Abstract
Breastfeeding is a dynamic biological and social process based on hormonal regulation involving oxytocin. While there is much work on the role of breastfeeding in infant development and on the role of oxytocin in socio-emotional functioning in adults, little is known about how breastfeeding impacts emotion perception during motherhood. We therefore examined whether breastfeeding influences emotion recognition in mothers. Using a dynamic emotion recognition task, we found that longer durations of exclusive breastfeeding were associated with faster recognition of happiness, providing evidence for a facilitation of processing positive facial expressions. In addition, we found that greater amounts of breastfed meals per day were associated with slower recognition of anger. Our findings are in line with current views of oxytocin function and support accounts that view maternal behaviour as tuned to prosocial responsiveness, by showing that vital elements of maternal care can facilitate the rapid responding to affiliative stimuli by reducing importance of threatening stimuli.
Breastfeeding is a dynamic process that is characterised by physical, hormonal, and psychosocial effects in both mothers and infants1,2,3. In particular, exclusive breastfeeding duration has been shown to play a vital role in bonding between mother and child through the promotion of positive affect and sensitive maternal behaviours4,5,6. Critically, exclusively breastfeeding mothers report lower stress, negative moods, and anxiety than formula-feeders7, and this anxiolytic impact is further evidenced through stronger cardiac vagal tone modulation, reduced heart rate reactivity8, blood pressure9 and reduced hypothalamic-pituitary-adrenal axis activity to psychosocial stress2. Reduced blood pressure has also been associated with the percentage of meals still breastfed9. At the hormonal level, lactation requires oxytocin, a neurohormone synthesised in the paraventricular and supraoptic nuclei of the hypothalamus. Milk let-down is a process in which infant suckling induces release of oxytocin into the bloodstream through the neurohypophysis, stimulating the extraction of milk through contraction of myoepithelial cells in the mammary gland8. Oxytocin is simultaneously released into the brain via projections from the paraventricular nucleus, allowing for the hormone to have a direct influence on behaviour11. The increase in peripheral oxytocin during breastfeeding has been well-documented in saliva and plasma of mothers during the feeding process12. Furthermore, breastfeeding mothers have higher levels of oxytocin in general as compared to formula-feeders13, making increased levels of oxytocin a characteristic trait of lactating mothers.
Oxytocin is well-known for its peripheral role in the female reproductive system; however research over the last two decades has demonstrated that it also plays a critical role in affiliative and prosocial behaviour14,15. Intranasal oxytocin administration increases the salience of eyes in faces, enhances trust, generosity, social memory, and emotion recognition in humans16,17,18. There is accumulating evidence that oxytocin might foster affiliative behaviour through both the perceptual enhancement of positive emotional cues and the perceptual reduction of negative, threatening cues19,20,21,22,23,24. This may be due to a mechanism in which oxytocin promotes the facilitation of approach-behaviours and reduces the tendency of withdrawal-behaviours25. Neuroimaging studies suggest this might be due to an attenuation of the amygdala as well as a reduction in functional coupling to regions of the brainstem in response to threatening scenes21,26,27.
As breastfeeding mothers exhibit an extended release of oxytocin12, the current study investigated whether breastfeeding experience promotes enhanced socio-emotional perception. Using a dynamic facial expression recognition task28, we explored whether breastfeeding behaviour, as indexed through both exclusive breastfeeding duration and current breastfeeding exposure (percentage of infant's diet still breastfed), is associated with individual differences in emotion processing in mothers. Based on theoretical accounts that assign a primary role to breastfeeding and oxytocin in maternal sensitivity4 and prior empirical work relying on intranasal administration of oxytocin18,19,20,21,22,23,24, we hypothesised that a greater exposure to breastfeeding would be associated with greater sensitivity to happy expressions while reducing sensitivity to expressions of threat (especially anger). As the majority of the aforementioned breastfeeding studies examined outcomes of exclusive breastfeeding duration, we used this index as our main variable of interest. Furthermore, focusing our investigation on differences in exclusive breastfeeding allowed us to examine variation within a group of breastfeeding mothers rather than coarsely contrasting formula feeding mothers to breastfeeding mothers.
