Abstract
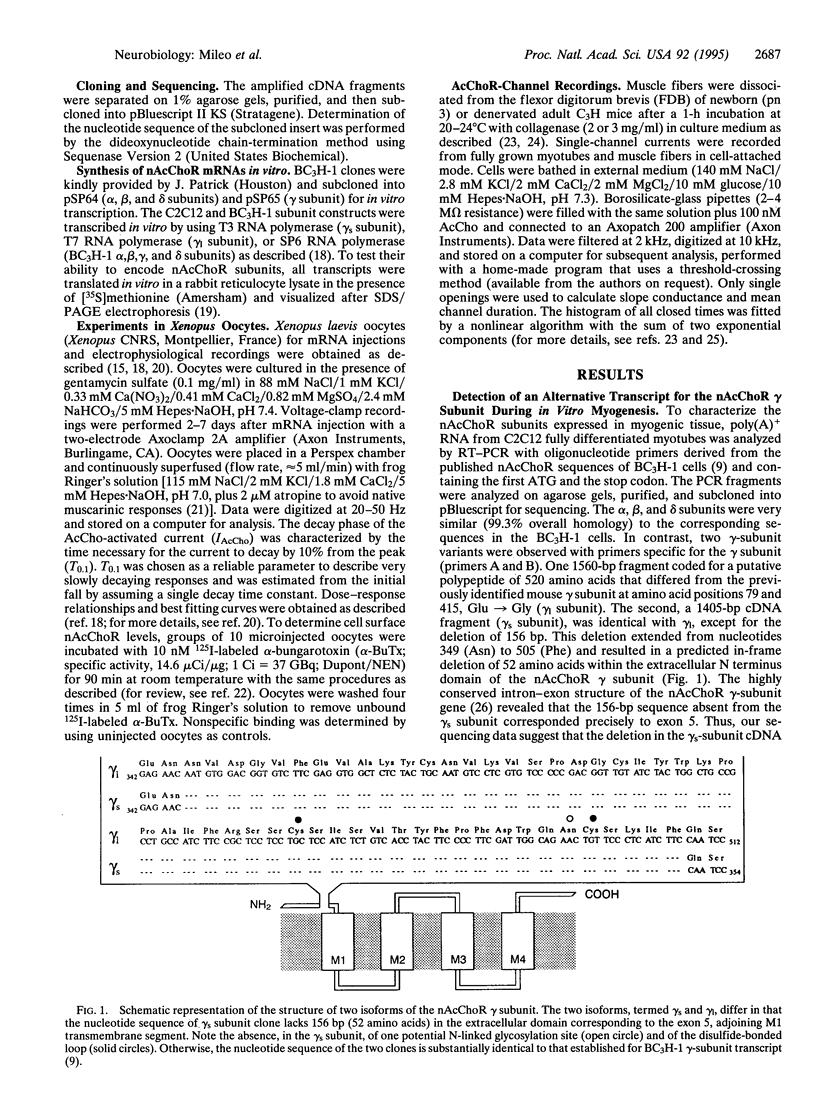
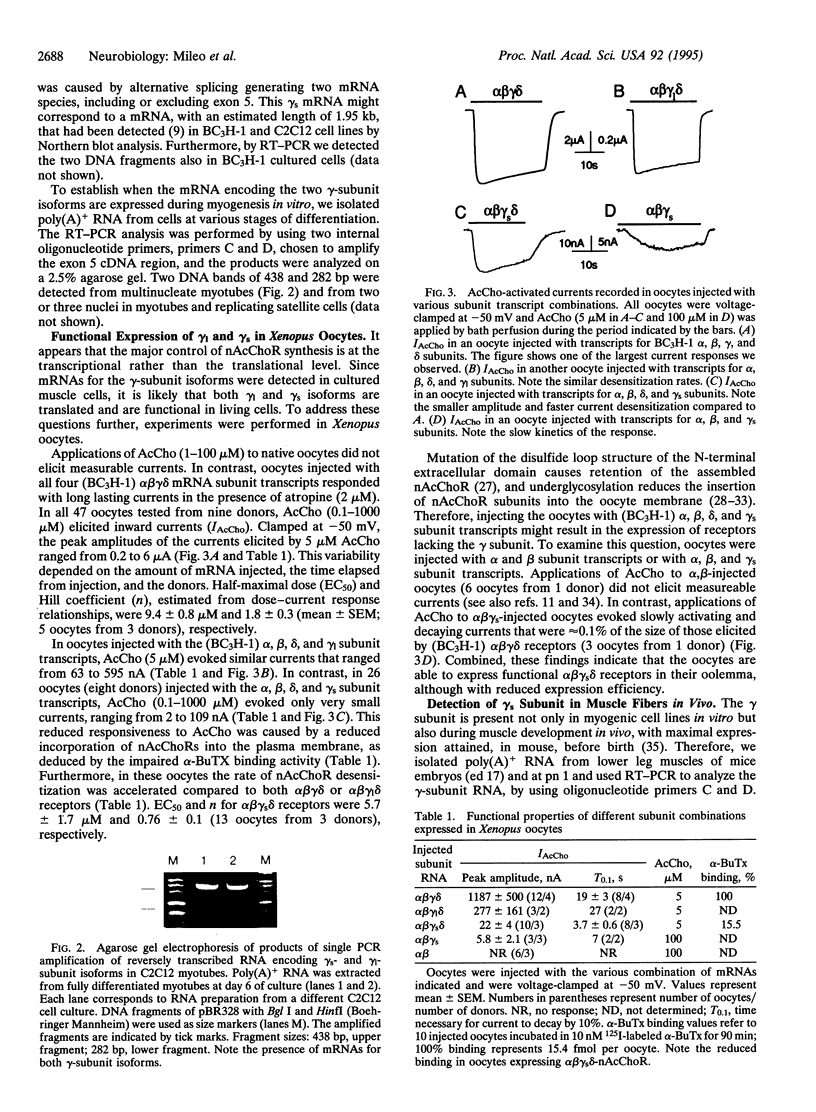
Nicotinic acetylcholine receptors (nAcChoRs) of skeletal muscle are heterosubunit ligand-gated channels that mediate signal transmission from motor nerves to muscle. While cloning murine nAcChoR subunits, to gain an insight into the receptor diversity across species, we detected two forms of gamma subunits in the myogenic C2C12 cell line. Both forms are functional when expressed in Xenopus oocytes. One gamma subunit [long gamma (gamma 1)] was almost identical to that previously cloned in the murine BC3H-1 tumor cell line. The second form of gamma subunit [short gamma (gamma s)] lacked 156 bp (52 amino acids) in the extracellular N terminus, adjoining the hydrophobic segment M1, which corresponds to the fifth exon of the gamma-subunit gene. The two forms of gamma subunit coexist during myogenesis in vitro and in 17-day embryonic and denervated adult muscle fibers in vivo. However, the gamma s variant was the only form of gamma subunit in newborn muscle. In dissociated muscle fibers of newborn mice, AcCho-evoked channel openings were more prolonged when compared with C2C12 myotubes or denervated adult muscle fibers. The gamma s subunit may, thus, contribute to the structural and functional diversity of nAcChoRs in muscle cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buller A. L., White M. M. Altered patterns of N-linked glycosylation of the Torpedo acetylcholine receptor expressed in Xenopus oocytes. J Membr Biol. 1990 May;115(2):179–189. doi: 10.1007/BF01869456. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Merlie J. P. Isolation and characterization of the beta and epsilon subunit genes of mouse muscle acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7611–7616. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Czajkowski C., Kaufmann C., Karlin A. Negatively charged amino acid residues in the nicotinic receptor delta subunit that contribute to the binding of acetylcholine. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6285–6289. doi: 10.1073/pnas.90.13.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Farini D., Grassi F., Monaco L., Ruzzier F. Effects of calcitonin gene-related peptide on synaptic acetylcholine receptor-channels in rat muscle fibres. Proc R Soc Lond B Biol Sci. 1988 Aug 23;234(1276):333–342. doi: 10.1098/rspb.1988.0052. [DOI] [PubMed] [Google Scholar]
