Abstract
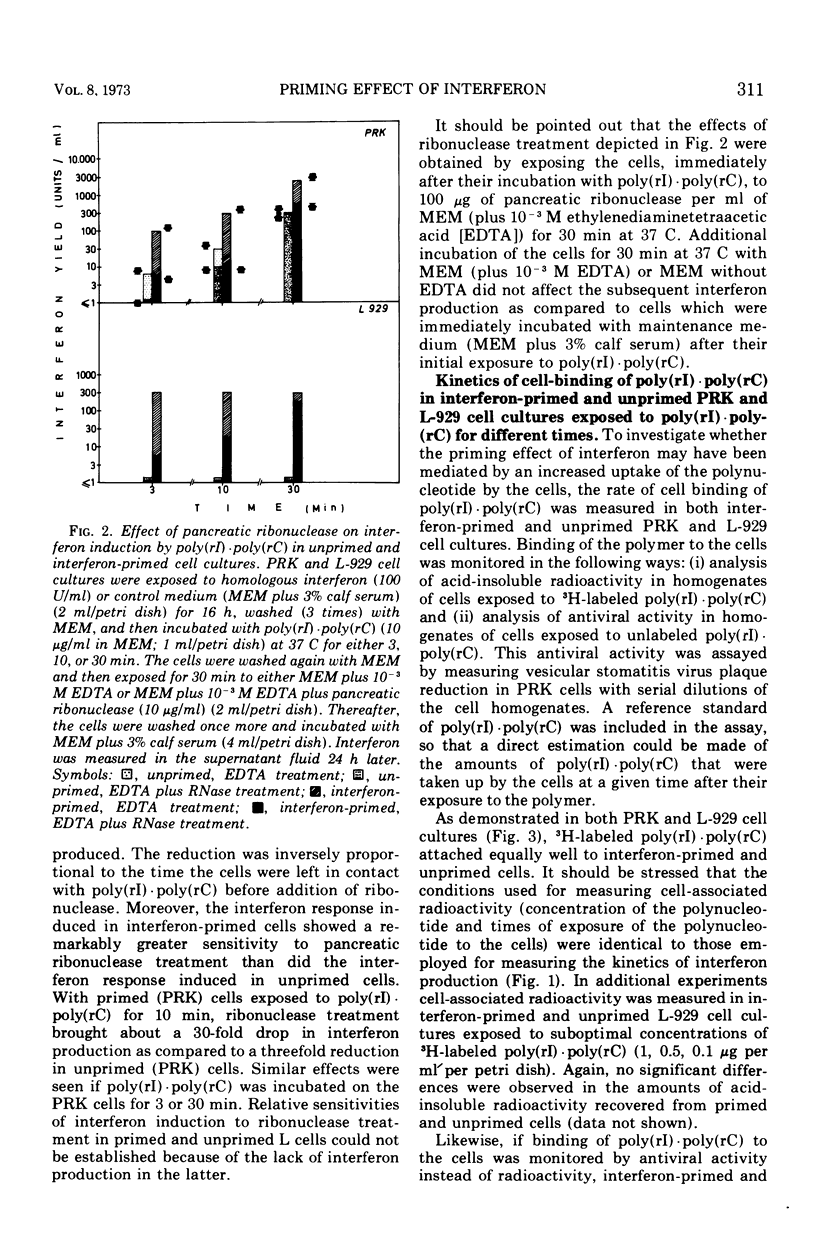
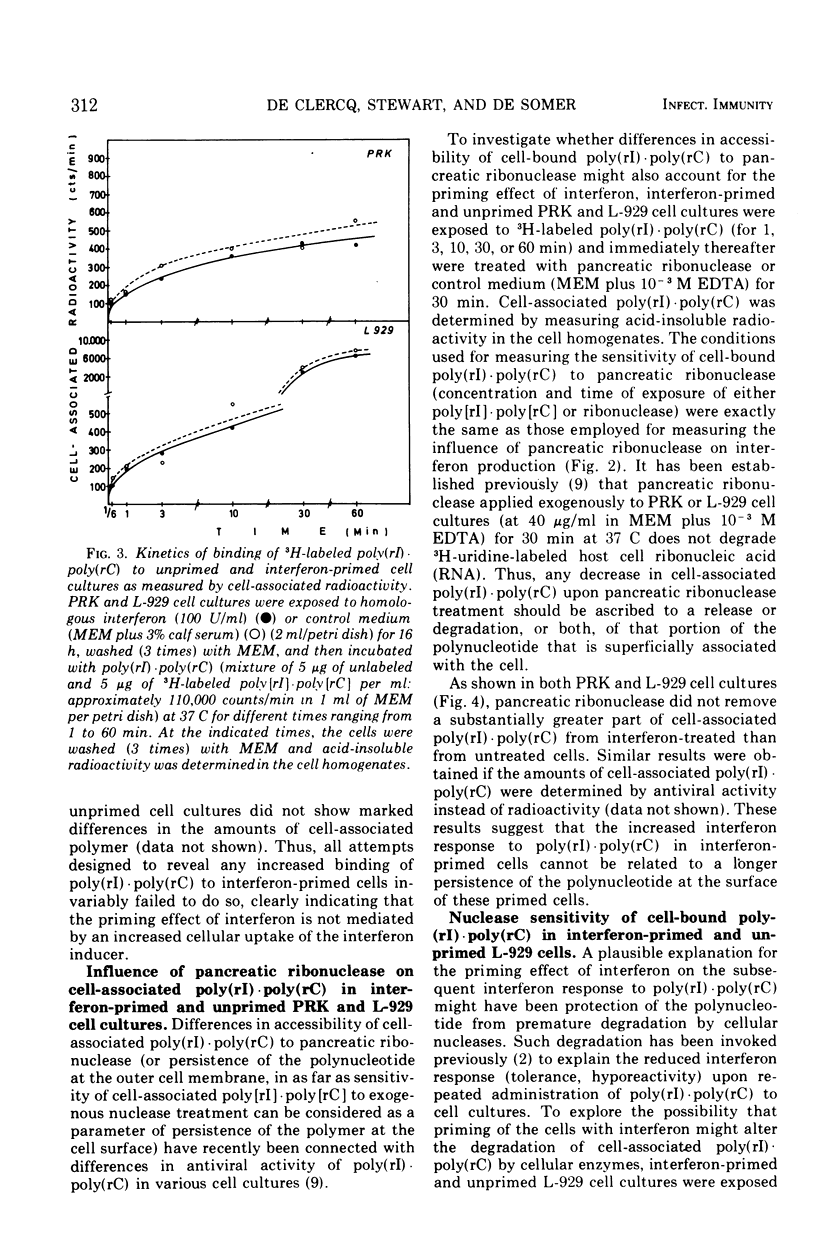
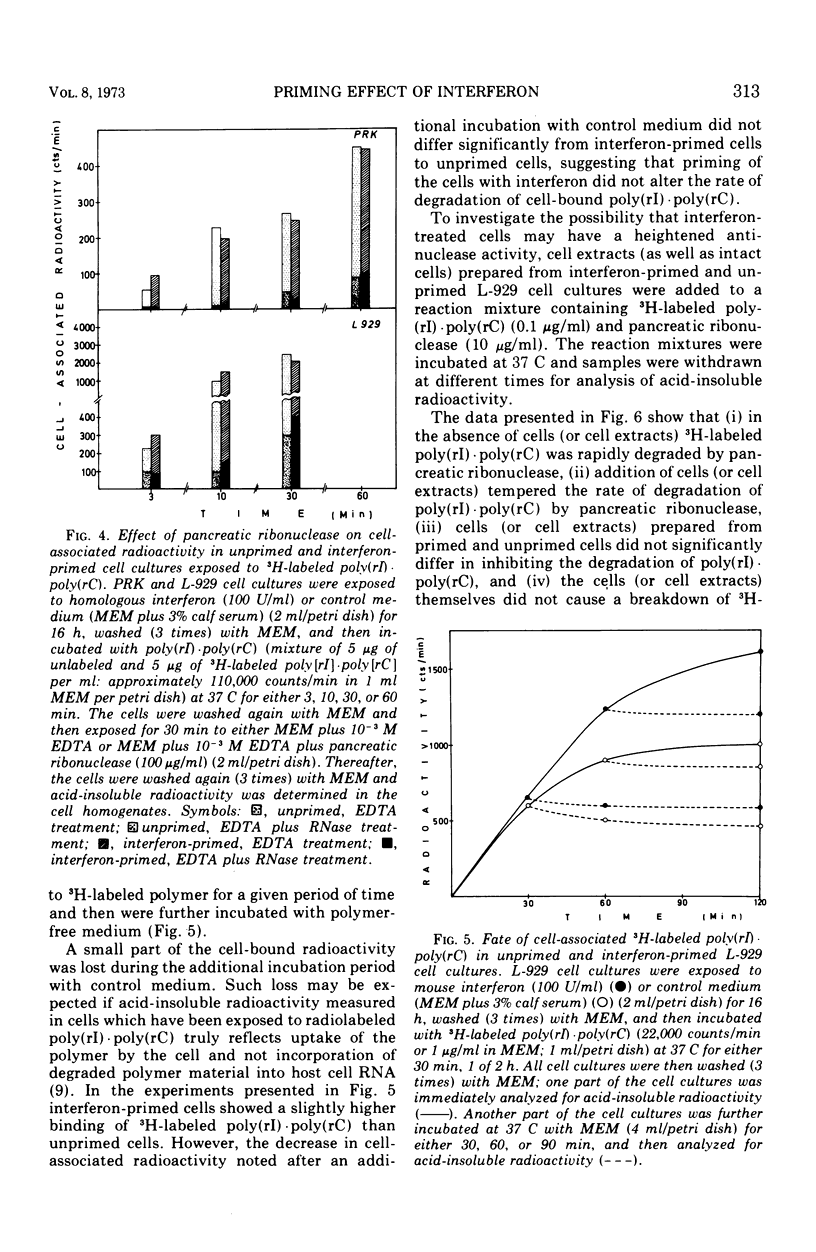
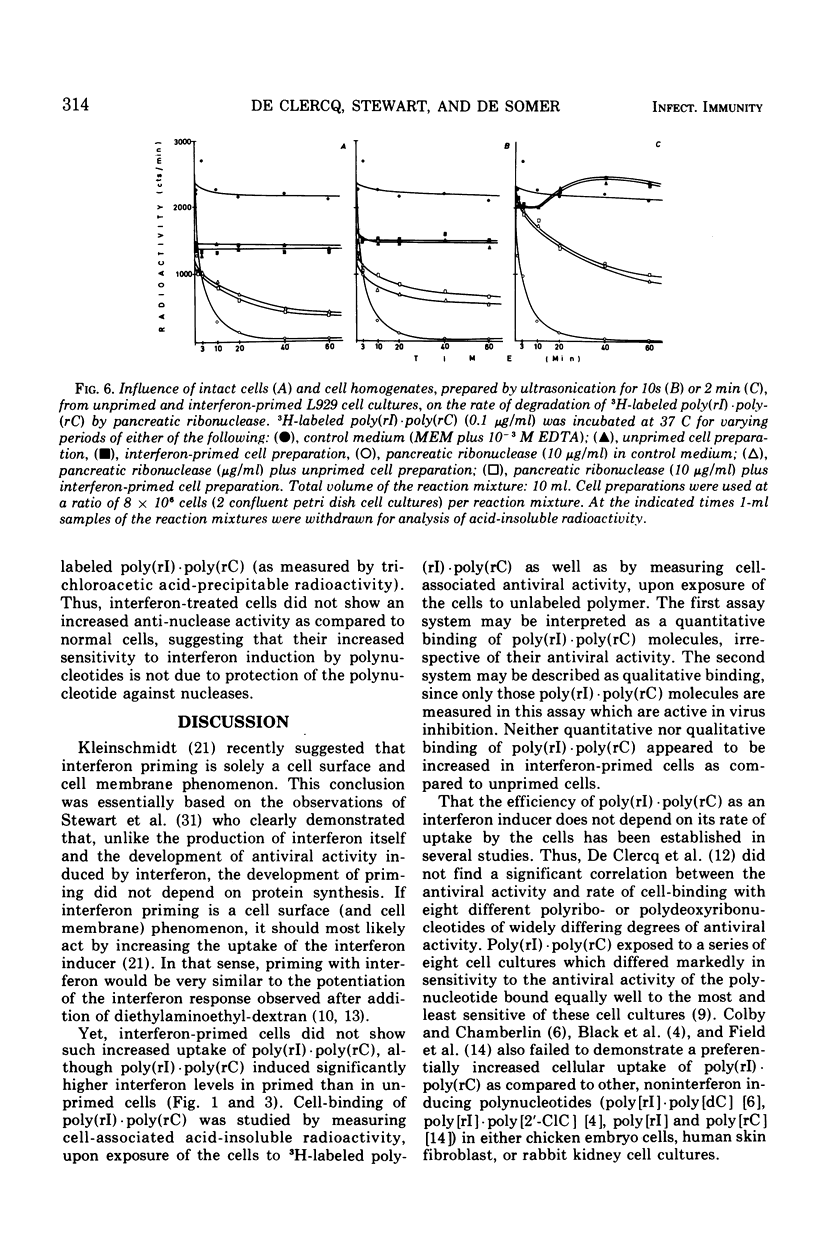
Interferon induction by poly(rI)·poly(rC) in primary rabbit kidney and mouse L-929 cell cultures was markedly increased if the cells were previously treated with homologous interferon. This priming effect has been established with different times of exposure of the cells to poly(rI)·poly(rC), and was most pronounced for short pulses of contact of the polynucleotide with the cells (10 s, 1 min). Treatment of the cells with pancreatic ribonuclease immediately after their exposure to poly(rI)·poly(rC) brought about a relatively greater reduction of the interferon response in interferon-primed cells than it did in unprimed cell cultures. Priming of the cells with interferon did not increase cell-binding of poly(rI)·poly(rC), whether this cell-binding was measured quantitatively (by radioactivity, upon exposure of the cells to radiolabeled polymer) or qualitatively (by antiviral activity, by assaying the cell extract for virus plaque reduction). Similarly, interferon priming did not alter the sensitivity of cell-associated poly(rI)·poly(rC) to extraneous ribonuclease treatment. Finally, priming with interferon did not decrease the rate of degradation of cell-bound poly(rI)·poly(rC) by cellular nucleases nor did it increase the anti-nuclease potency of the cells. The exact mechanism by which previous exposure of the cells to interferon enhances subsequent interferon production, induced by either synthetic polynucleotides or viruses, has not yet been resolved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bausek G. H., Merigan T. C. Cell interaction with a synthetic polynucleotide and interferon production in vitro. Virology. 1969 Nov;39(3):491–498. doi: 10.1016/0042-6822(69)90097-x. [DOI] [PubMed] [Google Scholar]