Results
Breastfeeding characteristics
Out of 62 mothers included in our sample, the great majority (53 mothers: 85.5%), were still breastfeeding their infants on a daily basis. All mothers breastfed exclusively for at least 3 months (range = 91–213 days; M = 159.21, SD = 28.91) (Table 1). Using correlations, we examined the possibility that empathic concern, positive or negative affect, infant positive affect (smiling and laughter), maternal education, or parity impact breastfeeding behaviour. The duration of exclusive breastfeeding did not relate to any of these measures (all p-values > .18). However, the percentage of breastfed meals per day related to maternal education (r[62] = .32, p = .007), infant age (r[62] = −.346, p = .006), and exclusive breastfeeding duration (r[62] = .662, p < .001). Therefore years of maternal education and infant age were included as regression predictors in all analyses.
Table 1. Infant and maternal characteristics.
| Descriptives | Pearson's r | |||
|---|---|---|---|---|
| Range | M(SE) | EBF | %BFM | |
| EBF duration (days) | 91–213 | 159.21 (3.67) | .662*** | |
| Breastfed meals (%) | 0–100 | 63.75 (4.23) | .662*** | |
| Maternal | ||||
| Age (years) | 22–42 | 32.33 (0.53) | .135 | .201 |
| Education (years) | 10–26 | 15.28 (0.42) | −.016 | .340** |
| Other children (count) | 0–2 | 0.53 (0.07) | −.131 | −.035 |
| PANAS | ||||
| Positive affect | 19–38 | 14.75 (0.31) | −.174 | −.128 |
| Negative affect | 21–39 | 30.48 (0.81) | .228 | .201 |
| IRI | ||||
| Empathic concern | 10–20 | 14.75 (0.31) | .063 | −.019 |
| Perspective taking | 7–20 | 13.49 (0.39) | .009 | .039 |
| Personal distress | 5–17 | 11.36 (0.38) | −.116 | .029 |
| Infant | ||||
| Age (days) | 166–226 | 195.79 (2.45) | .060 | −.346** |
| IBQ-R | ||||
| Smiling & laughter | 2.50–5.89 | 4.23 (0.11) | −.185 | −.122 |
Note: EBF = Exclusive breastfeeding duration, PANAS = Positive and Negative Affect Schedule, IRI = Interpersonal Reactivity Index, IBQ-R = Revised Infant Behaviour Questionnaire;
**p < .01,
***p < .001.
DEER-T performance
In our analysis, we tested the hypothesis that greater exposure to breastfeeding would be associated with greater sensitivity to happy expressions and reduced sensitivity to threatening expressions (especially anger). As a first step, correlations were performed in order to investigate relationships between breastfeeding behaviour and DEER-T performance. All significant correlations were subsequently entered into multiple regression analyses. This initial correlation analysis mainly served a protective function, namely, to identify potentially confounding factors and include those in the regression model designed to test the main hypothesis. Exclusive breastfeeding duration correlated only with reaction time (RT) to happiness (r[62] = −.268, p = .035). The percentage of breastfed meals correlated significantly with false alarms (FAs) to anger and fear (r[62] = −.263, p = .039 and r[62] = −.348, p = .006, respectively) as well as RTs to anger and fear (r[62] = .287, p = .024 and r[62] = −.325, p = .010, respectively). With this information, forced-entry multiple regressions were conducted analysing FAs (anger, fear) and RTs (anger, fear, happiness). Exclusive breastfeeding duration, percentage of breastfed meals, infant age, and maternal education were entered as predictors. No predictors remained significant for FAs, suggesting that after including variance from other factors, FAs to anger and fear no longer related to the percentage of breastfed meals (all β-values < −.187, p-values > .240). Additionally, the percentage of breastfed meals no longer predicted the RT to fear (β < −.200, p > .343).
