Abstract
Thalidomide has emerged as an effective agent for treating multiple myeloma, however the precise mechanism of action remains unknown. Agents known to target the isoprenoid biosynthetic pathway (IBP) can have cytotoxic effects in myeloma cells. The interactions between thalidomide and IBP inhibitors in human multiple myeloma cells were evaluated. Enhanced cytotoxicity and induction of apoptosis was observed in RPMI-8226 cells. Examination of intracellular levels of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) revealed a wide variance in basal levels and response to IBP inhibitors. These findings provide a mechanism for the differential sensitivity of myeloma cells to pharmacologic manipulation of the IBP.
Keywords: Myeloma, isoprenoid, lovastatin, thalidomide, zoledronic acid, farnesyl pyrophosphate, geranylgeranyl pyrophosphate
1. Introduction
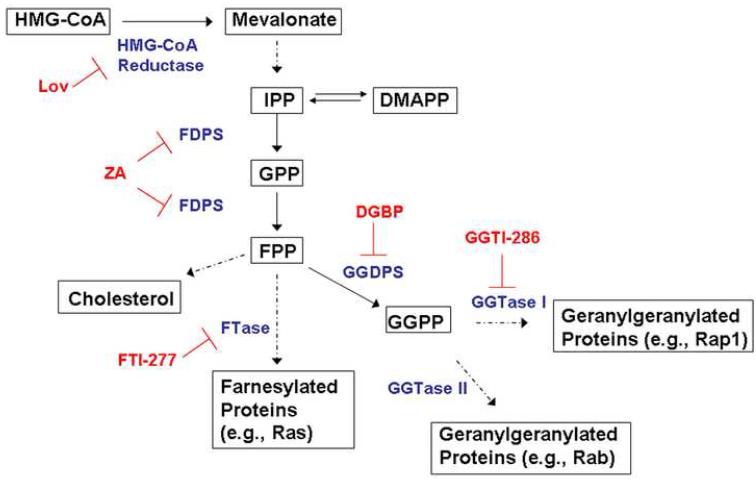
Multiple myeloma, a disorder of malignant plasma cells, accounts for approximately 10% of hematological malignancies and leads to 11,000 deaths per year in the United States. Thalidomide has been the standard of care for newly diagnosed multiple myeloma. Despite its wide-spread use, the precise mechanisms of action underlying thalidomide’s therapeutic effects in myeloma remain unknown. Thalidomide appears to have both direct and indirect effects on myeloma cells and in general, is thought to have immunomodulatory and antiangiogenic properties [1-4]. Zoledronic acid (ZA), an aminobisphosphonate, is widely used in the management of myeloma-induced bony disease. This agent has a very high affinity for bone mineral and causes inhibition of osteoclast function by inducing changes in the cytoskeleton, loss of the ruffled border, and apoptosis [5,6]. ZA also exerts direct effects on multiple myeloma cells, with prior studies showing that ZA can induce apoptosis in myeloma cell lines [7]. The mechanism of action for these effects is believed to be due to the ability of ZA and other aminobisphosphonates to inhibit farnesyl pyrophosphate synthase (FDPS), an enzyme which catalyzes a step in the isoprenoid biosynthetic pathway (IBP) (figure 1) [8-10].
Figure 1.
The isoprenoid biosynthetic pathway (IBP) and pharmacological inhibitors. Substrates and products of the pathway are shown in black, enzymes are shown in blue, and specific inhibitors are shown in red.
The IBP is responsible for the production of a variety of sterol and non-sterol moieties [11]. The rate limiting step in this pathway is catalyzed by the enzyme HMG-CoA reductase, leading to the production of mevalonate. FDPS catalyzes the conversion of the five-carbon units isopentyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) to the 10-carbon unit geranyl pyrophosphate (GPP), as well as the conversion of geranyl pyrophosphate to the 15-carbon unit farnesyl pyrophosphate (FPP). Geranylgeranyl pryophosphate (GGPP) synthase (GGDPS) catalyzes the addition of IPP to FPP, yielding the 20-carbon GGPP. Both FPP and GGPP serve as substrates for isoprenyltransferases, specifically farnesyl transferase (FTase) and geranylgeranyl transferases (GGTase) I and II, respectively. Prenylated proteins, including Ras and Rab family members, play central roles in diverse cellular processes, including cell survival, proliferation, differentiation, cytoskeletal organization, and membrane trafficking [12]. The function of these proteins is dependent on proper membrane localization, which is achieved in part through prenylation [13].
Preclinical studies have demonstrated that inhibitors of the IBP, including statins (HMG-CoA reductase inhibitors), aminobisphosphonates, and isoprenyltransferase inhibitors, can induce myeloma cell death [14-21]. The anti-myeloma effects of IBP inhibitors appear to be a consequence of disruption of geranylgeranylation, however the specific targets have not been delineated [21-24]. There has been a report evaluating the combination of thalidomide and lovastatin in ex vivo studies involving bone marrow mononuclear cells from patients with multiple myeloma [25]. In this study there appeared to be an increase in apoptosis with the combination of drugs. Increased cytotoxicity with the combination of ZA and thalidomide in RPMI-8226 cells, but not ARH-77 cells, has been demonstrated [26]. Finally, an interaction between simvastatin and lenalidomide, a second-generation immunomodulatory agent, has been observed in myeloma cells [27]. The mechanisms underlying these observations have yet to be defined.