- Fishburn C. S., Belleli D., David C., Carmon S., Fuchs S. A novel short isoform of the D3 dopamine receptor generated by alternative splicing in the third cytoplasmic loop. J Biol Chem. 1993 Mar 15;268(8):5872–5878. [PubMed] [Google Scholar]
- Gehle V. M., Sumikawa K. Site-directed mutagenesis of the conserved N-glycosylation site on the nicotinic acetylcholine receptor subunits. Brain Res Mol Brain Res. 1991 Aug;11(1):17–25. doi: 10.1016/0169-328x(91)90016-q. [DOI] [PubMed] [Google Scholar]
- Giovannelli A., Grassi F., Eusebi F., Miledi R. Tunicamycin increases desensitization of acetylcholine receptors in cultured mouse muscle cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1808–1811. doi: 10.1073/pnas.88.5.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L. Expression of ion channels by injection of mRNA into Xenopus oocytes. Methods Cell Biol. 1991;36:487–509. doi: 10.1016/s0091-679x(08)60293-9. [DOI] [PubMed] [Google Scholar]
- Goldman D., Tamai K. Coordinate regulation of RNAs encoding two isoforms of the rat muscle nicotinic acetylcholine receptor beta-subunit. Nucleic Acids Res. 1989 Apr 25;17(8):3049–3056. doi: 10.1093/nar/17.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Polenzani L., Mileo A. M., Caratsch C. G., Eusebi F., Miledi R. Blockage of nicotinic acetylcholine receptors by 5-hydroxytryptamine. J Neurosci Res. 1993 Apr 1;34(5):562–570. doi: 10.1002/jnr.490340508. [DOI] [PubMed] [Google Scholar]
- Green W. N., Ross A. F., Claudio T. Acetylcholine receptor assembly is stimulated by phosphorylation of its gamma subunit. Neuron. 1991 Oct;7(4):659–666. doi: 10.1016/0896-6273(91)90378-d. [DOI] [PubMed] [Google Scholar]
- Hartman D. S., Claudio T. Coexpression of two distinct muscle acetylcholine receptor alpha-subunits during development. Nature. 1990 Jan 25;343(6256):372–375. doi: 10.1038/343372a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. P., Lechleiter J. D., Brehm P. Single channel properties of newly synthesized acetylcholine receptors following denervation of mammalian skeletal muscle. J Gen Physiol. 1987 Jun;89(6):999–1014. doi: 10.1085/jgp.89.