Abstract
Hemichorea and generalized chorea are rare syndromes associated with nonketotic hyperglycemia. This disorder usually afflicts elderly females, and may herald the onset of new onset diabetes, usually type 2. There are conflicting reports of the underlying pathophysiology of this rare entity. Magnetic resonance imaging findings have been described in the past, and are characteristic. There are very few reports of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) findings of this unusual dyskinetic syndrome. This report describes the PET/CT features of this rare disease. Early detection and prompt correction of hyperglycemia may lead to complete or significant amelioration of symptoms.
Keywords: 18F-fluorodeoxyglucose positron emission tomography/computed tomography, chorea, nonketotic hyperglycemia
INTRODUCTION
Hemichorea and generalized chorea are rare syndromes associated with nonketotic hyperglycemia. They may occasionally be the presenting feature of new onset diabetes mellitus. The underlying pathophysiology is not clear at present. We describe a case of an elderly female patient who presented with acute onset of generalized choreoathetoid movements. Diabetes mellitus was detected for the first time in this case. She underwent a cranial magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan, which showed characteristic findings. On achieving euglycemia by insulin, the abnormal movements showed marked improvement. Early detection and prompt correction of hyperglycemia may lead to complete or significant amelioration of symptoms in this rare disease.
CASE REPORT
This was a case report of a 50-year-old female patient who presented with the complaints of acute onset quasi-purposive slow writhing movements mainly involving bilateral upper limbs, but more on the right side. On general physical examination, she was afebrile, normotensive, fully conscious, and oriented. Intermittent choreoathetotic involuntary movements were observed in the bilateral upper limbs and neck (more so on the right), which worsened on voluntary activity. There was no family history of movement disorders or any past history of diabetes mellitus, hypertension, stroke, or intake of neuroleptic drugs. Routine investigations were normal except that fasting blood glucose level was elevated (362 mg/dl) and HbA1c was 13.5%. Estimated blood osmolality was 304 mOsm/L and no ketones were detected on urinalysis.
Magnetic resonance imaging showed hyperintensity on T1-weighted (T1-W) sequences and a subtle hyperintense signal on T2-W sequences in the bilateral caudate nucleus and putamen [figure 1]. There was no surrounding edema or mass effect. 18F-FDG PET/CT study was subsequently performed on a Discovery STE16 camera (General Electric Medical Systems, Milwaukee, WI, USA). The PET/CT study showed diffuse hypometabolism in bilateral basal ganglia [figure 2]. Increased radiotracer accumulation was noted in the left motor cortex related to a recent episode of choreiform movements, more prominent on the right side [figure 3]. The patient was started on insulin therapy. The abnormal involuntary movements decreased gradually and she was discharged home.
Figure 1.
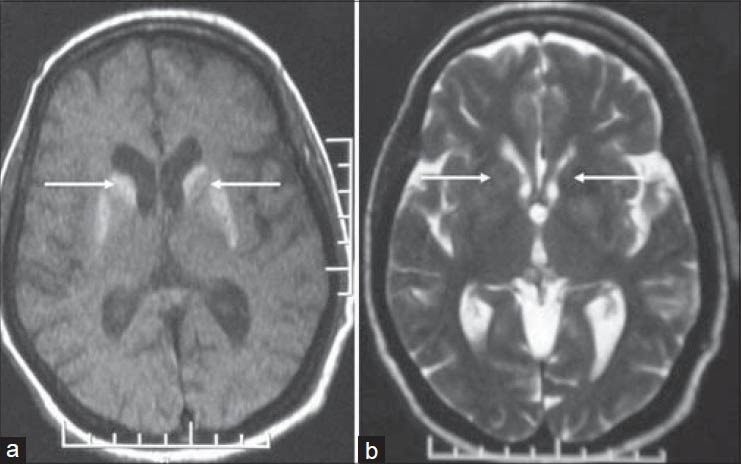
Transaxial T1-weighted (T1-W) (a) and T2-W (b) magnetic resonance imaging scans show diffuse hyperintense signal in bilateral caudate nucleus and putamen (arrows)
Figure 2.
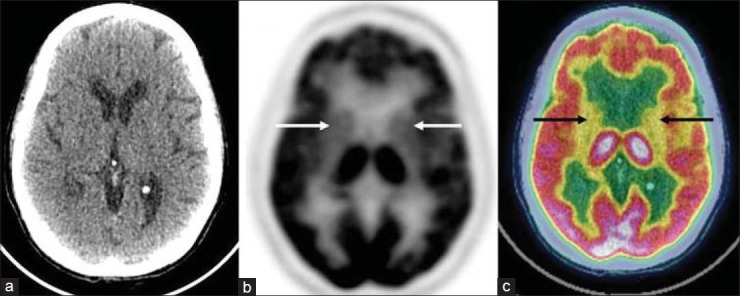
Transaxial computed tomography (CT) image (a) is unremarkable. Transaxial positron emission tomography (PET) (b) and fused PET/CT (c) images show diffuse hypometabolism in bilateral caudate nucleus and putamen (arrows)
Figure 3.