In the studies presented here, the effects of combining thalidomide with inhibitors of the IBP in human myeloma cells are examined. Agents which specifically inhibit discrete steps in the IBP (lovastatin as an HMG-CoA reductase inhibitor, ZA as a FDPS inhibitor, digeranylbisphosphonate (DGBP) as a GGDPS inhibitor) or directly inhibit the prenyltransferases (FTI-277 as a FTI and GGTI-286 as a GGTI-I inhibitor) are utilized. These studies reveal differential sensitivity of myeloma cell lines not only to inhibitors of the IBP, but also to the combination of thalidomide with IBP inhibitors. FPP and GGPP levels, both basal and in response to IBP inhibitors, were found to vary amongst cell lines, providing a mechanism for the differential sensitivity.
2. Materials and Methods
2.1 Materials
Lovastatin, DL-mevalonic acid lactone (converted to mevalonate prior to use), farnesyl pyrophosphate, geranylgeranyl pyrophosphate, and thalidomide were obtained from Sigma (St. Louis, MO). Zoledronate was purchased from Novartis (East Hanover, NJ). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), FTI-277, GGI-286 were obtained from Calbiochem (San Diego, CA). Digeranyl bisphosphonate [28] was supplied by Terpenoid Therapeutics, Inc (Coralville, IA). Anti-pan-Ras was obtained from InterBiotechnology (Tokyo, Japan). Anti-PARP, anti-β-tubulin, anti-Rap1a, anti-Rab6, and anti-goat IgG horseradish peroxidase (HRP) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-mouse and anti-rabbit HRP-linked antibodies were obtained from Amersham (GE Healthcare, Piscataway, NJ). Annexin V-FITC was obtained from BD Pharmingen (BD Biosciences, San Jose, CA). D*-GCVLS and D*-GCVLL (dansyl-labeled peptides) were obtained from Bio-Synthesis (Lewisville, TX). Rat recombinant FTase and GGTase I were purchased from Jena Biosciences (Jena, Germany). HPLC-grade water was prepared with a Milli-Q system (Millipore, Bedford, MA). All solvents were optima or HPLC grade.
2.2 Cell cultures
Human multiple myeloma cell lines (RPMI-8226, H929, U266) were purchased from American Type Culture Collection (Manassas, VA). Cells were grown in RPMI-1640 media with 10% (RPMI-8226, H929) or 15% (U266) heat-inactivated fetal calf serum (per ATCC suggestion) supplemented with glutamine and penicillin-streptomycin at 37 °C and 5% CO2.
2.3 MTT Assay
Cells were seeded (5 × 104 cells/150 μL per well) in 96-well flat-bottom plates. Cells were incubated with drugs for 24-48 hours at 37 °C and 5% CO2. The MTT assay was performed as previously described [29]. The absorbance for control cells treated with solvent only was defined as an MTT activity of 100%.
2.4 Annexin V staining and flow cytometry
Following incubation with drugs, cells (0.5-0.75 × 106 cells/sample) were washed with PBS, pelleted, and then resuspended in HEPES buffer solution (10 mM HEPES, 150 mM NaCl, 1 mM MgCl2, 5 mM KCl, 1.8 mM CaCl2). Annexin V-FITC (2.5 μg/mL) was added a 10-15 minute incubation at room temperature was performed. Propidium iodide solution (1 μg/mL) was then added. Flow cytometry was performed with a Becton Dickinson FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cellquest software (Becton Dickinson) was used for acquisition (Cellquest V3.3) and analysis (Cellquest Pro V4.0) of data. Forward scatter (FSC) and orthogonal scatter (SSC) were collected using linear amplification. Annexin V FITC and propidium iodide fluorescence were collected using log amplification. 10,000 events were collected in listmode. A bitmap gate was placed around the cell population on the basis of forward and orthogonal light scatter to eliminate small debris and aggregates. The bitmap was large enough so that apoptotic cells were not eliminated. Cells satisfying the bitmap gate were analyzed using quadrant statistics in an Annexin V FITC versus propidium iodide dual parameter histogram.
2.5 Western blot analysis
Cells (5 ×106/5 mL) were incubated drugs. At the conclusion of the incubations, cells were collected, washed with PBS, and lysed in RIPA buffer (0.15M NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton (v/v) X-100, 0.05 M Tris HCl) containing protease and phosphatase inhibitors. Protein content was determined using the bicinchoninic acid method (Pierce Chemical, Rockford, IL). Equivalent amounts of cell lysate were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membrane, probed with the appropriate primary antibodies, and detected using HRP-linked secondary antibodies and Amersham Pharmacia Biotech ECL Western blotting reagents per manufacturer’s protocols.
2.6 Intracellular FPP and GGPP measurements
Intracellular FPP and GGPP levels were measured using the previously reported reversed phase HPLC methodology [30]. Briefly, following incubation with drugs, cells were collected and counted using Trypan blue staining and a hemocytometer. Cells were then washed with PBS. Isoprenoid pyrophosphates were extracted from cell pellets (3-5 × 106 cells/sample) with 2 × 1.2 mL of extraction solvent (butanol /75 mM ammonium hydroxide/ethanol 1:1.25:2.75). After drying down by nitrogen gas, the FPP and GGPP in the residue were incorporated into fluorescent GCVLS or GCVLL peptides by farnesyltransferase or geranylgeranyl transferase I. The prenylated fluorescent peptides were separated and quantified by reversed phase HPLC with fluorescence detection.
2.7 Statistical analysis
Two-tailed t-testing was used to calculate statistical significance. An α of 0.05 was set as the level of significance. Comparisons were performed between IBP inhibitor alone versus IBP inhibitor plus thalidomide treatments.
