jnci/djt356.. [DOI] [PubMed] [Google Scholar]
- 53.Wei Q, Zhao Y, Yang Z-Q, Dong Q-Z, Dong X-J, Han Y, et al. Dishevelled family proteins are expressed in non-small cell lung cancer and function differentially on tumor progression. Lung Cancer. 2008;62:181–92. doi: 10.1016/j.lungcan.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Kafka A, Tomas D, Beroš V, Pećina HI, Zeljko M, Pećina-Šlaus N. Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down regulation of E-cadherin. Int J Mol Sci. 2014;15:10635–51. doi: 10.3390/ijms150610635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prasad CP, Gupta SD, Rath G, Ralhan R. Wnt signaling pathway in invasive ductal carcinoma of the breast: relationship between beta-catenin, dishevelled and cyclin D1 expression. Oncology. 2007;73:112–7. doi: 10.1159/000120999. [DOI] [PubMed] [Google Scholar]
- 56.Mizutani K, Miyamoto S, Nagahata T, Konishi N, Emi M, Onda M. Upregulation and overexpression of DVL1, the human counterpart of the Drosophila dishevelled gene, in prostate cancer. Tumori. 2005;91:546–51. doi: 10.1700/229.2674. [DOI] [PubMed] [Google Scholar]
- 57.Pulvirenti T, Van Der Heijden M, Droms LA, Huse JT, Tabar V, Hall A. Dishevelled 2 signaling promotes self-renewal and tumorigenicity in human gliomas. Cancer Res. 2011;71:7280–90. doi: 10.1158/0008-5472.CAN-11-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okino K, Nagai H, Hatta M, Nagahata T, Yoneyama K, Ohta Y, et al. Up-regulation and overproduction of DVL-1, the human counterpart of the Drosophila dishevelled gene, in cervical squamous cell carcinoma. Oncol Rep. 2003;10:1219–23. doi: 10.3892/or.10.5.1219. [DOI] [PubMed] [Google Scholar]
- 59.Zeljko M, Pecina-Slaus N, Nikuseva Martic T, Kusec V, Beros V, Tomas D. Molecular alterations of E-cadherin and beta-catenin in brain metastases. Front Biosci (Elite Ed) 2011;3:616–24. doi: 10.2741/e274. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of Wnt/β-catenin signal network. Cancer Lett. 2013;336:379–89. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 61.Pecina-Slaus N, Cicvara-Pecina T, Kafka A. Epithelial-to-mesenchymal transition: possible role in meningiomas. Front Biosci (Elite Ed) 2012;4:889–96. doi: 10.2741/E427. [DOI] [PubMed] [Google Scholar]
- 62.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–76. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen A, Rosner A, Milovanović T, Hope C, Planutis K, Saha B, et al. WNT pathway component LEF1 mediates tumor cell invasion and is expressed in human and murine breast cancers lacking ErbB2 (her-2/neu) overexpression. Int J Oncol. 2005;27:949–56. doi: 10.3892/ijo.27.4.949. [DOI] [PubMed] [Google Scholar]
- 64.Pećina-Šlaus N, Kafka A, Tomas D, Marković L, Okštajner PK, Sukser V, et al. Wnt signaling transcription factors TCF-1 and LEF-1 are upregulated in malignant astrocytic brain tumors Histol Histopathol 2014[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Shi C-S, Huang NN, Kehrl JH. Regulator of G-protein signaling isoform 1 (PDZ-RGS3) enhances canonical wnt signaling and promotes epithelial mesenchymal transition. J Biol Chem. 2012;287:33480–7. doi: 10.1074/jbc.M112.361873. [DOI] [PMC free article] [PubMed] [Google Scholar]