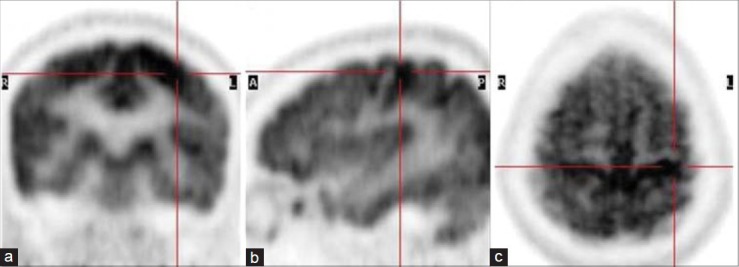
Coronal (a), sagittal (b) and axial (c) positron emission tomography images show increased radiotracer accumulation in the left motor cortex (cross-hairs)
DISCUSSION
Abnormal movements are well-known presenting signs of metabolic disturbances.
Most cases of choreiform movements secondary to nonketotic hyperglycemia are diagnosed in diabetic patients, and this entity has been reported in the past as one of the presenting symptoms of new-onset diabetes. This was reported for the first time by Bidwell in 1960.[1] A higher disease preponderance has been observed in elderly women of Asian origin, suggesting a genetic disposition.[2] A triad of nonketotic hyperglycemia, acute chorea, and a hyperdense and hyperintense putamen on CT and T1-W MRIs, respectively, is a known presentation.[3] These are usually not accompanied by signs of mass effect, edema, or volume loss. The abnormal signal seen on MRI may be unilateral, or bilateral with corresponding contralateral or bilateral body symptoms respectively. This entity can mimic ischemic stroke both clinically and radiologically.
The FDG PET findings in patients with nonketotic hyperglycemia have been described in the past.[4] Markedly reduced rates of cerebral glucose metabolism in the contralateral or bilateral striatum corresponding to lesions on T1-W MRI have been observed, as seen in the present case too. Our case demonstrated an additional PET/CT feature of increased radiotracer uptake in the left motor cortex, likely owing to a recent episode of choreiform movements, more prominent on the right side. Similar findings were documented in one previous report.[5] In a systematic study of six cases of nonketotic hyperglycemic chorea using single-photon emission CT,[6] the presence of hypoperfusion in the striatum contralateral to the symptomatic chorea was observed in five patients and hypoperfusion in both striatum in one.
The pathophysiological mechanism underlying the imaging appearance and clinical manifestations of nonketotic hyperglycemia is not fully understood. Previous studies, which have attempted to elucidate the pathological basis of this entity, have not been fully conclusive. The hypometabolism observed on 18F-FDG PET/CT suggests that hypofunction of the striatum is a possible common pathogenetic pathway in the development of chorea. Among the several hypotheses that have been put forward to explain the pathogenesis of movement disorders in this condition, the most prominent ones include relative dopaminergic hypersensitivity, undefined effect of hypersomolality, decrease in aminobutyric acid and hypometabolism of striatal cells due to hypoperfusion.[7] It is possible that underlying chronic focal cerebrovascular disease in diabetes and the synergetic metabolic effects during the hyperglycemic episode may precipitate these changes.[8] Hyperglycemia also produces a global decrease in regional cerebral blood flow with maximal reduction in the basal ganglia. This may contribute to the reduction of local amounts of g-aminobutyric acid, which may allow increased pallidal activity with resultant dyskinesia.[9] Biopsy performed in one patient with this disease revealed striatal changes in the form of severe thickening of all layers of arterioles with marked narrowing of vessel lumens and patchy necrotic tissue. Hyaline degeneration of the arteriolar walls, extravasation of erythrocytes, and prominent capillary proliferation were also notable, together with lymphocytic infiltration and macrophage invasion.[10] The authors concluded that the constellation of signs and symptoms and neuroimaging characteristics were due to obliterative vasculopathy with prominent vascular proliferation, vulnerability to which is restricted to the striatum.
