Abstract
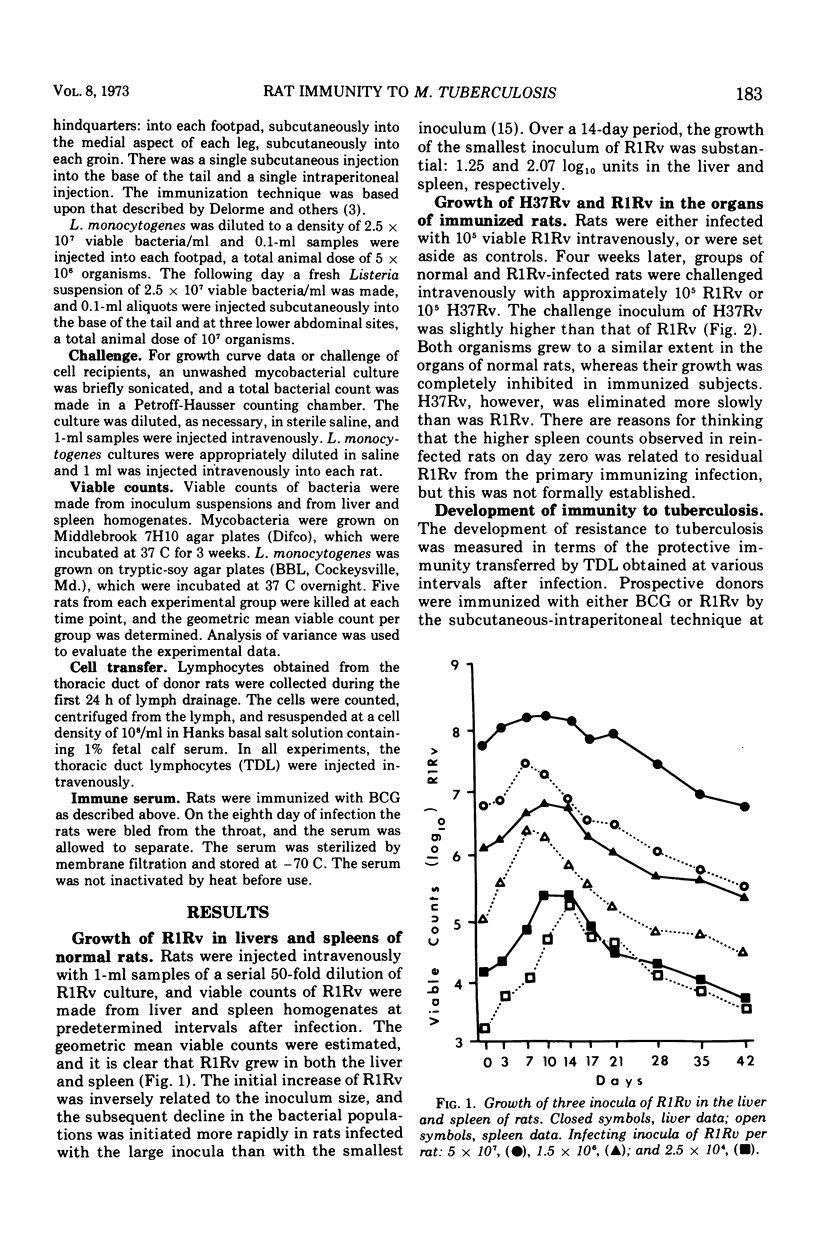
After intravenous injection into rats, both the attenuated strain R1Rv and the virulent strain H37Rv of Mycobacterium tuberculosis grow in the liver and spleen. However, the infected rats mount a specific immune response with great rapidity, giving a false impression of natural resistance to the tubercle bacillus. Adoptive immunity to tuberculosis was achieved by transferring thoracic duct cells from immunized donors to normal syngeneic recipients. The transferred immunity was vested in a population of lymphocytes uncontaminated with macrophages. The adoptive immunity was effectively expressed against both attenuated and virulent tubercle bacilli, and it was shown to be immunologically specific. Lymphocytes which conferred immunity to tuberculosis were not protective against Listeria monocytogenes infection, and vice versa. Immunity could not be transferred with either normal thoracic duct lymphocytes (TDL), heat-killed sensitized TDL, or serum from specifically immunized donors. The ability of TDL from BCG-immunized donors to confer immunity was maintained at an unimpaired level for at least 3 months after immunization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Increased antibacterial resistance and immunodepression during graft-versus-host reactions in mice. Transplantation. 1969 Jun;7(6):484–497. doi: 10.1097/00007890-196906000-00005. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme E. J., Hodgett J., Hall J. G., Alexander P. The cellular immune response to primary sarcomata in rats. I. The significance of large basophilic cells in the thoracic duct lymph following antigenic challenge. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):229–236. doi: 10.1098/rspb.1969.0089. [DOI] [PubMed] [Google Scholar]
- Everett N. B., Tyler R. W. Lymphopoiesis in the thymus and other tissues: functional implications. Int Rev Cytol. 1967;22:205–237. doi: 10.1016/s0074-7696(08)61836-7. [DOI] [PubMed] [Google Scholar]
- FLAX M. H., WAKSMAN B. H. Delayed cutaneous reactions in the rat. J Immunol. 1962 Oct;89:496–504. [PubMed] [Google Scholar]
- FONG J., CHIN D., ELBERG S. S. Studies on tubercle bacillus-histiocyte relationship. V. Passive transfer of cellular resistance. J Exp Med. 1962 Mar 1;115:475–489. doi: 10.1084/jem.115.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Gowans J. L. The traffic of lymphocytes. Semin Hematol. 1969 Jan;6(1):67–83. [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- GRAY D. F., NOBLE J. L., O'HARA M. Allergy in experimental rat tuberculosis. J Hyg (Lond) 1961 Dec;59:427–436. doi: 10.1017/s0022172400039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D. F. The relative natural resistance of rats and mice to experimental pulmonary tuberculosis. J Hyg (Lond) 1961 Dec;59:471–477. doi: 10.1017/s0022172400039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Migration of lymphocytes and thymocytes in the rat. II. Circulation of lymphocytes and thymocytes from blood to lymph. Lab Invest. 1968 Apr;18(4):397–406. [PubMed] [Google Scholar]
- Gowans J. L. Lymphocytes. Harvey Lect. 1968 1969;64:87–119. [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- MILLMAN I. Passive transfer of resistance to tuberculosis. Am Rev Respir Dis. 1962 Jan;85:30–32. doi: 10.1164/arrd.1962.85.1.30. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATCLIFFE H. L., PALLADINO V. S. Tuberculosis induced by droplet nuclei infection; initial homogeneous response of small mammals (rats, mice, guinea pigs, and hamsters) to human and to bovine bacilli, and the rate and pattern of tubercle development. J Exp Med. 1953 Jan;97(1):61–68. doi: 10.1084/jem.97.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATCLIFFE H. L. Tuberculosis induced by droplet nuclei infection; pulmonary tuberculosis of predetermined initial intensity in mammals. Am J Hyg. 1952 Jan;55(1):36–47. [PubMed] [Google Scholar]
- SEVER J. L. Passive transfer of resistance to tuberculosis through use of monocytes. Proc Soc Exp Biol Med. 1960 Feb;103:326–329. doi: 10.3181/00379727-103-25506. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Wiegeshaus E. H., Smith D. W. Biology of the mycobacterioses. Experimental models for study of immunity in tuberculosis. Ann N Y Acad Sci. 1968 Sep 5;154(1):194–199. doi: 10.1111/j.1749-6632.1968.tb16709.x. [DOI] [PubMed] [Google Scholar]