- Bausek G. H., Merigan T. C. Two mechanisms of cell resistance to repeated stimulation of interferon. Proc Soc Exp Biol Med. 1970 Jul;134(3):672–676. doi: 10.3181/00379727-134-34858. [DOI] [PubMed] [Google Scholar]
- Billiau A., Joniau M., De Somer P. Mass production of human interferon in diploid cells stimulated by poly-I:C. J Gen Virol. 1973 Apr;19(1):1–8. doi: 10.1099/0022-1317-19-1-1. [DOI] [PubMed] [Google Scholar]
- CANTELL K., PAUCKER K. QUANTITATIVE STUDIES ON VIRAL INTERFERENCE IN SUSPENDED L CELLS. IV. PRODUCTION AND ASSAY OF INTERFERON. Virology. 1963 Sep;21:11–21. doi: 10.1016/0042-6822(63)90298-8. [DOI] [PubMed] [Google Scholar]
- Colby C., Chamberlin M. J. The specificity of interferon induction in chick embryo cells by helical RNA. Proc Natl Acad Sci U S A. 1969 May;63(1):160–167. doi: 10.1073/pnas.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Mechanism of the antiviral activity resulting from sequential administration of complementary homopolyribonucleotides to cell cultures. J Virol. 1972 May;9(5):721–731. doi: 10.1128/jvi.9.5.721-731.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., De Somer P. Relationship between cell-interaction and antiviral activity of polyriboinosinic acid-polyribocytidylic acid in different cell cultures. J Gen Virol. 1973 Apr;19(1):113–123. doi: 10.1099/0022-1317-19-1-113. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Thermal activation of the antiviral activity of synthetic polyribonucleotides: influence of DEAE-dextran in various cell cultures. J Gen Virol. 1971 Feb;10(2):125–130. doi: 10.1099/0022-1317-10-2-125. