Abstract
Brain single photon emission computed tomography (SPECT) is a well-established and reliable method to assess brain function through measurement of regional cerebral blood flow (rCBF). It can be used to define a patient's pathophysiological status when neurological or psychiatric symptoms cannot be explained by anatomical neuroimaging findings. Though there is ample evidence validating brain SPECT as a technique to track human behavior and correlating psychiatric disorders with dysfunction of specific brain regions, only few psychiatrists have adopted brain SPECT in routine clinical practice. It can be utilized to evaluate the involvement of brain regions in a particular patient, to individualize treatment on basis of SPECT findings, to monitor the treatment response and modify treatment, if necessary. In this article, we have reviewed the available studies in this regard from existing literature and tried to present the evidence for establishing the clinical role of brain SPECT in major psychiatric illnesses.
Keywords: Brain perfusion, psychiatric disorders, regional cerebral blood flow, single photon emission computed tomography
INTRODUCTION
Brain single photon emission computed tomography (SPECT) is a well-established and reliable method for evaluating brain function through measurement of regional cerebral blood flow (rCBF).[1] It is being utilized for detection of various neurodegenerative diseases and their management for several years. Brain SPECT can be used to define a patient's pathological status when neurological or psychiatric symptoms cannot be explained by structural neuroimaging findings. Though there is ample evidence in the literature validating brain SPECT as a promising technique to track human behavior and correlating psychiatric disorders with dysfunction of specific brain regions, it is rarely utilized technique in routine psychiatric practice. Renowned medical bodies like the American College of Radiology, the Society of Nuclear Medicine and the European Society of Nuclear Medicine have published evidence-based guidelines for using brain SPECT to improve patient care. Commonly accepted clinical indications for brain SPECT include: Dementia (early diagnosis, differentiation from normal ageing, and differential diagnosis of Alzheimer's disease from other neurodegenerative diseases), epilepsy (localization of epileptic focus by ictal and interictal studies), movement disorders, traumatic brain injury, cerebrovascular diseases, brain tumor and brain infections.[2,3,4] Nearly two decades back, Holman and Devous in their study, highlighted brain SPECT as a powerful window into the function of the brain and asserted it as a promising tool which could become an important component of the routine clinical evaluation of patients with neurological and psychiatric diseases.[5] A consistently growing body of research supports brain SPECT's clinical utility. In 1996, Vasile concluded that, the clinical utility of SPECT in neuropsychiatry is well-established.[6] Camargo reviewed the utility of brain SPECT in 2001 and demonstrated its role in obsessive-compulsive disorder (OCD), Gilles de la Tourette's syndrome, schizophrenia, depression, panic disorder, and drug abuse, in addition to common neurological indications.[7] However, despite the evidence relevant to diagnosis and treatment, only few psychiatrists have adopted brain SPECT or other functional neuroimaging techniques in routine clinical practice. Brain SPECT can be utilized to evaluate the involvement of specific brain regions in different patients, to individualize the treatment, for monitoring the treatment response and to modify treatment, when warranted. Though specific perfusion patterns for various psychiatric diseases have not been definitely recognized, perfusion and receptor imaging findings may be used as an additional diagnostic tool to guide clinicians searching for a definitive diagnosis. In this review article we have tried to consolidate the facts from existing literature and our own clinical experience, as to the kind of role brain SPECT can play in different psychiatric diseases.
BRAIN PERFUSION SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY-TECHNICAL OVERVIEW
Radiopharmaceuticals
The tracers used for brain perfusion SPECT are technetium- 99m- hexamethylpropyleneamineoxime (99mTc-HMPAO) and technetium-99m-ethylcysteinate dimer (99mTc-ECD). The main differences between 99mTc-HMPAO and 99mTc-ECD relate to their in vitro stability, uptake mechanism, and dosimetry.[8,9,10] 99mTc-HMPAO is highly unstable in vitro and high radiochemical purity must be assured before injection.[11] Stabilized forms of 99mTc-HMPAO allow easier labeling and improvement of image quality by reducing background activity.[12] By contrast, 99mTc-ECD is stable up to at least 4 h in vitro, and freshly eluted 99mTc is not required. Higher gray-matter-to-white-matter ratio, contributes to the better image quality obtained with 99mTc-ECD. Although both of tracers are distributed proportionally to rCBF, their retention is not completely linear with rCBF because of an initial back diffusion. High blood flow may be underestimated and low blood flow may be overestimated with both tracers.[13,14] In normal brain tissue, the kinetic properties are similar for both the perfusion agents. They enter the brain cells because of their lipophilic nature and remain there because of conversion into hydrophilic compounds. However, in patients with brain disease, the distribution of these compounds may differ because of the biochemistry of lipophilic-to-hydrophilic conversion. Although a metabolic process of de-esterification accounts for hydrophilic conversion of 99mTc-ECD, instability of the lipophilic form have been proposed for 99mTc-HMPAO. A perfusion-metabolic (de-esterification) coupling is needed in case of 99mTc-ECD to be trapped within cell, whereas only perfusion matters in 99mTc-HMPAO. Thus, 99mTc-ECD would have a predominant cellular-metabolic uptake, and 99mTc-HMPAO would reflect blood flow arrival to cerebral regions.[15]
Patient preparation
Before arrival, patients should be instructed to avoid, if possible, caffeine, alcohol, or other drugs known to affect cerebral blood flow (CBF). Brain perfusion is sensitive to neuronal activities, hence, tracer injection to be done in a quiet room and no interaction with patients at this time is desirable, to avoid any sensorial and cognitive stimuli. To avoid head movement during scanning (20-30 min), the patient should be comfortable and relaxed. The uncooperative patients (those with severe cognitive impairment or with loss of insight) may need sedation. Tracer injection must precede sedation to avoid sedation-induced blood flow changes. Appropriate positioning is needed to keep the collimators as close as possible to the patient's head and to get entire brain within the center of field of view.[16,17]
Acquisition system and postprocessing software
Because of the small size of important anatomically and functionally independent cerebral structures, spatial resolution is the main concern in brain imaging. A good compromise is to fit a general purpose rotating camera with fan beam collimator. Addition of computed tomography scan improves the quality of images by attenuation correction and structural correlation. Software applications are available for image processing to quantify the results in terms of rCBF for each brain functional area. Many of them have the features to compare with normal population database and provide statistical parametric mapping, so that one can easily recognize the abnormally perfused area.[16,17]
MAJOR PSYCHIATRIC DISORDERS AND BRAIN PERFUSION SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY
Attention deficit hyperactivity disorder
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent disorders in child and adolescent psychiatry. Prevalence of ADHD in the general population is approximately 5% of school-age children.[18] ADHD is characterized by a developmentally inappropriate poor attention span or age-inappropriate features of hyperactivity and impulsivity or both. To meet the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) diagnostic criteria, the disorder should be present for at least 6 months, cause impairment in academic or social functioning, and occur before the age of 7 years. ADHD appears to be heterogeneous, with a variety of known etiologies such as head trauma, intrauterine exposure to toxins, and infections, but in the majority of cases no etiology can been determined.[19] A neurobiological basis of ADHD resulting from the involvement of the fronto-striatal system has been proposed.[20] Several studies [Table 1] analyzed the patterns of rCBF in ADHD, demonstrating decreases in brain perfusion, especially in the premotor cortex and the prefrontal cortex, and hypoperfusion of striatal and periventricular structures.[21,22,23,24] Daniel et al. found that, 65% of children and adolescents with ADHD revealed decreased perfusion in the prefrontal cortex with intellectual stress, though only 25% had decreased prefrontal lobe activity at rest.[25] There is a pattern of lateralization in prefrontal hypoperfusion from right to left with increase in age of patients as demonstrated by a study.[26] Many researcher demonstrated temporal lobe dysfunction as significant in patients with ADHD. Kaya et al. described temporal hypoperfusion being more frequent than in the frontal cortex.[27] An association of temporal lobe hypoperfusion with severity of symptoms and comorbidity have been demonstrated by some studies.[27,28] Studies were also undertaken to demonstrate the response to methylphenidate treatment. Responders usually normalize the prefrontal hypoperfusion and may have increase or decrease in striatal perfusion.[29,30] The nonresponders had significantly increased activity in anterior cingulated (AC) cortex at baseline.[31] We observed both prefrontal and temporal hypoperfusion in all of the seven ADHD patients scanned in our center [Figure 1] and there is evidence of prefrontal activity normalization in available post therapy scans (four out of seven in a time period of 6-9 months) after successful treatment.