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Wang J. B., Moss S. J., Huganir R. L., Burt D. R. Generation of two forms of the gamma-aminobutyric acidA receptor gamma 2-subunit in mice by alternative splicing. J Neurochem. 1991 Feb;56(2):713–715. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Hahn G. M. Ethanol-induced tolerance to heat and to adriamycin. Nature. 1978 Aug 17;274(5672):699–701. doi: 10.1038/274699a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Brehm P. Expression of subunit-omitted mouse nicotinic acetylcholine receptors in Xenopus laevis oocytes. J Physiol. 1993 Oct;470:349–363. doi: 10.1113/jphysiol.1993.sp019862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D. C., Pinkham J. L., Stevens C. F. Influence of the gamma subunit and expression system on acetylcholine receptor gating. Neuron. 1990 Dec;5(6):857–866. doi: 10.1016/0896-6273(90)90345-g. [DOI] [PubMed] [Google Scholar]
- Lorenzon P., Ruzzier F., Caratsch C. G., Giovannelli A., Velotti F., Santoni A., Eusebi F. Interleukin-2 lengthens extrajunctional acetylcholine receptor channel open time in mammalian muscle cells. Pflugers Arch. 1991 Oct;419(3-4):380–385. doi: 10.1007/BF00371120. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- MacLennan C., Beeson D., Vincent A., Newsom-Davis J. Human nicotinic acetylcholine receptor alpha-subunit isoforms: origins and expression. Nucleic Acids Res. 1993 Nov 25;21(23):5463–5467. doi: 10.1093/nar/21.23.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio M. L., Grenningloh G., Kuhse J., Schmieden V., Schmitt B., Prior P., Betz H. Alternative splicing generates two variants of the alpha 1 subunit of the inhibitory glycine receptor. J Biol Chem. 1991 Feb 5;266(4):2048–2053. [PubMed] [Google Scholar]
- Martinou J. C., Merlie J. P. Nerve-dependent modulation of acetylcholine receptor epsilon-subunit gene expression. J Neurosci. 1991 May;11(5):1291–1299. doi: 10.1523/JNEUROSCI.11-05-01291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzenbach B., Maulet Y., Sefton L., Courtier B., Avner P., Guénet J. L., Betz H. Structural analysis of mouse glycine receptor alpha subunit genes. Identification and chromosomal localization of a novel variant. J Biol Chem. 1994 Jan 28;269(4):2607–2612. [PubMed] [Google Scholar]