Basal ganglia hypometabolism is seen in other entities as well. Striatal hypometabolism is the hall-mark of Huntington's disease, seen not only in affected patients, but asymptomatic gene carriers as well.[11] It has also been observed in carbon-monoxide poisoning,[12] cyanide poisoning[13] and Fahr's disease.[14] In addition to areas of cortical hypometabolism seen in movement disorders, there is evidence of basal ganglia hypometabolism as well, in Parkinsonian syndromes like corticobasal syndrome and multisystem atrophy.[15,16] The choreiform movements associated with nonketotic hyperglycemia are potentially reversible as rapid detection and early correction of hyperglycemia can lead to complete recovery of these involuntary movements in several cases. Due to the reversible nature of the condition, ischemic injury to the basal ganglia, which may be more likely partial and reversible appears to be the most plausible explanation. The striatal changes detected on imaging may diminish or persist for months or years after resolution of the movement disorder. Familiarity with the imaging characteristics of this rare entity may facilitate prompt diagnosis and treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bidwell SF. Some observations on hemballismus. Neurol. 1960;10:619–26. [Google Scholar]
- 2.Lin JJ, Lin GY, Shih C, Shen WC. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by non-ketotic hyperglycemia: Report of seven new cases and a review of literature. J Neurol. 2001;248:750–5. doi: 10.1007/s004150170089. [DOI] [PubMed] [Google Scholar]
- 3.Yacoub HA. Abnormal magnetic resonance imaging and hemichorea associated with non-ketotic hyperglycemia. J Neurol Res. 2013;3:146–9. [Google Scholar]
- 4.Hsu JL, Wang HC, Hsu WC. Hyperglycemia-induced unilateral basal ganglion lesions with and without hemichorea. A PET study. J Neurol. 2004;251:1486–90. doi: 10.1007/s00415-004-0571-4. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen BD. Brain and upper extremity PET/CT findings of hyperglycemia-induced hemiballism-hemichorea. Clin Nucl Med. 2007;32:643–5. doi: 10.1097/RLU.0b013e3180a1acb0. [DOI] [PubMed] [Google Scholar]
- 6.Chang MH, Li JY, Lee SR, Men CY. Non-ketotic hyperglycaemic chorea: A SPECT study. J Neurol Neurosurg Psychiatry. 1996;60:428–30. doi: 10.1136/jnnp.60.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamide A, Kumarsamy R, Srimannarayana J, Mathew J, Das AK. Chorea due to nonketotic hyperglycemia. Neurol India. 2002;50:213–4. [PubMed] [Google Scholar]
- 8.Lai PH, Tien RD, Chang MH, Teng MM, Yang CF, Pan HB, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17:1057–64. [PMC free article] [PubMed] [Google Scholar]
- 9.McCall AL. The impact of diabetes on the CNS. Diabetes. 1992;41:557–70. doi: 10.2337/diab.41.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Yamamoto T, Soeda T, Kumagai T, Tanno Y, Kubo J, et al. Diabetic striatal disease: Clinical presentation, neuroimaging, and pathology. Intern Med. 2009;48:1135–41. doi: 10.2169/internalmedicine.48.1996. [DOI] [PubMed] [Google Scholar]
- 11.Antonini A, Leenders KL, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington's disease. Brain. 1996;119:2085–95. doi: 10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 12.Hon KL, Yeung WL, Ho CH, Leung WK, Li AM, Chu WC, et al. Neurologic and radiologic manifestations of three girls surviving acute carbon monoxide poisoning. J Child Neurol. 2006;21:737–41. doi: 10.1177/08830738060210090401. [DOI] [PubMed] [Google Scholar]
- 13.Zaknun JJ, Stieglbauer K, Trenkler J, Aichner F. Cyanide-induced akinetic rigid syndrome: Clinical, MRI, FDG-PET, beta-CIT and HMPAO SPECT findings. Parkinsonism Relat Disord. 2005;11:125–9. doi: 10.1016/j.parkreldis.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Benke T, Karner E, Seppi K, Delazer M, Marksteiner J, Donnemiller E. Subacute dementia and imaging correlates in a case of Fahr's disease. J Neurol Neurosurg Psychiatry. 2004;75:1163–5. doi: 10.1136/jnnp.2003.019547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turaga SP, Mridula R, Borgohain R. Cerebral glucose metabolism, clinical, neuropsychological, and radiological profile in patients with corticobasal syndrome. Neurol India. 2013;61:7–11. doi: 10.4103/0028-3886.107916. [DOI] [PubMed] [Google Scholar]
- 16.Lyoo CH, Jeong Y, Ryu YH, Lee SY, Song TJ, Lee JH, et al. Effects of disease duration on the clinical features and brain glucose metabolism in patients with mixed type multiple system atrophy. Brain. 2008;131:438–46. doi: 10.1093/brain/awm328. [DOI] [PubMed] [Google Scholar]


