Abstract
Neovascular age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV) are leading causes of irreversible blindness in developed countries. In this study, we investigated the associations of haplotype-tagging single nucleotide polymorphisms (SNPs) in the complement component 3 (C3) gene with both neovascular AMD and PCV, and potential epistatic effects on C3. Eight tagging SNPs in C3 were genotyped in 708 unrelated study subjects: 200 neovascular AMD patients, 233 PCV patients and 275 controls. Among the eight C3 SNPs, rs17030 was associated with PCV after adjusted for gender and SNP-gender interaction (P = 0.008, OR = 2.94; 95% CI: 1.32-6.52). Moreover, an interaction between rs17030 and gender was identified in PCV (P = 0.02). After stratification by gender, the rs17030 G allele was found to confer an increased risk for PCV in male (P = 0.010, OR = 1.56) but not in female. The haplotype AG defined by the major alleles of rs17030 and rs344555 was also associated with PCV in male (P = 0.010, OR = 0.64). In contrast to PCV, none of the eight SNPs was significantly associated with neovascular AMD. This study shows an association of C3 rs17030 with PCV in male, indicating that C3 may have an epistatic effect with gender in the pathogenesis of PCV.
Age-related macular degeneration (AMD) is a degenerative disease at the central region of the retina – the macula. AMD is a leading cause of irreversible blindness among the elderly. Age, genetic susceptibility and environmental factors are the major risk factors. Advanced AMD is associated with poor central vision, and can be subdivided into geographic atrophy (GA) and neovascular AMD. In the Chinese population, GA is relatively rare while neovascular AMD is the main cause of vision loss1. Polypoidal choroidal vasculopathy (PCV) is characterized by inner choroidal vascular networks ending in polypoidal lesions diagnosed by indocyanine green angiography (ICGA)2. The incidence of PCV in neovascular AMD is high in Asian populations, ranging from 24.5% to 54.7%3. Moreover, PCV is more common in male than females in Asians, with a male to female ratio of about 33. Clinically, it remains debatable whether PCV is a subtype of neovascular AMD or a distinct disease entity, and whether it represents inner choroidal vascular abnormalities or a variant of choroidal neovascularization (CNV)4. Genetically, diversities also exist between AMD and PCV. The complement factor H gene (CFH) and the ARMS2-HTRA1 locus have been associated with AMD and PCV in different populations, but their effect sizes were different between the two diseases5,6. Recently, we also found that the association profiles of the superkiller viralicidic activity 2-like (SKIV2L)7 and cholesteryl ester transfer protein (CETP)8 genes are different between AMD and PCV. Moreover, we identified a significant interaction between CFH and CETP in both AMD and PCV8, suggesting epistasis could have played a role in their disease mechanisms. In the study, we found that the CFH rs800292 G allele conferred a significantly increased risk of the diseases only in individuals carrying the risk allele T of CETP rs3764261, suggesting CETP may exert an epistatic effect on CFH in the genetic mechanisms of AMD and PCV8.
Apart from CFH, other components in the complement pathway have also been suggested to play a role in AMD pathogenesis, such as involvement in drusen formation, retinal pigment epithelium deterioration, photoreceptor degeneration, and CNV progression9. The complement component 3 (C3) is the central component of the complement cascade. A nonsynonymous single-nucleotide polymorphism (SNP) in C3, rs2230199 (R102G), was found to be strongly associated with AMD10. However, this association was not identified in Chinese11,12. In Japanese, instead of rs2230199, a more common SNP rs2241394 was reported to be associated with AMD and PCV13,14. Therefore, the reported association profiles of C3 with AMD and PCV are inconsistent among populations. Recently, a rare missense variant (K155Q) in C3 was identified to confer a strong risk toward AMD15,16. Therefore, C3 could play an important role in AMD genetics, while having ethnic diversities in allelic distributions. So far however, little is known about the genetic profile of C3 in PCV.
In this study, we investigated the associations of the C3 gene with both neovascular AMD and PCV by using haplotype-tagging SNP analysis in a Han Chinese population. Also, we explored potential epistatic effects between different factors and C3 in the both diseases.