Table 1.
Review summary of brain perfusion studies in patients with ADHD
Figure 1.
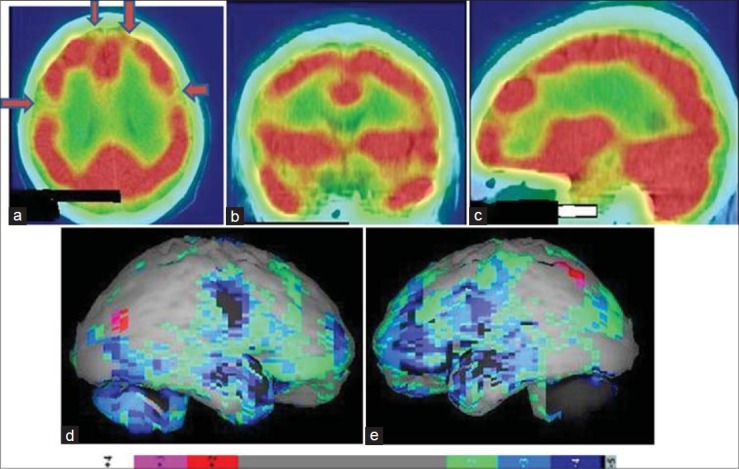
Technetium-99m-hexamethylpropyleneamineoxime brain perfusion single photon emission computed tomography-computed tomography (SPECT-CT) images of a 17 year old attention deficit hyperactivity disorder patient, showing hypoperfusion in bilateral frontal cortices and bilateral medial temporal lobes. (a) Transverse view, (b) sagittal view, (c) coronal view of the SPECT-CT, (d) right lateral, (e) left lateral surface projection views of “Neurogam” processed (compared with normal population adult database) images with color scale below (d and e)
Obsessive-compulsive disorder
Obsessive-compulsive disorder is rare (5% of psychiatric patients), with a usually gradual onset in adolescence or early adult life and a slightly greater prevalence in females. Family history shows a high incidence in other members. Obsessions are imperative, distressing thoughts that persist despite the desire to resist them and may take various forms: Intellectual (phrases, rhymes, ideas, images), impulsive (killing, stabbing, performing abject acts), or inhibiting. Compulsions are acts that result from obsessions, such as checking rituals, repeated hand washing, and wiping objects. Brain SPECT findings in patients with OCD have been investigated by several authors [Table 2]. A study suggested involvement of prefrontal-striatal-thalamic and limbic circuitry in the pathophysiology of OCD.[32] Hyperperfusion of the anterior portion of the cingulate gyrus; bilateral orbito-frontal regions; and in some patients, basal ganglia, before therapy has been described.[33,34,35] These changes returned to normal after treatment with fluoxetine.[34,35] In contrast, hypoperfusion of the frontal lobes, right caudate nucleus, and right thalamus has also been found.[36] Patients with poor insight on their condition or with schizo-obsessive behavior probably will display hypoperfusion of the frontal lobes, whereas patients with adequate insight tend to display hyperperfusion of frontal lobes and cingulate gyrus. Impulsive issues are often from low activity in the prefrontal cortex and compulsive tendencies are usually due to high activity in the anterior cingulate gyrus. In our patients, we observed hypoperfusion of prefrontal, temporal and AC cortex in majority, probably due to our study group, which comprises chronic patients under long term treatment [Figure 2]. Anterior cingulate cortex seems to be an important structure in the pathogenesis of OCD symptoms and anterior cingulotomy is an approach for symptomatic improvement.[37] The patients having increased activity in frontal and AC cortex also respond well to selective serotonin reuptake inhibitors.[38,39] There is possible role of brain SPECT in tracking hereditary OCD or predicting future development of OCD in offspring.[40]
Table 2.
Review summary of important brain perfusion study findings in patients with OCD
Figure 2.
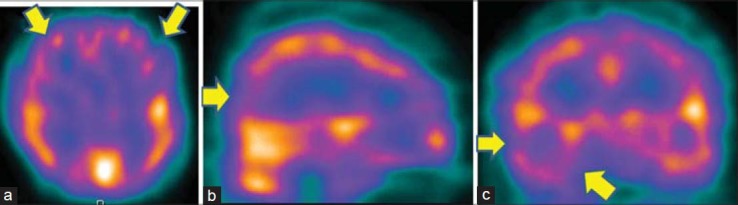
Technetium-99m-hexamethylpropyleneamineoxime brain perfusion single photon emission computed tomography (SPECT) in a 40-year-old male patient with obsessive-compulsive disorder revealed hypoperfusion in bilateral prefrontal cortices, with right temporal and occipital lobe. (a) Transverse view, (b) sagittal view, (c) coronal view of the SPECT images
Schizophrenia
Schizophrenia comprises a group of closely related disorders characterized by a particular type of disordered affect, behavior, and thinking. Symptoms are usually categorized as positive (auditory, tactile, visual, or olfactory hallucinations; persecutory, grandiose, or religious delusions; aggressiveness; bizarre appearance; abnormal sexual behavior; disordered thoughts) or negative (poor eye contact, speech, or hygiene; inappropriate affect; blocking; apathy; social inattentiveness). Though there is conflict among several studies [Table 3], brain SPECT most frequently shows hypofrontality, especially during a specific task; perfusion changes in the basal ganglia, possibly related to the use of neuroleptic drugs; and temporal lobe hypoperfusion, usually on the left side and frequently associated with ipsilateral frontal lobe hypoperfusion[41] [Figure 3]. However, patients who are not receiving medication may show hyperfrontality and depending on positive or negative symptoms may show conflicting findings (hypo and hyperperfusion).[42] Patients with positive symptoms have demonstrated increased precuneus activity.[43] Hypofrontality and temporal hypoperfusion related with negative symptoms and aggression in schizophrenia.[44,45,46,47,48] Studies on treatment response evaluation demonstrated improvement of blood flow in frontal, temporal, basal ganglia region with increased activity in motor cortex.[49,50,51,52] Involvement of inferior parietal cortex, cuneus and posterior temporal lobe are noted in chronic and progressive disease.[53] Injection of perfusion agents at the time of visual or auditory hallucinations shows hyperperfusion of the primary visual or auditory cortex, respectively.[54] Cognitive activation also significantly increases frontal activity in schizophrenia cases.[55] We have studied >50 patients of chronic schizophrenia and found significant hypoperfusion in prefrontal cortex mainly dorsolateral prefrontal cortex, and orbitofrontal cortex (OFC), temporal lobe, mainly temporopolar and superior temporal cortex, and inferior parietal lobule. There was also involvement of basal ganglia in 50% cases with occasional involvement of cerebellum, and sometimes global hypoperfusion [Figure 4] in severe cases.