- Morris A., Beeson D., Jacobson L., Baggi F., Vincent A., Newsom-Davis J. Two isoforms of the muscle acetylcholine receptor alpha-subunit are translated in the human cell line TE671. FEBS Lett. 1991 Dec 16;295(1-3):116–118. doi: 10.1016/0014-5793(91)81399-s. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Gundersen C. B., Miledi R. Expression of ACh-activated channels and sodium channels by messenger RNAs from innervated and denervated muscle. Proc R Soc Lond B Biol Sci. 1988 Apr 22;233(1272):235–246. doi: 10.1098/rspb.1988.0021. [DOI] [PubMed] [Google Scholar]
- Prives J. M., Olden K. Carbohydrate requirement for expression and stability of acetylcholine receptor on the surface of embryonic muscle cells in culture. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5263–5267. doi: 10.1073/pnas.77.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives J., Bar-Sagi D. Effect of tunicamycin, an inhibitor of protein glycosylation, on the biological properties of acetylcholine receptor in cultured muscle cells. J Biol Chem. 1983 Feb 10;258(3):1775–1780. [PubMed] [Google Scholar]
- Sakmann B., Methfessel C., Mishina M., Takahashi T., Takai T., Kurasaki M., Fukuda K., Numa S. Role of acetylcholine receptor subunits in gating of the channel. Nature. 1985 Dec 12;318(6046):538–543. doi: 10.1038/318538a0. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Claudio T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. J Biol Chem. 1991 Oct 15;266(29):19369–19377. [PubMed] [Google Scholar]
- Sumikawa K., Gehle V. M. Assembly of mutant subunits of the nicotinic acetylcholine receptor lacking the conserved disulfide loop structure. J Biol Chem. 1992 Mar 25;267(9):6286–6290. [PubMed] [Google Scholar]
- Talib S., Okarma T. B., Lebkowski J. S. Differential expression of human nicotinic acetylcholine receptor alpha subunit variants in muscle and non-muscle tissues. Nucleic Acids Res. 1993 Jan 25;21(2):233–237. doi: 10.1093/nar/21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P., McKernan R. M., Iversen L. L. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Mori H., Araki K., Mori K. J., Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992 Mar 23;300(1):39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- Yu L., LaPolla R. J., Davidson N. Mouse muscle nicotinic acetylcholine receptor gamma subunit: cDNA sequence and gene expression. Nucleic Acids Res. 1986 Apr 25;14(8):3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]