Results
A total of 708 unrelated study subjects were included, consisting of 200 patients with neovascular AMD, 233 patients with PCV, and 275 controls (Table 1). Notably, gender was not matched between the both disease groups and control group; therefore gender was adjusted in the association analysis using logistic regression. The mean age of the control individuals was significantly greater than that of the PCV patients (P<0.05). This is because we purposely recruited subjects older than 60 years as controls, with a view to reduce the confounding effects from younger subjects; thus age was not adjusted in association analysis.
Table 1. Characteristics of the Study Subjects.
| Comparison | |||||
|---|---|---|---|---|---|
| AMD (n = 200) | PCV (n = 233) | Control (n = 275) | AMD-Control | PCV-Control | |
| Gender (Male/Female) | 110/90 | 162/71 | 121/154 | P = 0.02 | P<0.05 |
| Mean age ± SD (years) | |||||
| general | 75.3 ± 7.7 | 68.5 ± 9.0 | 74.3 ± 7.6 | P = 0.16 | P<0.05 |
| male | 73.9 ± 7.4 | 68.9 ± 8.8 | 73.6 ± 7.1 | P = 0.75 | P<0.05 |
| female | 77.0 ± 7.7 | 67.6 ± 9.6 | 74.8 ± 7.9 | P = 0.04 | P<0.05 |
| Age range (years) | |||||
| general | 50–94 | 43–90 | 60–94 | NA | NA |
| male | 50–94 | 48–90 | 60–89 | NA | NA |
| female | 56–94 | 43–85 | 60–94 | NA | NA |
AMD: age related macular degeneration; NA: not applicable; PCV: polypoidal choroidal vasculopathy; SD: standard deviation.
According to the International HapMap Project for the Chinese Han population, the 8 tagging SNPs evaluated in this study captured 96% of alleles in the C3 locus with minor allele frequencies greater than 0.1 and a mean r2 of 0.97. All of the tested SNPs followed HWE in the control group (P>0.05). In single marker analyses, none of the 8 SNPs showed a significant allelic association with neovascular AMD or PCV (Table 2). Also, no SNP showed a significant association with the diseases in the dominant or recessive genetic models (data not shown). Moreover, no SNP showed a significant difference between neovascular AMD and PCV (Table 2).
Table 2. Allelic association of SNPs in C3 region with neovascular AMD and PCV.
| Minor allele frequency | Allelic association a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMD-control | PCV-control | AMD-PCV | |||||||||
| SNP | Location (residue change) | Minor allele | AMD (n = 200) | PCV (n = 233) | Control (n = 275) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) |
| rs2250656 | Intron 2 | G | 0.28 | 0.27 | 0.24 | 0.11 | 1.27 (0.95–1.71) | 0.24 | 1.19 (0.89–1.58) | 0.64 | 0.93 (0.69–1.26) |
| rs2230205 | Exon 14 (T612T) | A | 0.39 | 0.42 | 0.42 | 0.26 | 0.86 (0.66–1.12) | 0.94 | 0.99 (0.77–1.27) | 0.31 | 1.15 (0.88–1.51) |
| rs11672613 | Intron 17 | G | 0.42 | 0.44 | 0.43 | 0.67 | 0.95 (0.73–1.23) | 0.68 | 1.05 (0.82–1.35) | 0.43 | 1.12 (0.85–1.46) |
| rs428453 | Exon19 (V807V) | C | 0.20 | 0.17 | 0.16 | 0.11 | 1.32 (0.94–1.84) | 0.67 | 1.07 (0.77–1.50) | 0.25 | 0.82 (0.58–1.15) |
| rs2241392 | Intron 29 | G | 0.31 | 0.33 | 0.31 | 0.99 | 1.00 (0.76–1.32) | 0.45 | 1.11 (0.85–1.44) | 0.49 | 1.11 (0.83–1.47) |
| rs2241393 | Intron 29 | G | 0.36 | 0.36 | 0.34 | 0.72 | 1.05 (0.80–1.38) | 0.53 | 1.09 (0.84–1.41) | 0.81 | 1.03 (0.78–1.37) |
| rs344555 | Intron 37 | A | 0.28 | 0.26 | 0.25 | 0.40 | 1.13 (0.85–1.52) | 0.64 | 1.07 (0.81–1.42) | 0.71 | 0.95 (0.70–1.28) |
| rs17030 | Exon 41 (P1632P) | G | 0.43 | 0.45 | 0.41 | 0.69 | 1.06 (0.81–1.37) | 0.28 | 1.15 (0.90–1.47) | 0.53 | 1.09 (0.83–1.43) |
athe allelic association was adjusted for gender imbalance but not for SNP*gender interaction.