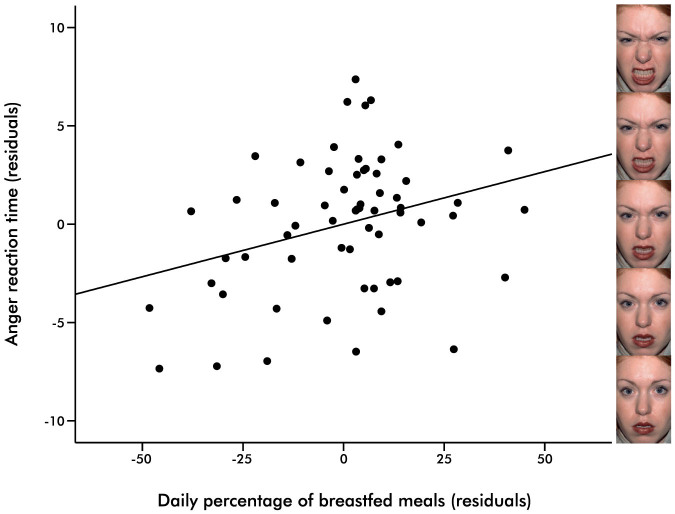
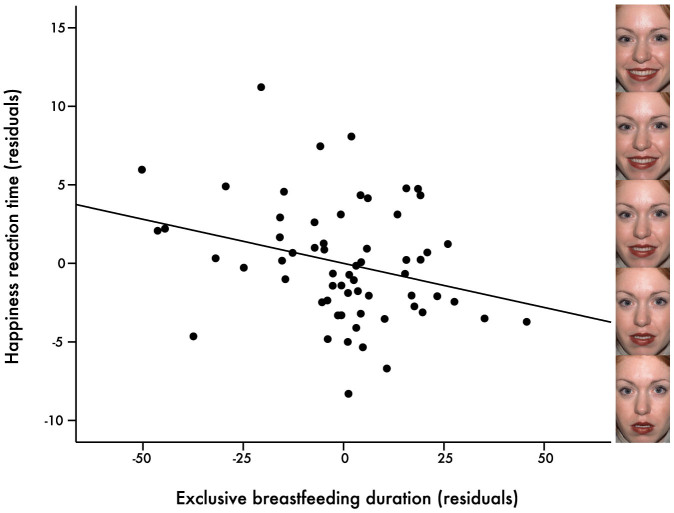
A significant impact of breastfeeding exposure remained for RTs to anger and happiness. The percentage of breastfed meals was the strongest predictor for the reaction time to anger (Table 2, Figure 1), in which the higher the percentage, the slower the RT to anger. Exclusive breastfeeding duration was the strongest predictor for the speed of processing happy facial expressions (Table 3, Figure 2). Specifically, our analysis revealed an acceleratory effect of exclusive breastfeeding on the RT to happiness, providing evidence for a facilitation of processing positive emotional signals. Note that all p-values reported here are uncorrected and would not survive a conservative Bonferroni correction.
Table 2. Summary of multiple regression analyses for variables predicting reaction time to anger, R2 = .161, N = 62.
| Variable | B | SE B | β | t | p-value |
|---|---|---|---|---|---|
| (Constant) | 42.23 | 7.21 | 5.86 | .000 | |
| EBF (in days) | −.05 | .03 | −.30 | −1.60 | .115 |
| Breastfed meals (%) | .07 | .03 | .53 | 2.54 | .014 |
| Infant age | .03 | .03 | .13 | .86 | .392 |
| Maternal education | −.01 | .18 | −.01 | −.05 | .961 |
Figure 1. Current breastfeeding behaviour and anger recognition.
Partial regression plot in which the percentage of currently breastfed meals predicts RT to anger, β = .53, p = .014. RT increases with a larger percentage of breastfed meals. Panel on the right depicts snapshots at five time points during a dynamic morph from neutral to anger. Note: This image is not covered by the CC BY-NC-ND licence. Photographs are from the NimStim Face Stimulus Set. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. (http://www.macbrain.org/resources.htm).
Table 3. Summary of multiple regression analyses for variables predicting reaction time to happiness, R2 = .142, N = 62.
| Model | B | SE B | β | t | p-value |
|---|---|---|---|---|---|
| (Constant) | 54.41 | 6.44 | 8.44 | .000 | |
| EBF (in days) | −.06 | .03 | −.42 | −2.25 | .029 |
| Breastfed meals (%) | .03 | .02 | .24 | 1.17 | .246 |
| Infant age | −.03 | .03 | −.15 | −1.06 | .293 |
| Maternal education | −.19 | .16 | −.16 | −1.15 | .254 |
Figure 2. Exclusive breastfeeding duration and happiness recognition.
Partial regression plot in which EBF significantly predicts RT to happiness, β = −.42, p = .029. RT decreases with a longer EBF duration. Panel on the right depicts snapshots at five time points during a dynamic morph from neutral to happiness. Note: This image is not covered by the CC BY-NC-ND licence. Photographs are from the NimStim Face Stimulus Set. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. (http://www.macbrain.org/resources.htm).