Table 3.
Review summary of important brain perfusion study findings in patients with schizophrenia
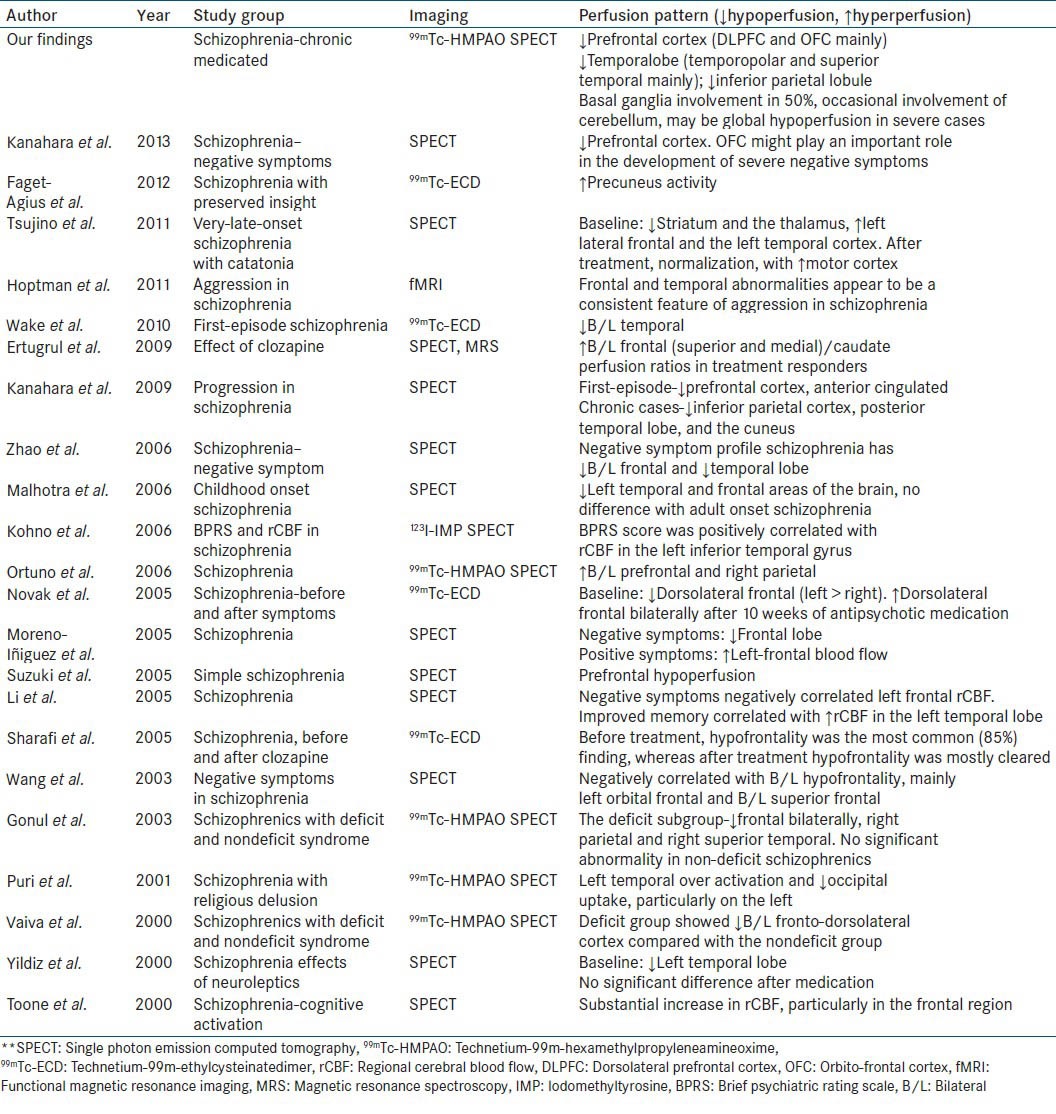
Figure 3.
A 45-year-old female with paranoid schizophrenia on antipsychotic treatment have bilateral frontal, and temporal hypoperfusion in technetium-99mhexamethylpropyleneamineoxime brain perfusion single photon emission computed tomography-computed tomography images. (a) Transverse view, (b) sagittal view, (c) coronal view
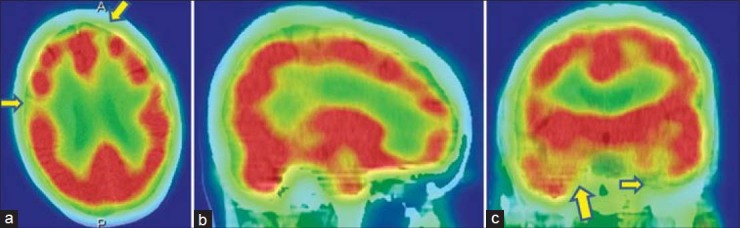
Figure 4.
A 26-year-old male with disorganized schizophrenia was under treatment for last 1-year, showing global cortical hypoperfusion with relative increase in basal ganglia and cerebellar activity in technetium-99m-hexamethylpropyleneamineoxime brain perfusion single photon emission computed tomography-computed tomography images. (a) Transverse view, (b) sagittal view, (c) coronal view
Anxiety and depression
Anxiety and depression are extremely common public health problems in today's world. The loss to our society from these illnesses is staggering: Individual pain, family strife, school and relationship failure, lost work productivity, and death. People actively seek out a cure for anxiety and depression, and are put on prescription medications that can harm them in other ways. Loss of interest or pleasure is the key symptom of depression. Other symptoms include feelings of hopelessness, worthlessness, and emotional pain; reduced energy and motivation; trouble sleeping; decreased appetite; and weight loss.[56] Brain SPECT with perfusion agents in patients free of medication has shown hypoperfusion of the following areas: The prefrontal area and temporal lobes, cingulate gyrus, and left caudate nucleus.[57,58,59] There is evidence of prefrontal, limbic, and paralimbic hypoperfusion in both unipolar and bipolar depression;[60] and the lateral frontal area involvement in acute depression in the elderly.[61] Hypofrontality was shown to be associated with severe negative symptoms[62] [Figure 5]. In many occasions, both anxiety and depression coexist. Increased activity in the basal ganglia and frontal lobe may be seen in patients with anxiety [Figure 6]. Severity of depression is inversely correlated with rCBF in left cingulate cortex, lentiform nucleus, and parahippocampal gyrus, and directly correlated with right posterolateral parietal cortex. Anxiety directly correlated with right anterolateral OFC, while cognitive performance correlated with right posteromedial OFC and left lentiform nucleus.[63,64] Cognitive decline in postmenopausal women is also associated with hypofrontality.[65] In major depressive disorders, sadness is related to decrease activity in dorsolateral prefrontal and dorsal cingulated cortex, with increased activity in ventromedial prefrontal and ventral cingulated cortex; whereas anxiety is associated with left AC cortex.[66] Whole brain blood flow also correlated positively with anxiety.[67] When recurrent depressions progressed to melancholies, involvement of left posterior parieto-temporal region is seen in addition to hypofrontality.[68] Findings of brain SPECT in anxiety depression disorders from different studies are summarized in Table 4.
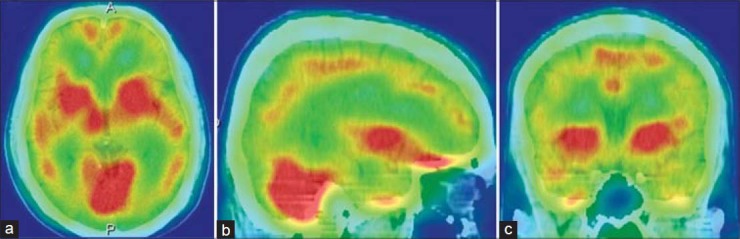
Figure 5.