AMD: age related macular degeneration; C3: complement component 3; CI: confidence interval; OR: odds ratio; PCV: polypoidal choroidal vasculopathy; SNP: single nucleotide polymorphism.
In the epistatic analysis, no SNP*SNP interaction was detected between each C3 SNP and CFH rs800292, HTRA1 rs11200638, or CETP rs3764261 (data not shown). However, logistic regression analyses revealed that C3 rs17030 was significantly associated with PCV after adjusted for gender and SNP-gender interaction (P = 0.008, OR = 2.94, 95% CI: 1.32–6.52), and there was a significant interaction between rs17030 and gender in PCV (P = 0.02, Table 3). In contrast, gender independently was not associated with PCV (P = 0.06). Stratification by gender revealed that rs17030 was significantly associated with PCV in male but not in female (Table 4). The minor allele G of rs17030 showed a risk effect toward PCV in male (P = 0.010, OR = 1.56, 95% CI: 1.11–2.20). This gender difference was further confirmed by the homogeneity test of the ORs between men and women (Breslow-Day test: P = 0.019, Table 4). In the genotypic analysis, rs17030 showed a nominal association with PCV in male in the dominant and recessive models (P = 0.044 and 0.027, respectively). However, the P values could not withstand the Bonferroni correction (P>0.025). In female, this SNP was not associated with PCV in any models. Breslow-Day test showed a statistically significant difference in the ORs between male and female in the recessive model (P = 0.014) but not the dominant model (P = 0.15; Table 4). Such gender difference was not observed in neovascular AMD (Table 4). The other C3 SNPs were not associated with neovascular AMD or PCV after adjusted by gender and SNP-gender interaction (data not shown).
Table 3. Logistic regression analysis of C3 rs17030, gender and C3 rs17030*gender interaction.
| AMD | PCV | |||
|---|---|---|---|---|
| Variable | P Value | OR (95% CI) | P Value | OR (95% CI) |
| C3 rs17030 G | 0.46 | 1.37 (0.60–3.12) | 0.008 | 2.94 (1.32–6.52) |
| Gender, female | 0.25 | 0.71 (0.40–1.26) | 0.06 | 0.58 (0.33–1.02) |
| C3 rs17030*gender | 0.58 | 0.87 (0.52–1.45) | 0.02 | 0.54 (0.32–0.90) |
AMD: age related macular degeneration; C3: complement component 3; CI: confidence interval; OR: odds ratio; PCV: polypoidal choroidal vasculopathy.
Table 4. Association of C3 rs17030 with neovascular AMD and PCV by gender stratification.
| Minor allele frequency | Allelic association | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMD-control | PCV-control | |||||||||
| SNP | Description | Gender | Allele/genotype | AMD | PCV | Control | P | OR (95% CI) | P | OR (95% CI) |
| rs17030 | Exon 41 (P1632P) | Male | G | 87 (0.40) | 150 (0.46) | 86 (0.36) | 0.37 | 1.19 (0.81–1.73) | 0.010 | 1.56 (1.11–2.20) |
| A | 133 (0.60) | 174 (0.54) | 156 (0.64) | |||||||
| GG | 20 (0.18) | 35 (0.22) | 14 (0.12) | 0.35a | - | 0.034a | - | |||
| GA | 47 (0.43) | 80 (0.49) | 58 (0.48) | 0.83b | 1.06 (0.63–1.80) | 0.044b | 1.67 (1.01–2.74) | |||
| AA | 43 (0.39) | 47 (0.29) | 49 (0.40) | 0.16c | 1.70 (0.81–3.55) | 0.027c | 2.11 (1.08–4.12) | |||
| Female | G | 84 (0.47) | 59 (0.42) | 142 (0.46) | 0.9 | 1.02 (0.71–1.48) | 0.37 | 0.83 (0.56–1.24) | ||
| A | 96 (0.53) | 83 (0.58) | 166 (0.54) | |||||||
| GG | 18 (0.20) | 12 (0.17) | 38 (0.25) | 0.29a | - | 0.41a | - | |||
| GA | 48 (0.53) | 35 (0.49) | 66 (0.43) | 0.34b | 1.32 (0.74–2.35) | 0.84b | 0.94 (0.52–1.71) | |||
| AA | 24 (0.27) | 24 (0.34) | 50 (0.32) | 0.40c | 0.76 (0.41–1.44) | 0.19c | 0.62 (0.30–1.28) | |||
| Breslow-Day test (P value) | Allele model | 0.58 | 0.019 | |||||||
| Dominant model | 0.58 | 0.15 | ||||||||
| Recessive model | 0.11 | 0.014 | ||||||||
AMD: age related macular degeneration; C3: complement component 3; CI: confidence interval; OR: odds ratio; PCV: polypoidal choroidal vasculopathy.