Discussion
In the current study, we examined whether breastfeeding exposure affects emotion recognition in mothers. Using a dynamic emotional face recognition task, we found that faster recognition of happiness was associated with longer exclusive breastfeeding duration in mothers. Furthermore, a greater percentage of currently breastfed meals was associated with slower reaction times toward anger. Thus, our data show that breastfeeding behaviour is related to an increased sensitivity to positive emotional cues and a decreased sensitivity to negative, threat-related cues. This pattern of results is in line with existing accounts of oxytocin function, according to which oxytocin is considered to enhance approach tendencies and inhibit withdrawal tendencies in an effort to facilitate prosocial behaviour25. Moreover, these findings generally support the notion that breastfeeding behaviour might be associated with what has been referred to as a positivity bias in emotion perception29. Critically, these findings cannot be explained by other variables such as maternal education, maternal mood, parity, or infant temperament, suggesting that variation in breastfeeding itself accounts for differences in emotion processing in motherhood.
Our findings support the view according to which maternal behaviour is tuned to prosocial responsiveness and bonding, and its vital elements can facilitate the rapid and accurate responding to affiliative cues30. In this context, it is important to note that the mothers in the current study showed this bias in their emotion recognition in response to unfamiliar persons. This suggests that the breastfeeding behaviour, while certainly having specific effects on the relationship between infant and mother3,4,5, is also linked to more general emotion processing differences that can be observed in response to unfamiliar persons. To find that specific aspects of maternal behaviour can have general effects on prosocial responsiveness is in line with accounts that assign an important role to maternal behaviours in the evolution of human prosociality31.
Oxytocin release is required for the process of milk let-down10, and its heightened release during breastfeeding has been well-documented12. Our results are in agreement with a host of studies showing that exogenous oxytocin administration impacts the perception of emotional expressions18,19,20,21,22,23,24. Specifically, the current findings are in line with oxytocin administration studies in that these studies also find an enhanced responding to positive emotional cues (related to approach tendencies) and a reduced responding to negative, threat-related cues (related to withdrawal tendencies)25. It is thus conceivable that our current findings are related to the endogenous release of oxytocin during breastfeeding. However, it must be stressed that we did not directly measure oxytocin levels in our sample of mothers. Therefore, until directly tested, increased oxytocin release must only be considered a potential pathway (among others) that can account for our current results.
The bias in emotion recognition observed in the current study may also be related to the noted anxiolytic effect of breastfeeding8. Indeed, there is evidence that anxiolytic drugs such as diazepam increase the sensitivity towards happiness32 while decreasing recognition of fear and anger in facial expressions33. This is also seen during administration of selective serotonin reuptake inhibitors (SSRIs), which exert anxiolytic effects34. Oxytocin itself exerts anxiolytic effects in both mice35 and humans36. It has close ties to the serotonergic system, and facilitates serotonin secretion in the raphe nucleus35. Moreover, breastfeeding involves skin-to-skin contact and pleasant touch, which has been found to influence emotion regulation, attention, joint engagement, and pain analgesia37,38. In this context, it should be emphasized that breastfeeding is a highly complex and dynamic biological and psychological process, and more systematic research is required to understand what exactly underpins the effects observed in the current study. Therefore, we would like to stress that our study is only a first step. Future work using a systematic assessment of hormonal, genetic, neural, and behavioural variables in mothers will be vital in order to achieve a more detailed and possibly more mechanistic understanding of the effects of breastfeeding on socio-emotional processing.
Another important issue raised by the current data is the question why exclusive breastfeeding duration is associated with recognition of happy faces, while current breastfeeding exposure relates to differences in anger recognition. One possibility is that there might be differences in emotion perception due to chronic (exclusive breastfeeding) versus acute (current breastfeeding) effects of breastfeeding exposure. More specifically, while long-term breastfeeding might lead to changes in receptor affinities or distributions (perhaps in the oxytocinergic system), more acute effects of breastfeeding might have the greatest impact on arousal systems important for threat detection, such as the amygdala and brainstem regions. To our knowledge, there is currently no research that has directly assessed this issue by comparing between current breastfeeding behaviour and exclusive breastfeeding duration. However, animal research confirms that chronic oxytocin exposure is capable of changing oxytocin receptor distributions39, and most imaging studies indicate that acute oxytocin administration seems to primarily target reactivity of the amygdala21,26,27,40. In future work it will be important to distinguish between long-term and short-term effects of breastfeeding.