A 62-year-old female with severe depression, showing severe bilateral hypofrontality in technetium-99m-hexamethylpropyleneamineoxime brain perfusion single photon emission computed tomography (SPECT). (a) Transaxial and (b) saggital view brain SPECT of the patient
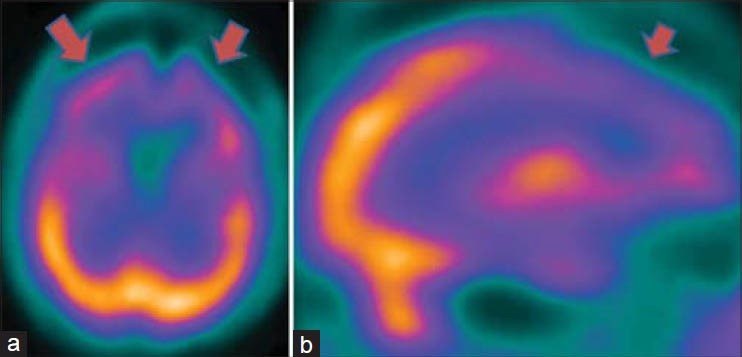
Figure 6.
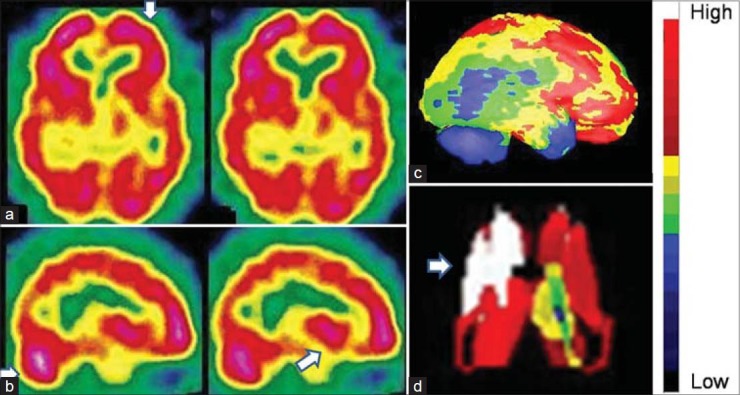
An 18-year-old male with severe anxiety neurosis revealed hyperactive prefrontal cortices and basal ganglia in technetium-99m-hexamethylpropyleneamineoxime brain perfusion single photon emission computed tomography images. (a) Transverse view, (b) sagittal view, (c) right lateral view of three-dimensional Talairach cortical perfusion report, (d) extracted basal ganglia and thalamus by “Neurogam” processing, (e) color scale for (c and d)
Table 4.
Summary of important brain perfusion study findings in patients suffering from anxiety-depression disorder
Substance abuse and addiction
Psychoactive substance abuse and dependence are disorders defined by patterns of maladaptive behavior related to the procurement and ingestion of substances of abuse (marijuana, hallucinogens, inhalants, cocaine, crack, heroin, stimulants, alcohol, and others).[69] Short and long-term substance abuse affects blood flow and metabolism, which negatively affect the way our central nervous system works [Table 5]. Fortunately, some researchers report that the damage associated with chronic use of alcohol, nicotine, inhalants, and solvents is at least partially reversible with de-addiction treatment. Brain SPECT, has shown disseminated CBF defects in abusers of cocaine, crack, heroin and alcohol.[70,71,72,73,74,75] [Figures 7 and 8]. Disappearance or improvement of the defects after a period of abstinence has been described, suggesting that arterial spasms may cause the defects.[70,71,74,75] Some studies in cocaine abusers described abnormality in the OFC and superior temporal cortex, with evidence of minute differences between men and women.[76,77] Patients with a history of inhalation of industrial solvents, such as glue, paint, and gasoline, have similar perfusion abnormalities.[78]
Table 5.
Summary of important brain perfusion study findings in patients suffering from substance abuse disorders
Figure 7.
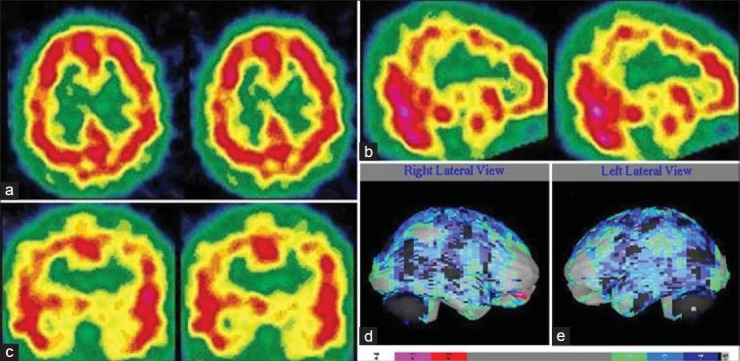
A 19-year-old female with multiple substance abuse disorder (predominantly alcohol and organic solvent) with induced-withdrawal behavioral problem showing diffuse cortical hypoperfusion on both side of cerebral cortex. (a) Transverse view, (b) sagittal view, (c) coronal view of technetium-99m-hexamethylpropyleneamineoxime brain single photon emission computed tomography, (d) right lateral, (e) left lateral surface projection views of “Neurogam” processed (compared with normal population adult database) images with color scale below (d and e)
Figure 8.