3. Results
3.1 Cytotoxic effects of IBP inhibitors and thalidomide
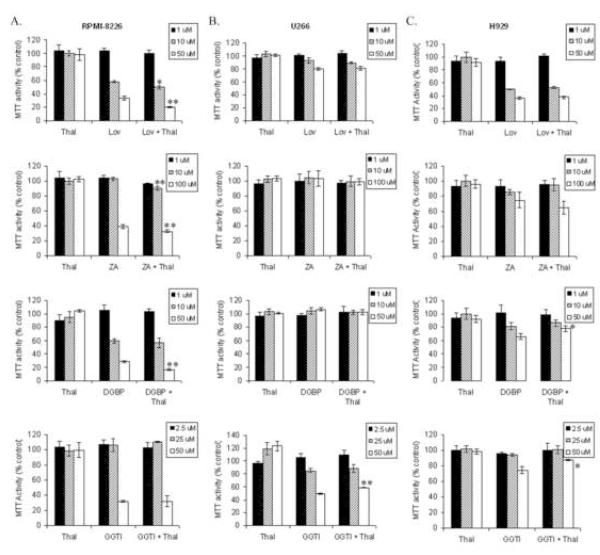
MTT assays, which involve the measurement of mitochondrial dehydrogenase activity [31], were performed to evaluate the cytotoxic effects of combining thalidomide with IBP inhibitors in multiple myeloma cells. As shown in figure 2, treatment with thalidomide (1-100 μM) alone does not diminish MTT activity. Treatment with lovastatin (1-50 μM) for 48 hours results in a concentration-dependent decrease in MTT activity in all three cell lines, with the RPMI-8226 cells (figure 2A) displaying the most sensitivity and the U266 cells (figure 2C) displaying the least sensitivity. While ZA (1-100 μM) and DGBP (1-50 μM) decrease MTT activity in the RPMI-8226 and H929 cells, no effect was seen in the U266 cell line. The H929 cells were more sensitive to lovastatin than to ZA or DGBP. Addition of thalidomide to lovastatin, ZA, or DGBP resulted in enhanced cytotoxicity in the RPMI-8226 cells (p<0.05), but not the U266 or H929 cells. These effects were most evident at 48 hours, but the interactions between thalidomide and lovastatin or DGBP were also observed at 24 hours in the RPMI-8226 cell line (data not shown). A farnesyl transferase inhibitor, FTI-277 (2.5-50 μM), either alone or in combination with thalidomide, did not decrease MTT activity in any cell line (Supplementary figure 1). In contrast, GGTI-286 (2.5-50 μM), a geranylgeranyl transferase I inhibitor, diminishes MTT activity in a concentration-dependent manner in each cell line. Interestingly, however, addition of thalidomide abrogated these effects at higher concentrations of GGTI-286 (p<0.05).
Figure 2.
The cytotoxic effects of thalidomide and IBP inhibitors in myeloma cells. Cells (RPMI-8226 (A), H929 (B), U266 (C) were incubated with equimolar concentrations of drugs (lovastatin, Lov; GGTI-286, GGTI) for 48 hours prior to the addition of MTT. Data are expressed as a percentage of control. The * denotes p<0.05 and ** denotes p<0.01 per unpaired two-tailed t-test comparing thalidomide + IBP inhibitor to IBP inhibitor alone.
3.2 Induction of apoptosis by IBP inhibitors and thalidomide
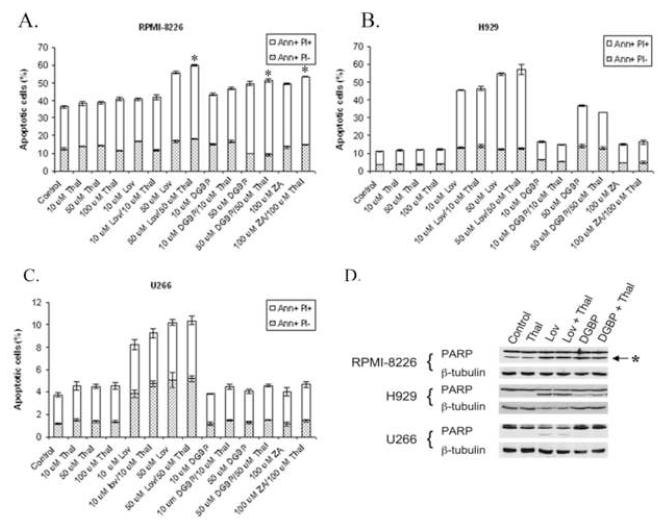
In order to determine whether the cytotoxicity observed in the MTT studies correlates with induction of apoptosis, Annexin V/propidium iodide (PI) flow cytometry studies were performed. During apoptosis, phosphatidylserine changes location in the cell membrane, and this can be detected with Annexin V [32]. Co-staining with Annexin V and propidium iodide (PI) differentiates among viable cells (Annexin V-negative, PI-negative), early apoptotic cells (Annexin V-positive, PI-negative) and late apoptotic/necrotic cells (Annexin V-positive, PI-positive). As shown in figure 3, incubation with lovastatin for 48 hours results in a concentration-dependent increase in apoptotic cells in all three cell lines (figure 3A-C). Consistent with the findings from the MTT studies, neither ZA nor DGBP induces apoptosis in the U266 cell line. In the H929 line, higher concentrations of DGBP did induce apoptosis, with only minor effects seen with ZA. Treatment with thalidomide alone did not induce an increase in Annexin V-positive cells, compared with control. Addition of thalidomide to lovastatin, ZA, or DGBP enhances the induction of apoptosis in the RPMI-8266 cells (p<0.05), but not in the U266 or H929 cells.
Figure 3.