Relatedly, it is unclear whether the positive and negative biases found in the current sample persist after the cessation of breastfeeding. Our study was not designed to directly address this question as only nine mothers in the current sample had ceased breastfeeding. However, an exploratory correlation analysis conducted with the nine mothers that had stopped breastfeeding revealed an association between exclusive breastfeeding duration and reaction time to happy expressions (r[9] = −.624, p = .037, one-tailed), similar to the mothers that were still breastfeeding. This suggests that some of the effects may persist after the cessation of breastfeeding, but clearly more work is needed to directly test this possibility. Finally, one limitation of the current study is that, as stated in the results section, we did not control for multiple comparisons and only report uncorrected results. However, it is important to note that the current study tested and provides support for a specific a priori hypothesis formulated on the basis of previous work19,20,21,22,23,24.
In conclusion, our results provide evidence that mothers who exclusively breastfeed for longer durations show an increased sensitivity to positive facial expressions. Moreover, mothers with an increased percentage of daily breastfed meals show a decreased sensitivity to anger. The finding that such emotional biases occur in the context of the psychological and biological processes associated with breastfeeding is testament for a need to better understand the impact that maternal behaviours in general and breastfeeding in particular have on socio-emotional functioning during motherhood.
Methods
Participants
62 healthy women of European descent (Mage = 32.33 years, SD = 4.17) participated in the study. All were mothers of 5- to 7-month-old infants (Mage = 6.43 months, SD = .63). All mothers were on maternity leave up to the point of testing. All gave informed consent and were compensated with travel money and a toy for their infant. Procedures were approved by the ethics committee of the Leipzig University Medical School and were conducted in accordance with the Declaration of Helsinki.
Questionnaires
We obtained information concerning maternal characteristics (age, years of education, number and age of other children, immigration history, highest academic/professional qualification) as well as the following information concerning breastfeeding: the duration a mother exclusively breastfed her child, at what age the child was introduced to other foods, and/or at what age the mother stopped breastfeeding, through an in-house developed questionnaire. As part of this questionnaire, a table was provided in which mothers could describe a feeding schedule over the course of a typical day. Based on this information, the frequency and percentage of breastfed meals per day was calculated. Durations provided in months were converted into days. If mothers were still exclusively breastfeeding, infants' age on testing day was used as exclusive breastfeeding duration to be as specific as possible. In addition, the Infant Behaviour Questionnaire in its revised form (IBQ-R), the Interpersonal Reactivity Index (IRI), and the Positive and Negative Affect Schedule (PANAS)41,42,43 were administered in order to investigate potential influences of infant temperament, maternal affect, and maternal empathy on both emotion recognition performance and breastfeeding behaviour. The IBQ-R is commonly used instrument to assess infant temperament through parental report. It includes subscales assessing infant expression of smiling and laughter, approach tendency, perceptual sensitivity, cuddliness, fear, rate of recovery from distress, and many others. Gartstein and Rothbart (2003) provide evidence supporting the instrument's high reliability and validity. The IRI (Davis, 1983) uses a multi-dimensional approach to assess empathy in adults. It assesses the tendency one has to take the point of view of others (PT), the tendency to experience feelings of sympathy and compassion for those less fortunate (EC), the tendency to experience feelings of distress in response to discomfort in others (PD), and the tendency to transpose oneself into fictional situations such as novels or movies (FS). The IRI has also been reported to be a reliable and valid instrument that assesses empathy in adults (Davis, 1983). The PANAS measures affect on two subscales in adults: positive affect (i.e., enthusiasm and alertness), and negative affect (i.e., aversive mood states and general distress). Watson and colleagues (1988) report high reliability and validity of the PANAS.