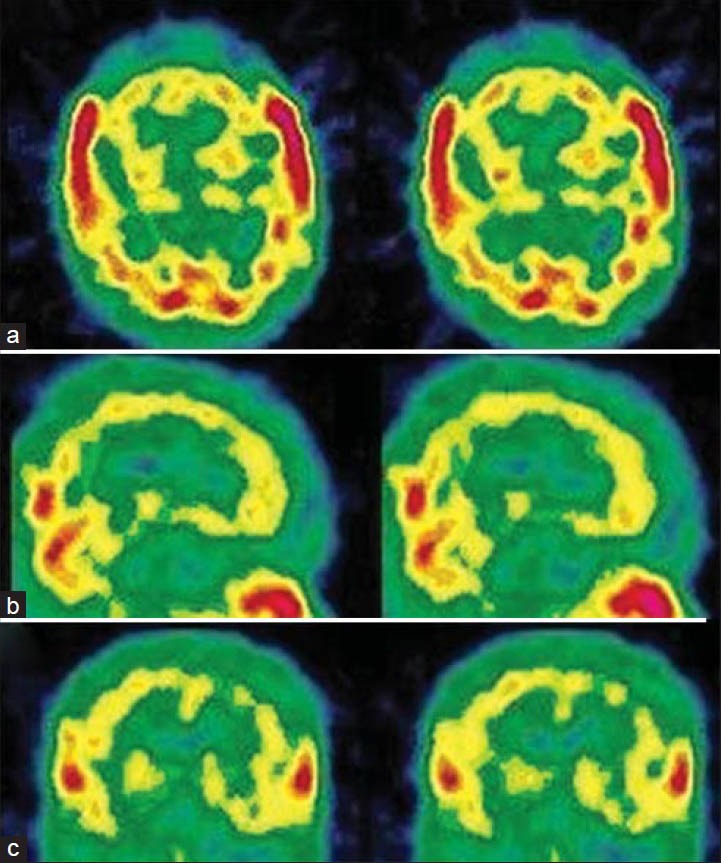
A 41-year-old male with multiple substance abuse disorder with significant decline in social and occupational performance showing severely decreased cortical perfusion globally. (a) Transverse view, (b) sagittal view, (c) coronal view of technetium-99m-hexamethylpropyleneamineoxime brain single photon emission computed tomography
Autism spectrum disorders
Autism spectrum disorders (ASD) are diagnosed today more than ever before. It has incidence rate of 2-5/10,000 births, males 1.5 times more commonly affected than females. This disorder is an early and severe development disorder, characterized by deficits in verbal and nonverbal languages, social skills, cognitive functioning and abnormal repetition of behavior (DSM-III R criteria). All children, teens, and adults with ASD are individuals and have unique brain patterns-one size does not fit all when it comes to ASD. Though SPECT studies are normal in many of the autism patients, it may show decreased temporal lobe perfusion. Up to 30% of autistic children eventually develop temporal lobe epilepsy.[79] A study by Degirmenci et al. suggested the existence of regional brain perfusion alterations in frontal, temporal, and parietal cortex and in caudate nucleus in autistic children and in their first-degree family member.[80]
SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY TO PERSONALISE TREATMENT IN PSYCHIATRIC DISORDERS
When brain SPECT scans detect the hyperfrontality pattern, it opens new avenues for intervention since this finding has been associated with predicting a positive treatment response to serotonergic medications in depression[38,81,82,83] and OCD,[84,85] predicting a positive response to sleep deprivation[86,87] and repetitive transcranial magnetic stimulation[88] for depression, predicting treatment response to a cingulotomy in OCD,[89] and help in distinguishing OCD from ADHD.[90] Hypofrontality, that is, decreased perfusion or activity in the prefrontal cortex, is another important brain SPECT finding that is often helpful in understanding and targeting treatment in individual patients. Hypofrontality is associated with a negative response to serotonergic medication in depression[91] and clozapine in schizophrenia[92] as well as with predicting relapse in alcoholics,[93] improved response to acetylcholine-esterase inhibitors for memory and behavior in AD,[94,95] predicting a poor response to ketamine in fibromyalgia patients[96] and improved response to stimulants in patients with ADHD symptoms during a concentration challenge.[97] Hypofrontality is also associated with antisocial symptoms, impulsive behaviors, and murder[98] as well as with completed suicide, which is often an impulsive act.[99] When hypofrontality is present in depressed patients, it is important to be vigilant in their care, as well as involve family support, as they may be less likely to respond to typical antidepressant medications and they may not have the cognitive resources to follow through with recommendations.[100] When abnormalities in the temporal lobes are seen (either hypo or hyperperfusion) and mood instability or temper problems are present, anticonvulsants provide a rational treatment option.[101] If there are memory or learning issues (and low temporal lobe perfusion), acetylcholine-esterase inhibitors may be helpful,[102] always taking into consideration the clinical picture.
CONCLUSIONS
Brain perfusion SPECT is a valuable tool in management of psychiatric disorders. It has a role in the diagnosis, therapeutic management, and follow-up of these patients. In addition, brain SPECT is a useful tool for research, because it is widely available and provides noninvasive in vivo assessment of human brain function. We can use this tool in psychiatric practice to evaluate the involvement of brain regions in a patient for a particular clinical condition, can individualize the treatment on basis of brain SPECT findings, can monitor the treatment response and modify the treatment, if necessary. There are a number of important areas where brain SPECT has the potential to provide relevant information to help personalize treatment to patients’ specific brain system pathophysiology rather than rely solely on general diagnostic and/or therapeutic categories. Brain SPECT should always be evaluated in conjunction with clinical assessment since it adds value to routine clinical assessment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Catafau AM, Parellada E, Lomeña F, Bernardo M, Pavía J, Ros D, et al. Baseline, visual deprivation and visual stimulation 99TCm-HMPAO-related changes in visual cortex can be detected with a single-head SPET system. Nucl Med Commun. 1996;17:480–4. doi: 10.1097/00006231-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 2.ACR Guidelines and Standards Committee. ACR-SPR Practice Guideline for the Performance of Single Photon Emission Computed Tomography (SPECT) Brain Perfusion and for Brain Death Examinations. Practice Guideline Revised. 2012:1–11. Resolution 25. [Google Scholar]
- 3.Juni JE, Waxman AD, Devous MD, Tikofsy RS, Ichise M, Van Heertum RL, et al. Society of Nuclear Medicine Procedure Guideline for Brain Perfusion Single Photon Emission Computed Tomography (SPECT) Using Tc-99m Radiopharmaceuticals, Version 2.0. Society of Nuclear Medicine Procedure Guidelines Manual. 2002 Jun;:113–8. [Google Scholar]
- 4.Kapucu OL, Nobili F, Varrone A, Booij J, Vander Borght T, Någren K, et al. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2093–102. doi: 10.1007/s00259-009-1266-y. [DOI] [PubMed] [Google Scholar]
- 5.Holman BL, Devous MD., Sr Functional brain SPECT: The emergence of a powerful clinical method. J Nucl Med. 1992;33:1888–904. [PubMed] [Google Scholar]
- 6.Vasile RG. Single photon emission computed tomography in psychiatry: Current perspectives. Harv Rev Psychiatry. 1996;4:27–38. doi: 10.3109/10673229609030519. [DOI] [PubMed] [Google Scholar]
- 7.Camargo EE. Brain SPECT in neurology and psychiatry. J Nucl Med. 2001;42:611–23. [PubMed] [Google Scholar]
- 8.Neirinckx RD, Canning LR, Piper IM, Nowotnik DP, Pickett RD, Holmes RA, et al. Technetium-99m d, l-HM-PAO: A new radiopharmaceutical for SPECT imaging of regional cerebral blood perfusion. J Nucl Med. 1987;28:191–202. [PubMed] [Google Scholar]
- 9.Vallabhajosula S, Zimmerman RE, Picard M, Stritzke P, Mena I, Hellman RS, et al. Technetium-99m ECD: A new brain imaging agent: In vivo kinetics and biodistribution studies in normal human subjects. J Nucl Med. 1989;30:599–604. [PubMed] [Google Scholar]
- 10.Nakamura K, Tukatani Y, Kubo A, Hashimoto S, Terayama Y, Amano T, et al. The behavior of 99mTc-hexamethylpropyleneamineoxime (99mTc-HMPAO) in blood and brain. Eur J Nucl Med. 1989;15:100–7. doi: 10.1007/BF00702628. [DOI] [PubMed] [Google Scholar]
- 11.Piera C, Martínez A, Ramírez I. Radiochemical purity of technetium-99m-HMPAO depends on specific activity. J Nucl Med. 1995;36:706. [PubMed] [Google Scholar]
- 12.Barthel H, Kämpfer I, Seese A, Dannenberg C, Kluge R, Burchert W, et al. Improvement of brain SPECT by stabilization of Tc-99m-HMPAO with methylene blue or cobalt chloride. Comparison with Tc-99m-ECD. Nuklearmedizin. 1999;38:80–4. [PubMed] [Google Scholar]