The effect of thalidomide and IBP inhibitors on induction of apoptosis in myeloma cells. Annexin V, propidium iodide (PI) flow cytometric experiments were performed in RPMI-8226 (A), H929 (B), and U266 (C) cells. Cells were incubated for 48 hours. The percentages of cells in the early apoptotic (Annexin V positive, PI negative (Ann+ PI-)) and late apoptotic/necrotic (Annexin V positive, PI positive (Ann+ PI+)) fractions are shown (n =3). * denotes p<0.05 per unpaired two-tailed t-test comparing thalidomide + IBP inhibitor to IBP inhibitor alone. D) Immunoblots depicting PARP cleavage. Cells were incubated for 48 hours with combinations of thalidomide (Thal, 50 μM), lovastatin (Lov, 50 μM) or DGBP (50 μM). The “*” denotes the C-terminal PARP cleavage product. β-Tubulin is shown as a loading control.
Induction of apoptosis was confirmed by evaluating PARP cleavage. As shown in figure 3D, treatment of cells with lovastatin ± thalidomide results in PARP cleavage in all three cell lines. DGBP ± thalidomide induces PARP cleavage in RPMI-8226 cells, and to a lesser extent in H929 cells, but not in U266 cells.
3.3 IBP inhibitor-induced cytotoxicity and apoptosis is dependent on isoprenoid depletion
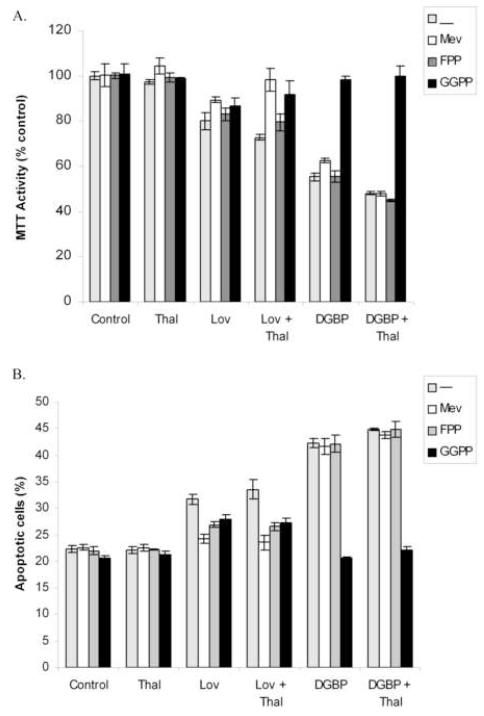
To determine whether the cytotoxicity and apoptosis induced by lovastatin or DGBP, with or without thalidomide, is due to isoprenoid depletion, add-back experiments were performed in the RPMI-8266 cells. As shown in figure 4A, mevalonate, FPP, or GGPP alone or in the presence of thalidomide did not induce a change in MTT activity. Lovastatin-induced cytotoxicity (with or without thalidomide) could be prevented by incubation with mevalonate or GGPP, with little effect of FPP. DGBP-induced decrease in MTT activity (in the presence of absence of thalidomide) was prevented only by co-incubation with GGPP. Similar results were obtained when Annexin-V/PI studies were performed (figure 4B). Incubation with mevalonate, FPP, or GGPP alone did not induce apoptosis. Mevalonate prevented lovastatin ± thalidomide-induced apoptosis, but not DGBP ± thalidomide-induced apoptosis. FPP and GGPP only partially prevented lovastatin ± thalidomide-induced apoptosis, while only GGPP prevented DGBP ± thalidomide-induced apoptosis.
Figure 4.
Add-back of select IBP intermediates prevents thalidomide/IBP inhibitor-induced cytotoxicity (A) and apoptosis (B) in RPMI-8226 cells. Cells were incubated for 48 hours in the presence or absence of thalidomide (10 μM) + IBP inhibitors (10 μM lovastatin or 10 μM DGBP) and IBP intermediates (1 mM mevalonate (Mev), 10 μM FPP, or 10 μM GGPP) prior to addition of MTT (A) or staining with Annexin V/PI (B). For the MTT assay, data are expressed as a percentage of control (n=4; mean ± standard error) and for the Annexin V/PI assay, data are expressed as the percentage of all Annexin-positive cells (n=2).
3.4 Effects of IBP inhibitors on protein prenylation
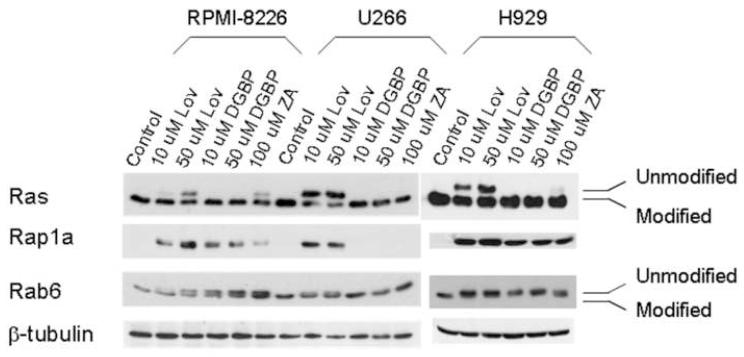
The cytotoxicity and apoptosis studies demonstrated differential effects of the IBP inhibitors, both alone and in combination with thalidomide, in multiple myeloma cells. Inhibition of protein prenylation is considered to be one of the major mechanisms of action underlying the cytotoxic effects of IBP inhibitors. Therefore, the effects of the IBP inhibitors on protein prenylation in these myeloma cell lines were examined. Ras is preferentially farnesylated by FTase, while Rap1a and Rab6 are preferentially geranylgeranylated via GGTase I and GGTase II respectively. As shown in figure 5, treatment with lovastatin results in inhibition of both farnesylation and geranylgeranylation in all three cell lines, as demonstrated by the appearance of a more slowly migrating band for Ras and Rab6 representing unmodified protein. The antibody used in the Rap1a immunoblots recognizes unmodified protein only. Treatment with the GGDPS inhibitor, DGBP, inhibits Rap1a and Rab6 geranylgeranylation, but does not affect Ras prenylation in RPMI-8226 cells. Similar effects were observed in the H929 cell line. Interestingly however, inhibition of geranylgeranylation (either Rap1a or Rab6) could not be detected in the U266 cell line. The FDPS inhibitor, ZA, inhibits farnesylation and geranylgeranylation in RPMI-8226 and H929 cells, but not U266 cells. The addition of thalidomide to IBP inhibitors did not alter the observed effects on protein prenylation (data not shown).