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Wells R. D., Grant R. C., Merigan T. C. Thermal activation of the antiviral activity of synthetic double-stranded polyribonucleotides. J Mol Biol. 1971 Feb 28;56(1):83–100. doi: 10.1016/0022-2836(71)90086-6. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Wells R. D., Merigan T. C. Studies on the antiviral activity and cell interaction of synthetic double-stranded polyribo- and polydeoxyribonucleotides. Virology. 1972 Feb;47(2):405–415. doi: 10.1016/0042-6822(72)90276-0. [DOI] [PubMed] [Google Scholar]
- De Clerq E., De Somer P. Production of interferon in rabbit cell cultures by mouse L cell-bound poly(rI).poly(rC). J Gen Virol. 1972 Sep;16(3):435–439. doi: 10.1099/0022-1317-16-3-435. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Cellular control of interferon production and release after treatment with poly I:C inducer. Proc Soc Exp Biol Med. 1972 Jun;140(2):710–714. doi: 10.3181/00379727-140-36537. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Effect of interferon treatment on interferon production. J Immunol. 1966 May;96(5):872–877. [PubMed] [Google Scholar]
- Giron D. J., Allen P. T., Pindak F. F., Schmidt J. P. Interferon-inducing characteristics of MM virus. Appl Microbiol. 1971 Mar;21(3):387–393. doi: 10.1128/am.21.3.387-393.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgher R. R., Paucker K. Blocking of interferon production by chromatographically purified L cell interferon. Proc Soc Exp Biol Med. 1973 Jan;142(1):167–174. doi: 10.3181/00379727-142-36981. [DOI] [PubMed] [Google Scholar]