- 13.Lassen NA, Andersen R, Friberg L, Paulson OB. The retention of [99mTc]-d, l-HM-PAO in the human brain after intracarotid bolus injection: A kinetic analysis. J Cereb Blood Flow Metab. 1988;8(Suppl 1):S13–22. doi: 10.1038/jcbfm.1988.28. [DOI] [PubMed] [Google Scholar]
- 14.Friberg L, Andersen AR, Lassen NA, Holm S, Dam M. Retention of 99mTc-bicisate in the human brain after intracarotid injection. J Cereb Blood Flow Metab. 1994;14(Suppl 1):S19–27. [PubMed] [Google Scholar]
- 15.Koyama M, Kawashima R, Ito H, Ono S, Sato K, Goto R, et al. SPECT imaging of normal subjects with technetium-99m-HMPAO and technetium-99m-ECD. J Nucl Med. 1997;38:587–92. [PubMed] [Google Scholar]
- 16.Juni JE, Waxman AD, Devous MD, Tikofsy RS, Ichise M, Van Heertum RL, et al. Procedure guideline for brain perfusion SPECT Using 99mTc Radiopharmaceuticals 3.0. J Nucl Med Technol. 2009;37:191–5. doi: 10.2967/jnmt.109.067850. [DOI] [PubMed] [Google Scholar]
- 17.Catafau AM. Brain SPECT in clinical practice. Part I: Perfusion. J Nucl Med. 2001;42:259–71. [PubMed] [Google Scholar]
- 18.Szatmari P, Offort DR, Boyle MH. Ontario child health study; prevalence of attention deficit disorder with hyperactivity. J Child Psychol Psychiatry. 1989;30:219–30. doi: 10.1111/j.1469-7610.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. 4th ed. Washington (DC): American Psychiatric Association; 1994. [last accessed on 03/07/2014]. Diagnostic and statistical manual of mental disorders: DSM-IV [Internet] p. 866. Available from: http://www.psychiatryonline.com/DSMPDF/dsm-iv.pdf . [Google Scholar]
- 20.Laufer MW, Denhoff E. Hyperkinetic behavior syndrome in children. J Pediatr. 1957;50:463–74. doi: 10.1016/s0022-3476(57)80257-1. [DOI] [PubMed] [Google Scholar]
- 21.Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323:1361–6. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]
- 22.Zametkin AJ, Liebenauer LL, Fitzgerald GA, King AC, Minkunas DV, Herscovitch P, et al. Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1993;50:333–40. doi: 10.1001/archpsyc.1993.01820170011002. [DOI] [PubMed] [Google Scholar]
- 23.Lou HC, Henriksen L, Bruhn P, Børner H, Nielsen JB. Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol. 1989;46:48–52. doi: 10.1001/archneur.1989.00520370050018. [DOI] [PubMed] [Google Scholar]
- 24.Zametkin AJ, Liotta W. The neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 1998;59(Suppl 7):17–23. [PubMed] [Google Scholar]
- 25.Daniel G, Amen MD, Blake D. Carmichael, high-resolution brain SPECT imaging in ADHD. Ann Clin Psychiatry. 1997;9:81–6. doi: 10.1023/a:1026201218296. [DOI] [PubMed] [Google Scholar]
- 26.Oner O, Oner P, Aysev A, Küçük O, Ibis E. Regional cerebral blood flow in children with ADHD: Changes with age. Brain Dev. 2005;27:279–85. doi: 10.1016/j.braindev.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Kaya GC, Pekcanlar A, Bekis R, Ada E, Miral S, Emiroglu N, et al. Technetium-99m HMPAO brain SPECT in children with attention deficit hyperactivity disorder. Ann Nucl Med. 2002;16:527–31. doi: 10.1007/BF02988629. [DOI] [PubMed] [Google Scholar]
- 28.Lorberboym M, Watemberg N, Nissenkorn A, Nir B, Lerman-Sagie T. Technetium 99m ethylcysteinate dimer single-photon emission computed tomography (SPECT) during intellectual stress test in children and adolescents with pure versus comorbid attention-deficit hyperactivity disorder (ADHD) J Child Neurol. 2004;19:91–6. doi: 10.1177/08830738040190020201. [DOI] [PubMed] [Google Scholar]
- 29.Kim BN, Lee JS, Cho SC, Lee DS. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42:19–29. doi: 10.3349/ymj.2001.42.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, Kim BN, Kang E, Lee DS, Kim YK, Chung JK, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: Comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24:157–64. doi: 10.1002/hbm.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SC, Hwang JW, Kim BN, Lee HY, Kim HW, Lee JS, et al. The relationship between regional cerebral blood flow and response to methylphenidate in children with attention-deficit hyperactivity disorder: Comparison between non-responders to methylphenidate and responders. J Psychiatr Res. 2007;41:459–65. doi: 10.1016/j.jpsychires.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Huyser C, Veltman DJ, de Haan E, Boer F. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev. 2009;33:818–30. doi: 10.1016/j.neubiorev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Machlin SR, Harris GJ, Pearlson GD, Hoehn-Saric R, Jeffery P, Camargo EE. Elevated medial-frontal cerebral blood flow in obsessive-compulsive patients: A SPECT study. Am J Psychiatry. 1991;148:1240–2. doi: 10.1176/ajp.148.9.1240. [DOI] [PubMed] [Google Scholar]
- 34.Hoehn-Saric R, Pearlson GD, Harris GJ, Machlin SR, Camargo EE. Effects of fluoxetine on regional cerebral blood flow in obsessive-compulsive patients. Am J Psychiatry. 1991;148:1243–5. doi: 10.1176/ajp.148.9.1243. [DOI] [PubMed] [Google Scholar]
- 35.Hoehn-Saric R, Harris GJ, Pearlson GD, Cox CS, Machlin SR, Camargo EE. A fluoxetine-induced frontal lobe syndrome in an obsessive compulsive patient. J Clin Psychiatry. 1991;52:131–3. [PubMed] [Google Scholar]
- 36.Lucey JV, Costa DC, Blanes T, Busatto GF, Pilowsky LS, Takei N, et al. Regional cerebral blood flow in obsessive-compulsive disordered patients at rest. Differential correlates with obsessive-compulsive and anxious-avoidant dimensions. Br J Psychiatry. 1995;167:629–34. doi: 10.1192/bjp.167.5.629. [DOI] [PubMed] [Google Scholar]
- 37.Chang JW, Kim CH, Lee JD, Chung SS. Single photon emission computed tomography imaging in obsessive-compulsive disorder and for stereotactic bilateral anterior cingulotomy. Neurosurg Clin N Am. 2003;14:237–50. doi: 10.1016/s1042-3680(03)00006-8. [DOI] [PubMed] [Google Scholar]
- 38.Hoehn-Saric R, Schlaepfer TE, Greenberg BD, McLeod DR, Pearlson GD, Wong SH. Cerebral blood flow in obsessive-compulsive patients with major depression: Effect of treatment with sertraline or desipramine on treatment responders and non-responders. Psychiatry Res. 2001;108:89–100. doi: 10.1016/s0925-4927(01)00114-7. [DOI] [PubMed] [Google Scholar]
- 39.Karadag F, Kalkan Oguzhanoglu N, Yüksel D, Kiraç S, Cura C, Ozdel O, et al. The comparison of pre-and post-treatment (99m) Tc HMPAO brain SPECT images in patients with obsessive-compulsive disorder. Psychiatry Res. 2013;213:169–77. doi: 10.1016/j.pscychresns.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Santra A, Thukral RK. Brain perfusion single photon emission computed tomography with (99m) Tc-hexamethylpropyleneamineoxime in hereditary obsessive compulsive disorder. Indian J Nucl Med. 2013;28:256–7. doi: 10.4103/0972-3919.121989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods SW. Regional cerebral blood flow imaging with SPECT in psychiatric disease: Focus on schizophrenia, anxiety disorders, and substance abuse. J Clin Psychiatry. 1992;53(Suppl):20–5. [PubMed] [Google Scholar]
- 42.Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet. 1997;349:1735–9. doi: 10.1016/S0140-6736(96)08380-8. [DOI] [PubMed] [Google Scholar]