Figure 5.
Effects of IBP inhibitors on protein prenylation in myeloma cells. Cells were incubated for 24 hours in the presence or absence of drugs. The Rap1a antibody recognizes only the unmodified form of Rap1a. β-Tubulin is shown as a loading control.
3.5 Effects of IBP inhibitors on intracellular FPP and GGPP levels
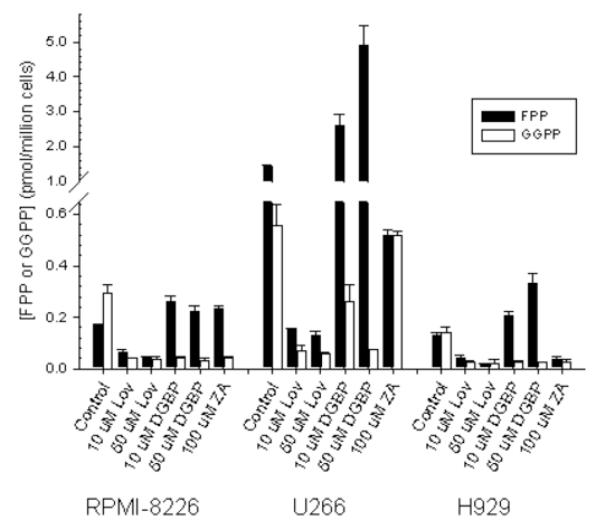
The mechanism underlying the resistance to inhibition of protein prenylation observed in the U266 cells to ZA and DGBP, but not lovastatin, was unknown. These three agents, as a consequence of targeting different enzymes in the IBP, have predicted effects on FPP and GGPP levels. Lovastatin, as an HMG-CoA reductase inhibitor, decreases mevalonate production, and therefore both FPP and GGPP production. ZA, as a FDPS inhibitor, should decrease FPP synthesis and therefore decrease GGPP production as well. DGBP, as a GGDPS inhibitor, should decrease GGPP synthesis and lead to an accumulation of FPP [33]. In order to determine whether these agents induced the predicted effects, FPP and GGPP levels were measured in the three cell lines (figure 6). Basal levels of GGPP are higher than FPP in the RPMI-8226 cells. Treatment with lovastatin decreases both FPP and GGPP levels. Likewise, treatment with DGBP decreases GGPP levels but increases FPP levels. Interestingly, 100 μM ZA also increases FPP levels while decreasing GGPP levels in the RPMI-8266 line. Unexpectedly, U266 cells were found to have markedly elevated basal levels of FPP and GGPP (8.6-fold and 1.9-fold compared to RPMI-8226 cells for FPP and GGPP respectively). In RPMI-8226 cells, treatment with 10 μM DGBP results in a decrease in the GGPP level comparable to that induced by 10 μM lovastatin. However, in the U266 cell line, while 10 μM DGBP does lead to a reduction in GGPP, the level remains significantly higher than that induced by lovastatin. Only with a higher concentration of DGBP does the GGPP level fall to a level equivalent to lovastatin-treated cells. While DGBP induces a 1.36-fold increase in FPP levels in the RPMI-8226 cells, the induced accumulation of FPP in the U266 cells is even higher (3.5-fold increase). ZA does decrease FPP levels (by 63%), however the GGPP level is not affected. Finally, the H929 cells, in contrast to the other cell lines, have equal basal levels of FPP and GGPP. The three IBP inhibitors behave as would be predicted in this cell line, with lovastatin decreasing both FPP and GGPP, DGBP increasing FPP and decreasing GGPP, and ZA decreasing both FPP and GGPP. FPP and GGPP levels in cells treated with 100 uM of thalidomide alone were within 20 % of control levels (data not shown) in all three cell lines. Likewise, addition of equimolar thalidomide to an IBP inhibitor (10 uM lovastatin, 10 uM DGBP, or 100 uM ZA) did not significantly alter levels compared to the IBP-treated cells alone in RPMI-8226 cells (data not shown). Finally, as expected the peptidomimetic inhibitors FTI-277 and GGTI-286 (25 uM) do not alter levels of FPP or GGPP in RPMI-8226 cells (data not shown).
Figure 6.
Effect of IBP inhibitors on FPP and GGPP levels in myeloma cells. Cells were incubated for 24 hours in the presence or absence of drugs. Data are presented as mean and standard deviation (n = 2-4 independent experiments).
4. Discussion
Currently, the vast majority of patients with multiple myeloma will receive thalidomide at some point during the course of their disease. However, thalidomide’s mechanism of action against this disease remains unknown. Furthermore, the optimal combination of agents to treat myeloma has yet to be determined. Prior studies have revealed evidence for an interaction between thalidomide and HMG-CoA reductase inhibitors or FDPS inhibitors in myeloma cell lines or primary myeloma cells [25,26]. Our studies, which utilize all available inhibitors of the IBP, reveal an intriguing interaction between thalidomide and select inhibitors of the IBP which is not generalizable across all cell lines. This interaction provides insight not only into the relevance of the IBP and its products in myeloma pathophysiology, but also into thalidomide’s effects.