The dynamic emotional expression recognition task (DEER-T)
Emotion recognition was measured using the DEER-T, which has been used to study emotion recognition in various populations and is described in detail elsewhere28. Neutral colour photographs of twelve Caucasian actors (six women) were morphed to create six dynamic expressions: anger, happiness, fear, sadness, disgust, and neutrality. Images were taken from the NimStim set, which is freely available to the scientific community at http://www.macbrain.org/resources.htm44. Stimuli were displayed as dynamically morphing from neutral into a full-blown emotion over the course of 3000 milliseconds. Six labelled response keys corresponded to the emotions. To reduce memory load, an illustration of the keys in relation to finger positions was displayed on the monitor below the presented stimuli. Participants were instructed to press the key of the corresponding emotion as quickly and accurately as they could. Stimuli were presented on a 13-inch laptop.
Performance on the DEER-T was assessed using reaction time (RT), hits, and false alarms (FAs). RTs were analysed for correct answers only. Hits refer to the correct response frequency for a particular emotion, while FAs refer to the frequency a participant responded with an emotion when it was incorrect. RTs were square-root transformed to correct for positive skew. Data was analysed with IBM SPSS Statistics (Version 19).
Author Contributions
K.M.K. and T.G. conceived the study. K.M.K. conducted the experiment and analysed the data with supervision by T.G. K.M.K. and T.G. wrote the manuscript. S.K. and H.V.C. kindly supplied the DEER-T stimuli and provided comments on the manuscript.
Acknowledgments
This research was supported by funds awarded to T.G. by the Max Planck Society. We would like to thank all families who participated in this study.
References
- Deoni S. C. et al. Breastfeeding and early white matter development: A cross sectional study. NeuroImage 82, 77–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Neumann I. & Ehlert U. Lactation and stress: Protective effects of breast-feeding in humans. Stress 5, 195–203 (2002). [DOI] [PubMed] [Google Scholar]
- Raju T. N. Breastfeeding is a dynamic biological process—not simply a meal at the breast. Breastfeed. Med. 6, 257–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. et al. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiat. 52, 907–915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom R. Breastfeeding and infant-mother interaction. Acta Paediatr. 88, 1–6 (1999). [DOI] [PubMed] [Google Scholar]
- Brandt K. A., Andrews C. M. & Kvale J. Mother-infant interaction and breastfeeding outcome 6 weeks after birth. J. Obstet. Gynecol. Neonatal Nurs. 27, 169–174 (1998). [DOI] [PubMed] [Google Scholar]
- Groer M. W. Differences between exclusive breastfeeders, formula-feeders, and controls: A study of stress, mood, and endocrine variables. Biol. Res. Nurs. 15, 105–117 (2005). [DOI] [PubMed] [Google Scholar]
- Mezzacappa E. S., Kelsey R. M. & Katkin E. S. Breast feeding, bottle feeding, and maternal autonomic responses to stress. J. Psychosom. Res. 58, 351–365 (2005). [DOI] [PubMed] [Google Scholar]
- Hahn-Holbrook J., Holt-Lunstad J., Holbrook C., Coyne S. M. & Lawson E. T. Maternal defense: Breast feeding increases aggression by reducing stress. Psychol. Sci. 22, 1288–1295 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln D. W. & Paisley A. C. Neuroendocrine control of milk ejection. J. Reprod. Fertil. 65, 571–586 (1982). [DOI] [PubMed] [Google Scholar]
- Landgraf R. & Neumann I. D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrin. 25, 150–176 (2004). [DOI] [PubMed] [Google Scholar]
- Dawood M. Y., Khan-Dawood F. S., Wahi R. S. & Fuchs F. Oxytocin release and plasma anterior pituitary and gonadal hormones in women during lactation. J. Clin. Endocrinol. Metab. 52, 678–683 (1981). [DOI] [PubMed] [Google Scholar]
- Grewen K. M., Davenport R. E. & Light K. C. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology 47, 625–632 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. R. Oxytocin— A neuropeptide for affiliation: Evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 17, 3–35 (1992). [DOI] [PubMed] [Google Scholar]
- Heinrichs M., von Dawans B. & Domes G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 30, 548–557 (2009). [DOI] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P. J., Firschbacher U. & Fehr E. Oxytocin increases trust in humans. Nature 435, 673–676 (2005). [DOI] [PubMed] [Google Scholar]