aP values for the genotypic associations.
bP values in dominant genetic models.
cP values in recessive genetic models.
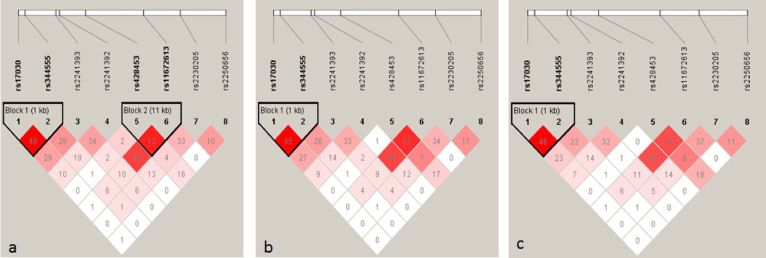
Linkage disequilibrium (LD) analysis revealed two haplotype blocks in neovascular AMD (Block 1 involves SNPs rs17030 and rs344555, Block 2 involves SNPs rs428453 and rs11672613) and one in PCV (involves SNPs rs17030 and rs344555; Figure 1). No haplotype was significantly associated with either disease. Since rs17030 showed an association with PCV in male, haplotype analyses were performed in different genders. The LD structure across C3 in male PCV patients was similar to that in all PCV patients, with rs17030 and rs344555 being in the same LD block (Figure 1). A haplotype AG, defined by the major alleles of the two SNPs, showed a significant protection for PCV in male (P = 0.010, permutation P = 0.030; OR = 0.64, 95% CI: 0.45–0.90). This haplotype was present in 53.7% of male patients and 64.5% of male controls. No significant haplotype association between C3 and PCV was detected in female.
Figure 1. Linkage disequilibrium (LD) structure of the C3 locus for neovascular AMD (a), PCV (b) and male PCV (c).
LD was measured using data from all controls and neovascular AMD or PCV in the present study. The haplotype blocks were defined by the confidence interval method implemented in the Haploview software. The LD (r2) between any two SNPs is listed in the cross cells. AMD, age related macular degeneration; C3, complement component 3; PCV, polypoidal choroidal vasculopathy.
Discussion
In this study, we have, for the first time, reported a haplotype tagging SNP analysis of the C3 gene concurrently in neovascular AMD and PCV. The 8 tagging SNPs capture the majority of common genetic variations in C3. Our results revealed that none of the tagging SNPs was associated with neovascular AMD, whereas rs17030 was associated with PCV after adjusted for gender and SNP-gender interaction. These findings suggest that in the Chinese population C3 may play a relatively more important role in PCV. Moreover, the male-specific association and SNP-gender interaction between rs17030 and PCV suggest additional risk factors should be required for C3 to exert its effect in the pathogenesis of PCV, likely through an epistatic function.
Results of this study enrich our knowledge of the genetic diversities of C3 in AMD and PCV. The nonsynonymous variant rs2230199 (R102G) in C3 was consistently associated with AMD in Caucasians, but not in Asian populations12. This is likely due to the difference in the frequencies of the risk allele of rs2230199 between Caucasians and Asians. The risk allele frequency of rs2230199 is relatively high in Caucasians (0.2)12, while being absent in Japanese and Korean (HapMap project), and rare in Chinese (~0.005)11,12. In contrast, SNP rs2241394, which has a minor allele frequency (MAF) of 0.11 in Japanese, was significantly associated with neovascular AMD (OR = 0.48)14. However, the MAF of rs2241394 is less than 0.05 in Chinese (HapMap project), therefore the statistical power to detect a significant association in our study would be less than 30%. Thus this SNP was not included in the present study. Notably, another SNP rs2250656 was reported to be associated with neovascular AMD in a Chinese cohort, with the minor allele showing a protective effect (allelic P = 0.02; OR = 0.61)17. However, our data did not support this association (allelic P = 0.11; OR = 1.27). Although our sample size only provided ~60% of power to detect an OR of 0.61 for rs2250656, the fact that our OR was toward a different direction suggested this SNP does not have a consistent effect on AMD.