The specific IBP inhibitors which yielded this interaction in the RPMI-8226 cells, namely an HMG-CoA reductase inhibitor, a FDPS inhibitor, and a GGDPS inhibitor, all share the ability of lowering intracellular GGPP levels, while having disparate effects on FPP levels (figure 6). This suggests that inhibition of geranylgeranylation, as a consequence of depletion of GGPP, is a necessary component of this interaction. An alternative hypothesis is that depletion of GGPP, and not an effect on protein geranylgeranylation, is important. That the interaction was not observed in cells treated with thalidomide and a GGTase I inhibitor, suggests that inhibition of GGTase II substrates (Rab proteins) may be the important target. Of note, a GGTase II inhibitor has been shown to induce myeloma cell apoptosis in vitro and to reduce bone loss in a murine model of multiple myeloma [34,35].
Interestingly, the enhanced cytotoxicity induced by thalidomide and IBP inhibitors was not observed in all cell lines. Therefore, although depletion of GGPP is necessary for the interaction, it does not appear to be sufficient. This may reflect intrinsic differences amongst cell lines and/or response to thalidomide. It is recognized that thalidomide has weak effects on proliferation (as measured by [3H]-thymidine incorporation into DNA) and apoptosis in cultured myeloma cells [2]. One clue into thalidomide’s mechanism action may come from the finding that IL-6 can inhibit thalidomide’s effects [2]. It has been well-delineated that IL-6 mediates its effects through activation of the Ras-MAPK pathway [36-38]. There are differences in the tested cell lines with respect to Ras mutation status (RPMI-8226 mutated N-Ras, H929 mutated K-Ras, U266 wildtype N- and K-Ras) [39]. However, statins (and presumably other IBP inhibitors which inhibit isoprenylation by depleting cells of FPP/GGPP) have been shown to inhibit cell growth regardless of Ras mutational status, presumably as these agents can disrupt Ras intracellular localization independent of the activation state of Ras [40,41]. The myeloma cell lines used in these studies have complex cytogenetic abnormalities and there may be other factors which contribute to their differential sensitivity to thalidomide.
Our results, for the first time, demonstrate marked variation amongst human multiple myeloma cell lines, not only with respect to basal levels of FPP/GGPP and the FPP/GGPP ratio, but also in the changes in these levels in response to IBP inhibitors. The markedly elevated levels of FPP and GGPP in the U266 cell line explains the observed resistance to IBP inhibitors, specifically those inhibitors targeting enzymes further downstream in the IBP. That this cell line is sensitive to GGTI-286 is likely a consequence of this agent’s mechanism of action—it is a competitive inhibitor of GGTase I with respect to the protein substrate and not the isoprenoid substrate. Therefore, the ability of GGTI-286 to inhibit geranylgeranylation is independent of isoprenoid pools. It is likely, however, that the high GGPP levels this cell line would render it resistant to a GGTase I inhibitor which was competitive with respect to GGPP.
Our previous work has demonstrated that pools of FPP and GGPP are detectable under steady state conditions in both cell lines and mammalian tissues [30,42]. Absolute levels vary across organ type as does the ratio of FPP to GGPP [42]. While one can speculate as to why certain tissue types might have higher requirements for isoprenoid intermediates based on specialized cell/organ function, it is unexpected to find the degree to which levels and ratios vary within a single malignancy. This raises the intriguing possibility that regulation of the IBP, as well as flux through the pathway, is altered in multiple myeloma. Whether this is a consequence of altered expression and/or activity of individual enzymes within the IBP remains to be determined.
Previous work has demonstrated differential sensitivity of multiple myeloma cell lines to lovastatin [43]. Microarray analysis revealed a number of differentially expressed genes in the sensitive cell lines, but no common differentially expressed genes in the three insensitive cell lines that were examined. Interestingly, IPP δ isomerase, responsible for the conversion between IPP and DMAPP (figure 1) was noted to be upregulated in several insensitive lines as well as a sensitive line [43]. Our results provide insight into the mechanism underlying differential sensitivity (i.e., lower basal levels correlates with increased sensitivity to IBP inhibitors) and offer a novel method for determining IBP inhibitor sensitivity.
The finding that ZA has disparate effects on FPP levels in the various cell lines (Figure 6) is unexpected. ZA is a potent inhibitor of FDPS [44] and, as such, would be expected to decrease both FPP and GGPP levels. That this effect was observed in only one cell line raises several hypotheses: 1) ZA has activity against other IBP enzymes, 2) FDPS activity varies amongst cell lines, or 3) treatment with ZA induces changes in FDPS activity. With regard to the first hypothesis, nitrogen-containing bisphosphonates (including ZA) have failed to show activity against IPP isomerase [44]. It is possible that ZA may have some activity against squalene synthase, which would lead to an increase in FPP levels. With regard to the second hypothesis, FDPS has been shown to be upregulated in several malignancies [45,46]. Thus one explanation is that FDPS activity or expression varies amongst the tested cell lines. Finally, there is evidence that ZA may upregulate FDPS. In a study involving rats, FDPS mRNA levels were found to increase in kidney tissue following treatment with ZA [47]. Further studies are required to explore the mechanisms underlying the effects observed with ZA, however, it seems likely that there is a complex relationship between isoprenoid pool sizes, IBP enzyme activity, and IBP inhibitors.
In conclusion, these studies demonstrate an interaction between thalidomide and IBP inhibitors which is dependent on depletion of GGPP. These studies reveal that the differential sensitivity of the myeloma cell lines to IBP inhibitors is a consequence of isoprenoid pool sizes. Further studies will determine the extent to which isoprenoid pool sizes vary in primary samples and may ultimately allow for the identification of multiple myeloma patients who would benefit from the addition of an IBP inhibitor to a thalidomide-containing regimen.