- 43.Faget-Agius C, Boyer L, Padovani R, Richieri R, Mundler O, Lançon C, et al. Schizophrenia with preserved insight is associated with increased perfusion of the precuneus. J Psychiatry Neurosci. 2012;37:297–304. doi: 10.1503/jpn.110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoptman MJ, Antonius D. Neuroimaging correlates of aggression in schizophrenia: An update. Curr Opin Psychiatry. 2011;24:100–6. doi: 10.1097/YCO.0b013e328342c8e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J, He X, Liu Z, Yang D. The effects of clozapine on cognitive function and regional cerebral blood flow in the negative symptom profile schizophrenia. Int J Psychiatry Med. 2006;36:171–81. doi: 10.2190/1AA0-UW9Q-1CNK-3E2N. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Iñiguez M, Ortuño F, Arbizu J, Millán M, Soutullo C, Cervera-Enguix S. Regional cerebral blood flow SPECT study, at rest and during Wisconsin Card Sorting Test (WCST) performance, in schizophrenia naive patients or treated with atypical neuroleptics. Actas Esp Psiquiatr. 2005;33:343–51. [PubMed] [Google Scholar]
- 47.Li X, Tang J, Wu Z, Zhao G, Liu C, George MS. SPECT study of Chinese schizophrenic patients suggests that cerebral hypoperfusion and laterality exist in different ethnic groups. World J Biol Psychiatry. 2005;6:98–106. doi: 10.1080/15622970510029821. [DOI] [PubMed] [Google Scholar]
- 48.Wang CS, Yang YK, Chen M, Chiu NT, Yeh TL, Lee IH. Negative symptoms and regional cerebral blood flow in patients with schizophrenia: A single photon emission computed tomography study. Kaohsiung J Med Sci. 2003;19:464–9. doi: 10.1016/S1607-551X(09)70492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsujino N, Nemoto T, Yamaguchi T, Katagiri N, Tohgi N, Ikeda R, et al. Cerebral blood flow changes in very-late-onset schizophrenia-like psychosis with catatonia before and after successful treatment. Psychiatry Clin Neurosci. 2011;65:600–3. doi: 10.1111/j.1440-1819.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 50.Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: Relationship with treatment response. Psychiatry Res. 2009;174:121–9. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Novak B, Milcinski M, Grmek M, Kocmur M. Early effects of treatment on regional cerebral blood flow in first episode schizophrenia patients evaluated with 99Tc-ECD-SPECT. Neuro Endocrinol Lett. 2005;26:685–9. [PubMed] [Google Scholar]
- 52.Sharafi M. Comparison of Classical and Clozapine Treatment on Schizophrenia Using Positive and Negative Syndrome Scale of Schizophrenia (PANSS) and SPECT Imaging. Int J Med Sci. 2005;2:79–86. doi: 10.7150/ijms.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanahara N, Shimizu E, Sekine Y, Uchida Y, Shibuya T, Yamanaka H, et al. Does hypofrontality expand to global brain area in progression of schizophrenia?: A cross-sectional study between first-episode and chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:410–5. doi: 10.1016/j.pnpbp.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Musalek M, Podreka I, Walter H, Suess E, Passweg V, Nutzinger D, et al. Regional brain function in hallucinations: A study of regional cerebral blood flow with 99m-Tc-HMPAO-SPECT in patients with auditory hallucinations, tactile hallucinations, and normal controls. Compr Psychiatry. 1989;30:99–108. doi: 10.1016/0010-440x(89)90123-5. [DOI] [PubMed] [Google Scholar]
- 55.Toone BK, Okocha CI, Sivakumar K, Syed GM. Changes in regional cerebral blood flow due to cognitive activation among patients with schizophrenia. Br J Psychiatry. 2000;177:222–8. doi: 10.1192/bjp.177.3.222. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan HI, Sadock BJ. 6th ed. Baltimore, MD: Williams and Wilkins; 1991. Synopsis of Psychiatry; pp. 278–84. (363-82). [Google Scholar]
- 57.Devous MD., Sr Comparison of SPECT applications in neurology and psychiatry. J Clin Psychiatry. 1992;53(Suppl):13–9. [PubMed] [Google Scholar]
- 58.Mayberg HS, Jeffery PJ, Wagner HN, Simpson SG. Regional cerebral blood flow in patients with refractory unipolar depression measured with Tc-99m HMPAO SPECT. J Nucl Med. 1991;32(Suppl):951. Abstract. [Google Scholar]
- 59.Van Heertum RL, O’Connell RA. Functional brain imaging in the evaluation of psychiatric illness. Semin Nucl Med. 1991;21:24–39. doi: 10.1016/s0001-2998(05)80077-7. [DOI] [PubMed] [Google Scholar]
- 60.Ito H, Kawashima R, Awata S, Ono S, Sato K, Goto R, et al. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J Nucl Med. 1996;37:410–4. [PubMed] [Google Scholar]
- 61.Vasile RG, Schwartz RB, Garada B, Holman BL, Alpert M, Davidson PB, et al. Focal cerebral perfusion defects demonstrated by 99mTc-hexamethylpropyleneamine oxime SPECT in elderly depressed patients. Psychiatry Res. 1996;67:59–70. doi: 10.1016/0925-4927(96)02689-3. [DOI] [PubMed] [Google Scholar]
- 62.Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39:608–12. [PubMed] [Google Scholar]
- 63.Périco CA, Skaf CR, Yamada A, Duran F, Buchpiguel CA, Castro CC, et al. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: A single photon emission computed tomography study using statistical parametric mapping. Neurosci Lett. 2005;384:265–70. doi: 10.1016/j.neulet.2005.04.088. [DOI] [PubMed] [Google Scholar]
- 64.Kim SJ, Song SH, Kim JH, Kwak IS. Statistical parametric mapping analysis of the relationship between regional cerebral blood flow and symptom clusters of the depressive mood in patients with pre-dialytic chronic kidney disease. Ann Nucl Med. 2008;22:201–6. doi: 10.1007/s12149-007-0108-x. [DOI] [PubMed] [Google Scholar]
- 65.Yao WJ, Pan HA, Yang YK, Chou YH, Wang ST, Yu CY, et al. Reduced frontal perfusion in depressed postmenopausal women: A SPECT study with WCST. Maturitas. 2008;59:83–90. doi: 10.1016/j.maturitas.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Brody AL, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry. 2001;6:102–12. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- 67.Lucey JV, Costa DC, Adshead G, Deahl M, Busatto G, Gacinovic S, et al. Brain blood flow in anxiety disorders. OCD, panic disorder with agoraphobia, and post-traumatic stress disorder on 99mTcHMPAO single photon emission tomography (SPET) Br J Psychiatry. 1997;171:346–50. doi: 10.1192/bjp.171.4.346. [DOI] [PubMed] [Google Scholar]
- 68.Fernández-Argüelles P, Castro Montaño J, García López O, Cambil Molina T. SPECT study of a group of patients with severe recurrent depression. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1998;26:223–32. [PubMed] [Google Scholar]
- 69.Holman BL, Carvalho PA, Mendelson J, Teoh SK, Nardin R, Hallgring E, et al. Brain perfusion is abnormal in cocaine-dependent polydrug users: A study using technetium-99m-HMPAO and ASPECT. J Nucl Med. 1991;32:1206–10. [PubMed] [Google Scholar]
- 70.Botelho MF, Relvas JS, Abrantes M, Cunha MJ, Marques TR, Rovira E, et al. Brain blood flow SPET imaging in heroin abusers. Ann N Y Acad Sci. 2006;1074:466–77. doi: 10.1196/annals.1369.047. [DOI] [PubMed] [Google Scholar]