- Goore M. Y., Dickson J. H., Lipkin S., DiCuollo C. J. The production of human leukocyte interferon in a serum-free medium. Proc Soc Exp Biol Med. 1973 Jan;142(1):46–49. doi: 10.3181/00379727-142-36954. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS A., BURKE D. C. Mode of action of interferon. Nature. 1958 Oct 18;182(4642):1073–1074. doi: 10.1038/1821073a0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt W. J. Biochemistry of interferon and its inducers. Annu Rev Biochem. 1972;41(10):517–542. doi: 10.1146/annurev.bi.41.070172.002505. [DOI] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr PRODUCTION OF AN INTERFERON BY L CELLS INFECTED WITH WESTERN EQUINE ENCEPHALOMYELITIS VIRUS. J Bacteriol. 1963 Mar;85:556–566. doi: 10.1128/jb.85.3.556-566.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Koenig R. E., Golgher R. R., Paucker K. Immunology of interferons. II. Heterospecific activities of human interferons and their neutralization by antibody. J Immunol. 1970 Apr;104(4):791–797. [PubMed] [Google Scholar]
- Levy H. B., Buckler C. E., Baron S. Effect of interferon on early interferon production. Science. 1966 May 27;152(3726):1274–1276. doi: 10.1126/science.152.3726.1274. [DOI] [PubMed] [Google Scholar]
- Margolis S. A., Oie H., Levy H. B. The effect of interferon, interferon inducers or interferon induced virus resistance on subsequent interferon production. J Gen Virol. 1972 May;15(2):119–128. doi: 10.1099/0022-1317-15-2-119. [DOI] [PubMed] [Google Scholar]
- Paucker K., Boxaca M. Cellular resistance to induction of interferon. Bacteriol Rev. 1967 Jun;31(2):145–156. doi: 10.1128/br.31.2.145-156.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha P. M., Marshall L. W., Carter W. A. Interferon induction: rate of cellular attachment of poly IC. J Gen Virol. 1972 Apr;15(1):89–92. doi: 10.1099/0022-1317-15-1-89. [DOI] [PubMed] [Google Scholar]