- Zak P. J., Stanton A. A. & Ahmadi S. Oxytocin increases generosity in humans. PLoS One 2, e1128 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A. J., Mitchell P. B. & Matthews F. Oxytocin enhances the encoding of positive social memories in humans. Biol. Psychiatry 64, 256–258 (2008). [DOI] [PubMed] [Google Scholar]
- Di Simplicio M., Massey-Chase R., Cowen P. J. & Harmer C. J. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J. Psychopharmacol. 23, 241–248 (2009). [DOI] [PubMed] [Google Scholar]
- Domes G., Steiner A., Porges S. W. & Heinrichs M. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology 38, 1198–1202 (2013). [DOI] [PubMed] [Google Scholar]
- Kirsch P. et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A. A., Yu H. H., Pine D. S. & Blair R. J. Oxytocin improves specific recognition of positive facial expressions. J. Psychopharmacol. 209, 225–232 (2010). [DOI] [PubMed] [Google Scholar]
- Parr L. A., Modi M., Siebert E. & Young L. J. Intranasal oxytocin selectively attenuates rhesus monkeys' attention to negative facial expressions. Psychoneuroendocrinology 38, 1748–1756 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L., Lischke A., Greif J., Herpertz S. C., Heinrichs M. & Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology 36, 1378–1382 (2011). [DOI] [PubMed] [Google Scholar]
- Kemp A. H. & Guastella A. J. The role of oxytocin in human affect: A novel hypothesis. Curr. Dir. Psychol. Sci. 20, 222–231 (2011). [Google Scholar]
- Gamer M., Zurowski B. & Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci. U.S.A. 107, 9400–9405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P., Kalisch R., Singer T. & Dolan R. J. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J. Neurosci. 28, 6607–6615 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B., Kamboj S., Morgan C. J. & Curran H. V. Processing dynamic facial affect in frequent cannabis-users: evidence of deficits in the speed of identifying emotional expressions. Drug Alcohol Depend. 112, 27–32 (2010). [DOI] [PubMed] [Google Scholar]
- Peeters G. The positive-negative asymmetry: On cognitive consistency and positivity bias. Eur. J. Soc. Psychol. 1, 455–474 (1971). [Google Scholar]
- Feldman R. Parent-infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monogr. Soc. Res. Child Dev. 77, 42–51. [Google Scholar]
- Hrdy S. B. Mothers and others: The evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press (2009). [Google Scholar]
- Murphy S. E., Downham C., Cowan P. J. & Harmer C. J. Direct effects of diazepam on emotional processing in healthy volunteers. J. Psychopharmacol. 199, 503–513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangara A., Blair R. & Curran H. V. A comparison of the effects of a β-adrenergic blocker and a benzodiazepine upon the recognition of human facial expressions. Psychopharmacology 163, 36–41 (2002). [DOI] [PubMed] [Google Scholar]
- Harmer C. J., Shelley N. C., Cowen P. J. & Goodwin G. M. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am. J. Psychiatry 161, 1256–1263 (2004). [DOI] [PubMed] [Google Scholar]
- Yoshida M. et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 29, 2259–2271 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira D. C. G., Zuardi A. W., Graeff F. G., Queiroz R. H. C. & Crippa J. A. S. Anxiolytic-like effect of oxytocin in the simulated public speaking test. J. Psychopharmacol. 26, 497–504 (2012). [DOI] [PubMed] [Google Scholar]
- Feldman R., Rosenthal Z. & Eidelman A. I. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry 75, 56–64 (2014). [DOI] [PubMed] [Google Scholar]
- Gray L., Watt L. & Blass E. M. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics 105, e14 (2000). [DOI] [PubMed] [Google Scholar]
- Insel T. R., Winslow J. T. & Witt D. M. Homologous regulation of brain oxytocin receptors. Endocrinology 130, 2602–2608 (1992). [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Glascher J., Buchel C., Braus D. F. & Herpertz S. C. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry 62, 1187–1190 (2007). [DOI] [PubMed] [Google Scholar]
- Gartstein M. A. & Rothbart M. K. Studying infant temperament via the Revised Infant Behaviour Questionnaire. Infant Behav. Dev. 26, 64–86 (2003). [Google Scholar]
- Davis M. H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126 (1983). [Google Scholar]
- Watson D., Clark L. A. & Tellegen A. Development and validation of brief measures of positive and negative affect: the Positive and Negative Affect Schedule scales. J. Pers. Soc. Psychol. 54, 1063–1070 (1988). [DOI] [PubMed] [Google Scholar]
- Tottenham N. et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]