Supplementary Material
Acknowledgements
This project was supported by the Roy J. Carver Charitable Trust as a Research Program of Excellence and the Roland W. Holden Family Program for Experimental Cancer Therapeutics. S.A.H. was supported through a NIH T32 training grant. Funding sources did not have a role in study design, collection/analysis/interpretation of data, writing of the manuscript, or decision to submit for publication.
We wish to acknowledge The University of Iowa Flow Cytometry Facility.
Footnotes
Contributions S.A.H. was responsible for the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, and final approval of the article.
H.T. was responsible for acquisition of data, drafting the article, and final approval.
R.J.H. was responsible for analysis and interpretation of data, drafting the article, and final approval.
Conflict of interest S.A.H: Celgene: Award.
H.T.: There are no relevant conflicts of interest to disclose.
R.J.H: Celgene: Consultancy, Speakers Bureau; Terpenoid Therapeutics: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees; Novartis: Consultancy, Speakers Bureau.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. others. [PubMed] [Google Scholar]
- 3.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 4.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. others. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi M, Nakaseko C, Ohwada C, Takeuchi M, Ozawa S, Shimizu N, Cho R, Nishimura M, Saito Y. Zoledronate has an antitumor effect and induces actin rearrangement in dexamethasone-resistant myeloma cells. Eur J Haematol. 2007;79:382–391. doi: 10.1111/j.1600-0609.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 8.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 9.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem Biophys Res Commun. 1999;255:491–494. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- 10.Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys. 2000;373:231–241. doi: 10.1006/abbi.1999.1502. [DOI] [PubMed] [Google Scholar]
- 11.Holstein SA, Hohl RJ. Isoprenoids: remarkable diversity of form and function. Lipids. 2004;39:293–309. doi: 10.1007/s11745-004-1233-3. [DOI] [PubMed] [Google Scholar]
- 12.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Seabra MC. Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 1998;10:167–172. doi: 10.1016/s0898-6568(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 14.Shipman CM, Croucher PI, Russell RG, Helfrich MH, Rogers MJ. The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. Cancer Res. 1998;58:5294–5297. [PubMed] [Google Scholar]
- 15.van de Donk NW, Kamphuis MM, Lokhorst HM, Bloem AC. The cholesterol lowering drug lovastatin induces cell death in myeloma plasma cells. Leukemia. 2002;16:1362–1371. doi: 10.1038/sj.leu.2402501. [DOI] [PubMed] [Google Scholar]
- 16.Gronich N, Drucker L, Shapiro H, Radnay J, Yarkoni S, Lishner M. Simvastatin induces death of multiple myeloma cell lines. J Investig Med. 2004;52:335–344. doi: 10.1136/jim-52-05-34. [DOI] [PubMed] [Google Scholar]
- 17.Janosi J, Sebestyen A, Bocsi J, Barna G, Nagy K, Valyi-Nagy I, Kopper L. Mevastatin-induced apoptosis and growth suppression in U266 myeloma cells. Anticancer Res. 2004;24:1817–1822. [PubMed] [Google Scholar]
- 18.Morgan MA, Sebil T, Aydilek E, Peest D, Ganser A, Reuter CW. Combining prenylation inhibitors causes synergistic cytotoxicity, apoptosis and disruption of RAS-to-MAP kinase signalling in multiple myeloma cells. Br J Haematol. 2005;130:912–925. doi: 10.1111/j.1365-2141.2005.05696.x. [DOI] [PubMed] [Google Scholar]
- 19.Otsuki T, Sakaguchi H, Hatayama T, Fujii T, Tsujioka T, Sugihara T, Takata A, Hyodoh F, Eto M. Effects of an HMG-CoA reductase inhibitor, simvastatin, on human myeloma cells. Oncol Rep. 2004;11:1053–1058. [PubMed] [Google Scholar]
- 20.Schmidmaier R, Simsek M, Baumann P, Emmerich B, Meinhardt G. Synergistic antimyeloma effects of zoledronate and simvastatin. Anticancer Drugs. 2006;17:621–629. doi: 10.1097/01.cad.0000215058.85813.02. [DOI] [PubMed] [Google Scholar]
- 21.van de Donk NW, Kamphuis MM, van Kessel B, Lokhorst HM, Bloem AC. Inhibition of protein geranylgeranylation induces apoptosis in myeloma plasma cells by reducing Mcl-1 protein levels. Blood. 2003;102:3354–3362. doi: 10.1182/blood-2003-03-0970. [DOI] [PubMed] [Google Scholar]
- 22.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 23.Baulch-Brown C, Molloy TJ, Yeh SL, Ma D, Spencer A. Inhibitors of the mevalonate pathway as potential therapeutic agents in multiple myeloma. Leuk Res. 2007;31:341–352. doi: 10.1016/j.leukres.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S, Helfrich MH, Sati HI, Greaves M, Ralston SH, Culligan DJ, Soutar RL, Rogers MJ. Pamidronate causes apoptosis of plasma cells in vivo in patients with multiple myeloma. Br J Haematol. 2002;119:475–483. doi: 10.1046/j.1365-2141.2002.03824.x. [DOI] [PubMed] [Google Scholar]
- 25.Dmoszynska A, Podhorecka M, Klimek P, Grzasko N. Lovastatin and thalidomide have a combined effect on the rate of multiple myeloma cell apoptosis in short term cell cultures. Eur J Clin Pharmacol. 2006;62:325–329. doi: 10.1007/s00228-006-0106-2. [DOI] [PubMed] [Google Scholar]