PCV is more common in males than in females among Asians3. Our interaction analysis revealed a significant interaction between rs17030 and gender in PCV. By stratification, the association of rs17030 with PCV was significant in male, in agreement with the male-predominance in the prevalence of PCV. Our results indicated a risk effect of the rs17030 G allele and a protective effect of a haplotype containing the rs17030 A allele in male PCV patients. In contrast, the rs17030 G allele showed a trend of protection in female (OR = 0.83). As far as we know, such a gender difference in the association of C3 with PCV is reported for the first time. However, gender differences in the genetic profiles have been reported in glaucoma18 and uveitis19. In the study of Wiggs et al., associations of primary open angle glaucoma with several CAV1/CAV2 SNPs were found to be significant mostly in women18. Similarly, in the study of Yang et al., the CFH 184G was found as a genetic risk marker for anterior uveitis in Chinese females19. Our finding that C3 rs17030 was associated with PCV only in men suggests that the C3 gene is likely to be a risk factor for the male predominance of PCV, at least in Chinese. However, how C3 interacts with gender in PCV pathogenesis is not known. C3 is the core of the complement system, with all of the complement pathways converge at C3 cleavage to C3a and C3b. Plasma concentrations of the des Arg form of complement C3a, a reflection of complement activation, were higher in patients with AMD compared with controls20. The level of C3 increases with age from puberty to up to ~55 years and the C3 levels were not different between male and female21. However, there is a correlation of serum C3 with LDL-cholesterol after the age of 40 years in men and 60 years in women22. Higher total serum cholesterol and LDL levels are associated with increased risk of AMD23. Thus, a higher serum C3 level in association with higher LDL-cholesterol, may predispose men to AMD in an earlier age. It is noteworthy that we found a significant association between C3 rs17030 and PCV in men, although the G allele also showed a trend towards an increased AMD risk (OR 1.19 in AMD vs. 1.56 in PCV, Table 4). Thus, whether rs17030 is associated with C3 serum level, and whether the C3-LDL-cholesterol level has a stronger correlation with PCV than with AMD or controls in men, should be investigated. Smoking is another risk factor of AMD and PCV. A higher prevalence of male smokers may explain partly the higher prevalence of AMD and PCV in males24. In this study information on smoking status of the study subjects was incomplete, thus an interaction analysis of smoking and C3 SNPs in PCV was not possible. Two variants, R102G and P314L, of the C3 gene were found to significantly increased the risk of AMD independent of smoking25, suggesting that smoking may not have epistatic effect with C3 in the pathgenesis of AMD. Nevertheless, the epistatic effect between smoking and C3 is worth to be investigated in future studies.
In the current study, as the PCV patients were recruited consecutively and the gender ratio was not purposely confined, the proportion of male subjects was significantly higher in the PCV group than the control group. We therefore corrected the potential impact of gender difference by using logistic regression, which, in return, suggested that gender was not a significant confounding factor (P>0.05). Thus, the male-specific association between rs17030 and PCV should not be artifact due to gender imbalance in this study. Meanwhile, although significant association between rs17030 and PCV was identified in male after Bonferroni correction (P<0.025), and the protective association of the haplotype AG defined by rs17030 and rs344555 with PCV in male maintained significant after correction by permutation (Pperm = 0.03), the significance in allelic association would have disappeared if a more stringent Bonferroni correction as adopted, such as adjusting the P values by the number of SNPs × number of strata (i.e., 8 × 2 = 16). This is likely due to the relative small sample size in our study. Therefore, the role of rs17030 in PCV should be verified in larger study cohorts with matched gender and exhaustive information of smoking status. Also, whether the gender specific association of C3 with PCV exists in other ethnic groups remain to be investigated.