- 71.Jordaan GP, Warwick JM, Nel DG, Hewlett R, Emsley R. Alcohol-induced psychotic disorder: Brain perfusion and psychopathology-before and after anti-psychotic treatment. Metab Brain Dis. 2012;27:67–77. doi: 10.1007/s11011-011-9273-7. [DOI] [PubMed] [Google Scholar]
- 72.Jordaan GP, Warwick JM, Hewlett R, Emsley R. Resting brain perfusion in alcohol-induced psychotic disorder: A comparison in patients with alcohol dependence, schizophrenia and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:479–85. doi: 10.1016/j.pnpbp.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 73.Chung YA, Choi SW, Joe KH, Jeong J, Cheon Y, Kim DJ. Regional cerebral blood flow in patients with alcohol-related dementia: A SPECT study. Int J Neurosci. 2009;119:2100–11. doi: 10.1080/00207450903170338. [DOI] [PubMed] [Google Scholar]
- 74.Holman BL, Mendelson J, Garada B, Teoh SK, Hallgring E, Johnson KA, et al. Regional cerebral blood flow improves with treatment in chronic cocaine polydrug users. J Nucl Med. 1993;34:723–7. [PubMed] [Google Scholar]
- 75.Miller BL, Mena I, Giombetti R, Villanueva-Meyer J, Djenderedjian AH. Neuropsychiatric effects of cocaine: SPECT measurements. J Addict Dis. 1992;11:47–58. doi: 10.1300/J069v11n04_04. [DOI] [PubMed] [Google Scholar]
- 76.Adinoff B, Braud J, Devous MD, Harris TS. Caudolateral orbitofrontal regional cerebral blood flow is decreased in abstinent cocaine-addicted subjects in two separate cohorts. Addict Biol. 2012;17:1001–12. doi: 10.1111/j.1369-1600.2011.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD., Sr Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med. 2006;3:206–22. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Küçük NO, Kiliç EO, Ibis E, Aysev A, Gençoglu EA, Aras G, et al. Brain SPECT findings in long-term inhalant abuse. Nucl Med Commun. 2000;21:769–73. doi: 10.1097/00006231-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Lele RD. 1st ed. New Delhi: Jaypee Brothers Medical Publishers Pvt; 2009. Principles and practice of nuclear medicine and correlative medical imaging; p. 175. [Google Scholar]
- 80.Degirmenci B, Miral S, Kaya GC, Iyilikçi L, Arslan G, Baykara A, et al. Technetium-99m HMPAO brain SPECT in autistic children and their families. Psychiatry Res. 2008;162:236–43. doi: 10.1016/j.pscychresns.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: A potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 82.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage. 2004;22:409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 83.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 84.Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161:1038–48. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- 85.Diler RS, Kibar M, Avci A. Pharmacotherapy and regional cerebral blood flow in children with obsessive compulsive disorder. Yonsei Med J. 2004;45:90–9. doi: 10.3349/ymj.2004.45.1.90. [DOI] [PubMed] [Google Scholar]
- 86.Wu JC, Gillin JC, Buchsbaum MS, Schachat C, Darnall LA, Keator DB, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107:181–6. doi: 10.1016/j.jad.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 87.Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156:1149–58. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 88.Langguth B, Wiegand R, Kharraz A, Landgrebe M, Marienhagen J, Frick U, et al. Pre-treatment anterior cingulate activity as a predictor of antidepressant response to repetitive transcranial magnetic stimulation (rTMS) Neuro Endocrinol Lett. 2007;28:633–8. [PubMed] [Google Scholar]
- 89.Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–7. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- 90.Oner P, Oner O, Aysev A, Küçük O, Ibis E. Comparison of cerebral blood flow in children with obsessive compulsive disorder and attention deficit hyperactivity disorder. Turk Psikiyatri Derg. 2008;19:13–8. [PubMed] [Google Scholar]
- 91.Brockmann H, Zobel A, Joe A, Biermann K, Scheef L, Schuhmacher A, et al. The value of HMPAO SPECT in predicting treatment response to citalopram in patients with major depression. Psychiatry Res. 2009;173:107–12. doi: 10.1016/j.pscychresns.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 92.Molina Rodríguez V, Montz Andreé R, Pérez Castejón MJ, Capdevila García E, Carreras Delgado JL, Rubia Vila FJ. SPECT study of regional cerebral perfusion in neuroleptic-resistant schizophrenic patients who responded or did not respond to clozapine. Am J Psychiatry. 1996;153:1343–6. doi: 10.1176/ajp.153.10.1343. [DOI] [PubMed] [Google Scholar]
- 93.Noël X, Sferrazza R, Van Der Linden M, Paternot J, Verhas M, Hanak C, et al. Contribution of frontal cerebral blood flow measured by (99m) Tc-Bicisate spect and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37:347–54. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- 94.Kanetaka H, Hanyu H, Hirao K, Shimizu S, Sato T, Akai T, et al. Prediction of response to donepezil in Alzheimer's disease: Combined MRI analysis of the substantia innominata and SPECT measurement of cerebral perfusion. Nucl Med Commun. 2008;29:568–73. doi: 10.1097/MNM.0b013e3282f5e5f4. [DOI] [PubMed] [Google Scholar]
- 95.Mega MS, Dinov ID, Lee L, O’Connor SM, Masterman DM, Wilen B, et al. Orbital and dorsolateral frontal perfusion defect associated with behavioral response to cholinesterase inhibitor therapy in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2000;12:209–18. doi: 10.1176/jnp.12.2.209. [DOI] [PubMed] [Google Scholar]
- 96.Guedj E, Cammilleri S, Colavolpe C, Taieb D, de Laforte C, Niboyet J, et al. Predictive value of brain perfusion SPECT for ketamine response in hyperalgesic fibromyalgia. Eur J Nucl Med Mol Imaging. 2007;34:1274–9. doi: 10.1007/s00259-007-0392-7. [DOI] [PubMed] [Google Scholar]
- 97.Amen DG, Hanks C, Prunella J. Predicting positive and negative treatment responses to stimulants with brain SPECT imaging. J Psychoactive Drugs. 2008;40:131–8. doi: 10.1080/02791072.2008.10400622. [DOI] [PubMed] [Google Scholar]
- 98.Goethals I, Audenaert K, Jacobs F, Van den Eynde F, Bernagie K, Kolindou A, et al. Brain perfusion SPECT in impulsivity-related personality disorders. Behav Brain Res. 2005;157:187–92. doi: 10.1016/j.bbr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 99.Amen DG, Hanks C, Prunella JR, Green A. An analysis of regional cerebral blood flow in impulsive murderers using single photon emission computed tomography. J Neuropsychiatry Clin Neurosci. 2007;19:304–9. doi: 10.1176/jnp.2007.19.3.304. [DOI] [PubMed] [Google Scholar]
- 100.Amen DG, Prunella JR, Fallon JH, Amen B, Hanks C. A comparative analysis of completed suicide using high resolution brain SPECT imaging. J Neuropsychiatry Clin Neurosci. 2009;21:430–9. doi: 10.1176/jnp.2009.21.4.430. [DOI] [PubMed] [Google Scholar]
- 101.Gescher DM, Malevani J. Mood stabilizer in the psychopharmacotherapy of borderline personality disorder. Fortschr Neurol Psychiatr. 2009;77:389–98. doi: 10.1055/s-0028-1109455. [DOI] [PubMed] [Google Scholar]
- 102.Freo U, Pizzolato G, Dam M, Ori C, Battistin L. A short review of cognitive and functional neuroimaging studies of cholinergic drugs: Implications for therapeutic potentials. J Neural Transm. 2002;109:857–70. doi: 10.1007/s007020200070. [DOI] [PubMed] [Google Scholar]