- 26.Ural AU, Yilmaz MI, Avcu F, Pekel A, Zerman M, Nevruz O, Sengul A, Yalcin A. The bisphosphonate zoledronic acid induces cytotoxicity in human myeloma cell lines with enhancing effects of dexamethasone and thalidomide. Int J Hematol. 2003;78:443–449. doi: 10.1007/BF02983818. [DOI] [PubMed] [Google Scholar]
- 27.van der Spek E, Bloem AC, Lokhorst HM, van Kessel B, Bogers-Boer L, van de Donk NW. Inhibition of the mevalonate pathway potentiates the effects of lenalidomide in myeloma. Leuk Res. 2009;33:100–108. doi: 10.1016/j.leukres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Shull LW, Wiemer AJ, Hohl RJ, Wiemer DF. Synthesis and biological activity of isoprenoid bisphosphonates. Bioorg Med Chem. 2006;14:4130–4136. doi: 10.1016/j.bmc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Holstein SA, Hohl RJ. Interaction of cytosine arabinoside and lovastatin in human leukemia cells. Leuk Res. 2001;25:651–660. doi: 10.1016/s0145-2126(00)00162-4. [DOI] [PubMed] [Google Scholar]
- 30.Tong H, Holstein SA, Hohl RJ. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Anal Biochem. 2005;336:51–59. doi: 10.1016/j.ab.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudakovic A, Wiemer AJ, Lamb KM, Vonnahme LA, Dietz SE, Hohl RJ. Inhibition of geranylgeranyl diphosphate synthase induces apoptosis through multiple mechanisms and displays synergy with inhibition of other isoprenoid biosynthetic enzymes. J Pharmacol Exp Ther. 2008;324:1028–1036. doi: 10.1124/jpet.107.132217. [DOI] [PubMed] [Google Scholar]
- 34.Roelofs AJ, Hulley PA, Meijer A, Ebetino FH, Russell RG, Shipman CM. Selective inhibition of Rab prenylation by a phosphonocarboxylate analogue of risedronate induces apoptosis, but not S-phase arrest, in human myeloma cells. Int J Cancer. 2006;119:1254–1261. doi: 10.1002/ijc.21977. [DOI] [PubMed] [Google Scholar]
- 35.Lawson MA, Coulton L, Ebetino FH, Vanderkerken K, Croucher PI. Geranylgeranyl transferase type II inhibition prevents myeloma bone disease. Biochem Biophys Res Commun. 2008;377:453–457. doi: 10.1016/j.bbrc.2008.09.157. [DOI] [PubMed] [Google Scholar]
- 36.Billadeau D, Jelinek DF, Shah N, LeBien TW, Van Ness B. Introduction of an activated N-ras oncogene alters the growth characteristics of the interleukin 6-dependent myeloma cell line ANBL6. Cancer Res. 1995;55:3640–3646. [PubMed] [Google Scholar]
- 37.Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL, Anderson KC. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 38.Ogata A, Chauhan D, Urashima M, Teoh G, Treon SP, Anderson KC. Blockade of mitogen-activated protein kinase cascade signaling in interleukin 6-independent multiple myeloma cells. Clin Cancer Res. 1997;3:1017–1022. [PubMed] [Google Scholar]
- 39.Intini D, Agnelli L, Ciceri G, Ronchetti D, Fabris S, Nobili L, Lambertenghi-Deliliers G, Lombardi L, Neri A. Relevance of Ras gene mutations in the context of the molecular heterogeneity of multiple myeloma. Hematol Oncol. 2007;25:6–10. doi: 10.1002/hon.801. [DOI] [PubMed] [Google Scholar]
- 40.Jackson JH, Cochrane CG, Bourne JR, Solski PA, Buss JE, Der CJ. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci U S A. 1990;87:3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumi S, Beauchamp RD, Townsend CM, Jr., Pour PM, Ishizuka J, Thompson JC. Lovastatin inhibits pancreatic cancer growth regardless of RAS mutation. Pancreas. 1994;9:657–661. doi: 10.1097/00006676-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Tong H, Wiemer AJ, Neighbors JD, Hohl RJ. Quantitative determination of farnesyl and geranylgeranyl diphosphate levels in mammalian tissue. Anal Biochem. 2008;378:138–143. doi: 10.1016/j.ab.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Wong WW, Clendening JW, Martirosyan A, Boutros PC, Bros C, Khosravi F, Jurisica I, Stewart AK, Bergsagel PL, Penn LZ. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Mol Cancer Ther. 2007;6:1886–1897. doi: 10.1158/1535-7163.MCT-06-0745. [DOI] [PubMed] [Google Scholar]
- 44.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 45.Notarnicola M, Messa C, Cavallini A, Bifulco M, Tecce MF, Eletto D, Di Leo A, Montemurro S, Laezza C, Caruso MG. Higher farnesyl diphosphate synthase activity in human colorectal cancer inhibition of cellular apoptosis. Oncology. 2004;67:351–358. doi: 10.1159/000082918. [DOI] [PubMed] [Google Scholar]
- 46.Jiang F, Yang L, Cai X, Cyriac J, Shechter I, Wang Z. Farnesyl diphosphate synthase is abundantly expressed and regulated by androgen in rat prostatic epithelial cells. J Steroid Biochem Mol Biol. 2001;78:123–130. doi: 10.1016/s0960-0760(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 47.Luhe A, Kunkele KP, Haiker M, Schad K, Zihlmann C, Bauss F, Suter L, Pfister T. Preclinical evidence for nitrogen-containing bisphosphonate inhibition of farnesyl diphosphate (FPP) synthase in the kidney: implications for renal safety. Toxicol In Vitro. 2008;22:899–909. doi: 10.1016/j.tiv.2008.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.