In summary, this present study shows that no SNP in C3 was significantly associated with neovascular AMD, whereas rs17030 was associated with PCV in male but not female. These findings reveal a gender-susceptibility pattern in the genetic association of C3 with PCV, suggesting an epistatic effect between C3 and gender in the pathogenesis of PCV. Further studies in larger cohorts and in other populations are warranted to confirm these new findings.
Methods
Study participants
All study subjects were Han Chinese recruited at the Hong Kong Eye Hospital and the Eye Centre of the Prince of Wales Hospital, Hong Kong. The study protocol was approved by the Ethics Committee on Human Research, the Chinese University of Hong Kong. Written informed consent was obtained from every subject. The study procedures were performed in accordance with the tenets of the Declaration of Helsinki.
The patients were given complete ophthalmic examinations, including ocular tonometry, best-corrected visual acuity measurement, slit-lamp biomicroscopy, color fundus photographs, fluorescein angiography, and ICGA. All AMD patients had neovascular AMD in at least one eye. PCV was diagnosed using ICGA. Patients with any eye with other causes of CNV, such as myopic maculopathy, or with both CNV and PCV lesions in the same or fellow eye, were excluded. Unrelated control subjects were recruited from people who attended the clinic for eye examinations. After complete ophthalmic examinations they were included on the following criteria: (1) age >60 years; (2) no age-related maculopathy or macular degeneration of any cause; and (3) no any other major eye diseases, except for mild senile cataracts and mild refractive errors (Table 1).
SNP selection and genotyping
Haplotype tagging SNPs across the C3 region were obtained from the International HapMap Project (http://hapmap.ncbi.nlm.nih.gov/, HapMap Genome Browser release #27, accessed Jun 20, 2012) for the Han Chinese in Beijing (CHB) population. Eight SNPs were selected by the tagger-pairwise method with R square and MAF values greater than 0.8 and 0.10 respectively. Genomic DNA was extracted from whole blood using a QIAamp Blood Kit (Qiagen, Hilden, Germany) according to the protocol from the manufacturer. The 8 candidate SNPs were genotyped using TaqMan genotyping assays (Applied Biosystems [ABI], Foster City, CA) on a Roche LightCycler® 480 Real-Time PCR System (Roche, Switzerland) according to the manufacturer's instructions.
Statistical analysis
Hardy-Weinberg Equilibrium (HWE) of individual SNP in the control group was tested using the exact test implemented in the software package PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/)26. Allelic or genotype association of each SNP was evaluated using the chi-square test or Fisher's exact test in PLINK. The odds ratio (OR) and corresponding 95% confidence interval (CI) were estimated with the major allele as reference. Pairwise linkage disequilibrium and haplotype associations were assessed using the Haploview software27. Haplotype blocks were determined using the confidence interval method in Haploview. Logistic regression was performed with gender and SNP-gender interaction to adjust the effect of gender on SNPs, referring to previously reported28. We stratified the study subjects according to gender and performed association analysis of the SNP in each gender stratum, and used the Breslow-Day test to examine the homogeneity of the OR in different gender strata. The SPSS software (ver.20.0, SPSS Inc., Chicago, IL) was used. Also, the epistasis algorithm in PLINK was applied to detect gene-gene interaction between the C3 SNPs and three major gene variants for AMD, CFH rs800292, HTRA1 rs11200638 and CETP rs3764261. Genotype data of the latter two SNPs were obtained from our previous studies7,8. For the significance threshold of P values, as no C3 SNP was found of significant association with overall AMD or PCV, correction for multiple testing was not required. In the interaction analysis, all items (either SNP*SNP or SNP*gender) with P<0.05 will be subjected to further stratification analysis. In the stratification analysis, the Bonferroni correction was applied to adjust the P values by the number of strata. In particular, since the SNP rs17030 was analyzed separately in males and females, a P value of less than 0.025 ( = 0.5/2) was considered statistically significant.
Author Contributions
K.L., C.P.P. and L.J.C. designed the experiments. K.L., S.C. and P.T. performed the experiments. K.L. performed the analysis and wrote the paper. T.L., V.C. and A.Y. contributed the clinical samples. C.P.P. and L.J.C. revised the paper. All authors contributed to the editing of the paper and to scientific discussions.
Acknowledgments
The authors express their highest gratitude to all the participants in this study. This study was supported in part by an Endowment Fund for Lim Por-Yen Eye Genetics Research Centre and the General Research Fund from the Research Grants Council (grant number: 473410), Hong Kong.