- Rosztóczy I. Interferon induced by polyinosinic-polycytidylic acid in interferon pretreated L cells in absence of DEAE-dextran. Acta Virol. 1971 Sep;15(5):431–431. [PubMed] [Google Scholar]
- Rosztóczy I., Mécs I. Enhancement of interferon synthesis by polyinosinic-polycytidylic acid in L cells pretreated with interferon. Acta Virol. 1970 Sep;14(5):398–400. [PubMed] [Google Scholar]
- Steward W. E., 2nd, Gosser L. B., Lockart R. Z., Jr The effect of priming with interferon on interferon production by two lines of L cells. J Gen Virol. 1972 Apr;15(1):85–87. doi: 10.1099/0022-1317-15-1-85. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Clercq E., Billiau A., Desmyter J., De Somer P. Increased susceptibility of cells treated with interferon to the toxicity of polyriboinosinic-polyribocytidylic acid. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1851–1854. doi: 10.1073/pnas.69.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Distinguishing characteristics of the interferon responses of primary and continuous mouse cell cultures. J Gen Virol. 1971 Oct;13(1):35–50. doi: 10.1099/0022-1317-13-1-35. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovell D., Cantell K. Kinetics of interferon production in human leukocyte suspensions. J Gen Virol. 1971 Dec;13(3):485–489. doi: 10.1099/0022-1317-13-3-485. [DOI] [PubMed] [Google Scholar]
- VILCEK J., RADA B. Studies on an interferon from tickborne encephalitis virus-infected cells (IF). III. Antiviral action of IF. Acta Virol. 1962 Jan;6:9–16. [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Inhibition of induction of interferon synthesis in L-cells pretreated with interferon. Virology. 1969 Mar;37(3):473–475. doi: 10.1016/0042-6822(69)90231-1. [DOI] [PubMed] [Google Scholar]


