Abstract
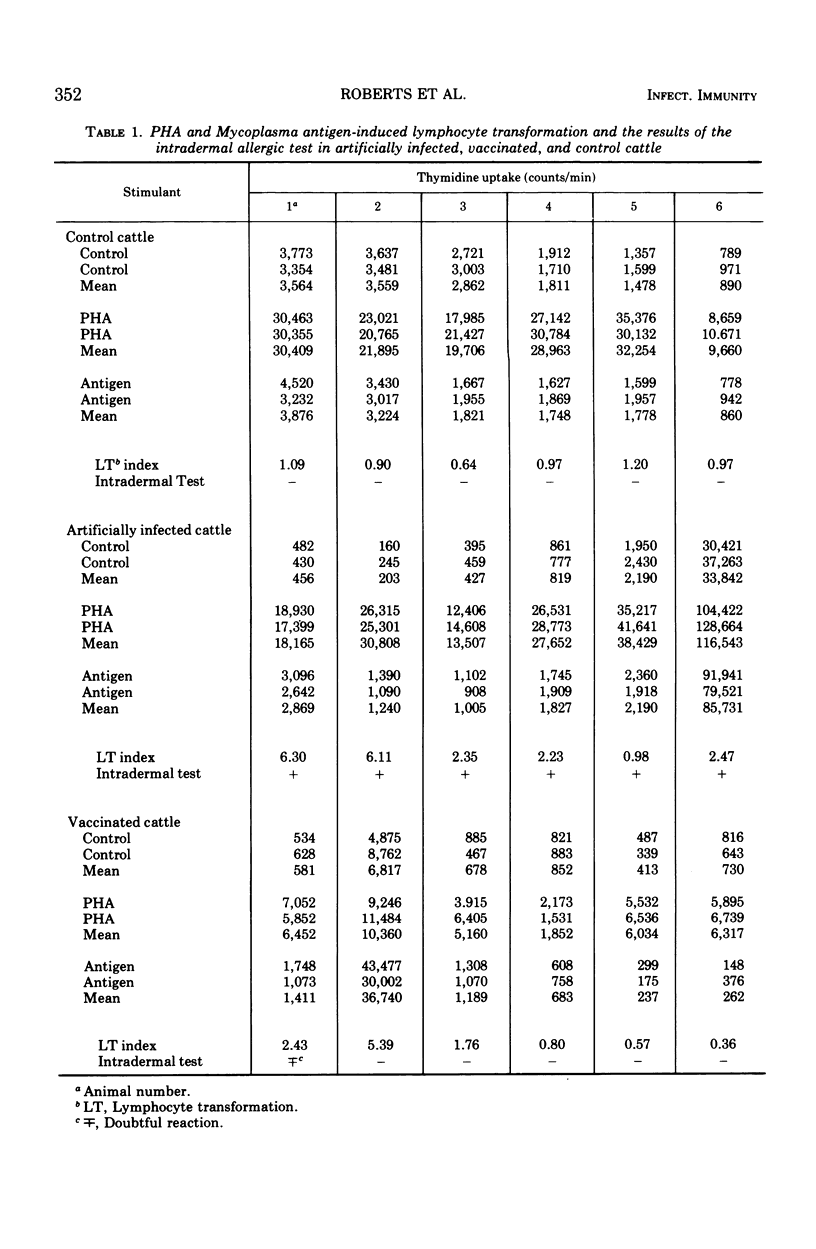
The cell-mediated immune response of cattle to Mycoplasma mycoides var. mycoides was studied. Sensitized lymphocytes in blood leukocyte preparations showed a significant degree of antigen-induced transformation, judged by the uptake of tritiated thymidine. The increase in tritiated thymidine uptake in sensitized lymphocytes in the presence of M. mycoides membrane antigen varied from 2- to 13-fold compared with the controls, and this increase in activity was observed from 3 days after artificial infection. Inhibition of leukocyte migration by M. mycoides membrane antigen commenced between 17 and 30 days after infection, and preliminary observations indicate that this test correlated with the intradermal allergic test. M. mycoides-induced unresponsiveness was demonstrated 23 and 30 days after infection. Unresponsiveness, in that the lymphocytes did not respond to phytohemagglutinin, was very marked in two of three animals and partial in the third animal, whereas the humoral antibody response did not appear to be affected. Antigen-induced transformation was demonstrated in only two out of six cattle vaccinated two months previously with M. mycoides T1 broth culture vaccine, and óne animal only gave a doubtful intradermal allergic reaction. A further six cattle vaccinated 15 months previously were negative to both the leukocyte migration inhibition test and the intradermal allergic test.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalund O., Hoerlein A. B., Adler H. C. The migration test on circulating bovine leukocytes and its possible application in the diagnosis of Johne's disease. Acta Vet Scand. 1970;11(2):331–334. doi: 10.1186/BF03547996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN R. D., GOURLAY R. N., MACLEOD A. K. THE PRODUCTION OF T1 BROTH CULTURE CONTAGIOUS BOVINE PLEUROPNEUMONIA VACCINE. Bull Epizoot Dis Afr. 1965 Jun;13:149–155. [PubMed] [Google Scholar]
- Barile M. F., Leventhal B. G. Possible mechanism for Mycoplasma inhibition of lymphocyte transformation induced by phytohaemagglutinin. Nature. 1968 Aug 17;219(5155):750–752. doi: 10.1038/219751a0. [DOI] [PubMed] [Google Scholar]
- Federlin K., Maini R. N., Russell A. S., Dumonde D. C. A micro-method for peripheral leucocyte migration in tuberculin sensitivity. J Clin Pathol. 1971 Sep;24(6):533–536. doi: 10.1136/jcp.24.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOURLAY R. N. THE ALLERGIC REACTION IN CONTAGIOUS BOVINE PLEUROPNEUMONIA. J Comp Pathol. 1964 Jul;74:286–299. doi: 10.1016/s0368-1742(64)80034-5. [DOI] [PubMed] [Google Scholar]
- Kaklamanis E., Pavlatos M. The immunosuppressive effect of mycoplasma infection. I. Effect on the humoral and cellular response. Immunology. 1972 Apr;22(4):695–702. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Windsor R. S., Boarer C. D. A method for the rapid enumeration of Mycoplasma species growing in broth culture. J Appl Bacteriol. 1972 Mar;35(1):37–42. doi: 10.1111/j.1365-2672.1972.tb03671.x. [DOI] [PubMed] [Google Scholar]