References
- Liu X. et al. Association study of complement factor H, C2, CFB, and C3 and age-related macular degeneration in a Han Chinese population. Retina 30, 1177–1184 (2010). [DOI] [PubMed] [Google Scholar]
- Sho K. et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 121, 1392–1396 (2003). [DOI] [PubMed] [Google Scholar]
- Laude A. et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res 29, 19–29 (2010). [DOI] [PubMed] [Google Scholar]
- Costa R. A. et al. Polypoidal choroidal vasculopathy: angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res 24, 560–586 (2005). [DOI] [PubMed] [Google Scholar]
- Liang X. Y. et al. Differentiation of exudative age-related macular degeneration and polypoidal choroidal vasculopathy in the ARMS2/HTRA1 locus. Invest Ophthalmol Vis Sci 53, 3175–3182 (2012). [DOI] [PubMed] [Google Scholar]
- Chen H. et al. Genetic associations in polypoidal choroidal vasculopathy: a systematic review and meta-analysis. Mol Vis 18, 816–829 (2012). [PMC free article] [PubMed] [Google Scholar]
- Liu K. et al. Associations of the C2-CFB-RDBP-SKIV2L locus with age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology 120, 837–843 (2013). [DOI] [PubMed] [Google Scholar]
- Liu K. et al. Genes in the high-density lipoprotein metabolic pathway in age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology 121, 911–916 (2014). [DOI] [PubMed] [Google Scholar]
- Hageman G. S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A 102, 7227–7232 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R. et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med 357, 553–561 (2007). [DOI] [PubMed] [Google Scholar]
- Tian J. et al. Association of Genetic Polymorphisms and Age-related Macular Degeneration in Chinese population. Invest Ophthalmol Vis Sci (2012). [DOI] [PubMed] [Google Scholar]
- Thakkinstian A. et al. Systematic review and meta-analysis of the association between complement component 3 and age-related macular degeneration: a HuGE review and meta-analysis. Am. J. Epidemiol. 173, 1365–1379 (2011). [DOI] [PubMed] [Google Scholar]
- Goto A. et al. Genetic analysis of typical wet-type age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese population. J Ocul Biol Dis Infor 2, 164–175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S. et al. A common complement C3 variant is associated with protection against wet age-related macular degeneration in a Japanese population. PLoS One 6, e28847 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason H. et al. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nat. Genet. 45, 1371–1374 (2013). [DOI] [PubMed] [Google Scholar]
- Zhan X. et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat. Genet. 45, 1375–1379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X. T. et al. Association of c3 gene polymorphisms with neovascular age-related macular degeneration in a chinese population. Curr Eye Res 34, 615–622 (2009). [DOI] [PubMed] [Google Scholar]
- Wiggs J. L. et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum. Mol. Genet. 20, 4707–4713 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. M. et al. CFH 184G as a genetic risk marker for anterior uveitis in Chinese females. Mol Vis 17, 2655–2664 (2011). [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S. et al. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol 125, 515–519 (2007). [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Kitajima H., Tanabe S., Ochi T. & Shinkai H. Effect of age on C1q and C3 levels in human serum and their presence in colostrum. Immunology 35, 523–530 (1978). [PMC free article] [PubMed] [Google Scholar]
- Muscari A. et al. Relationship between serum C3 levels and traditional risk factors for myocardial infarction. Acta Cardiol 53, 345–354 (1998). [PubMed] [Google Scholar]
- Reynolds R., Rosner B. & Seddon J. M. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology 117, 1989–1995 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cackett P. et al. Relationship of smoking and cardiovascular risk factors with polypoidal choroidal vasculopathy and age-related macular degeneration in Chinese persons. Ophthalmology 118, 846–852 (2011). [DOI] [PubMed] [Google Scholar]
- Despriet D. D. et al. Complement component C3 and risk of age-related macular degeneration. Ophthalmology 116, 474–480 (2009). [DOI] [PubMed] [Google Scholar]
- Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J. & Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005). [DOI] [PubMed] [Google Scholar]
- Liu L. Y., Schaub M. A., Sirota M. & Butte A. J. Sex differences in disease risk from reported genome-wide association study findings. Hum. Genet. 131, 353–364 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]