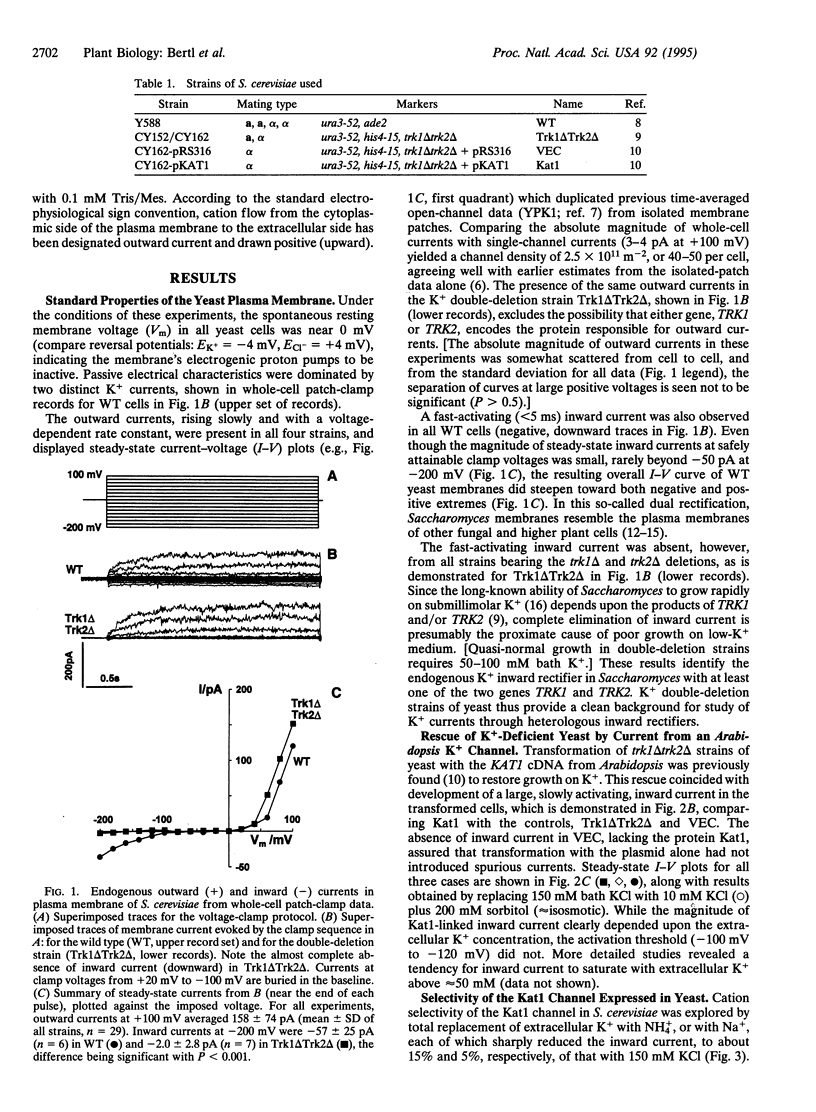
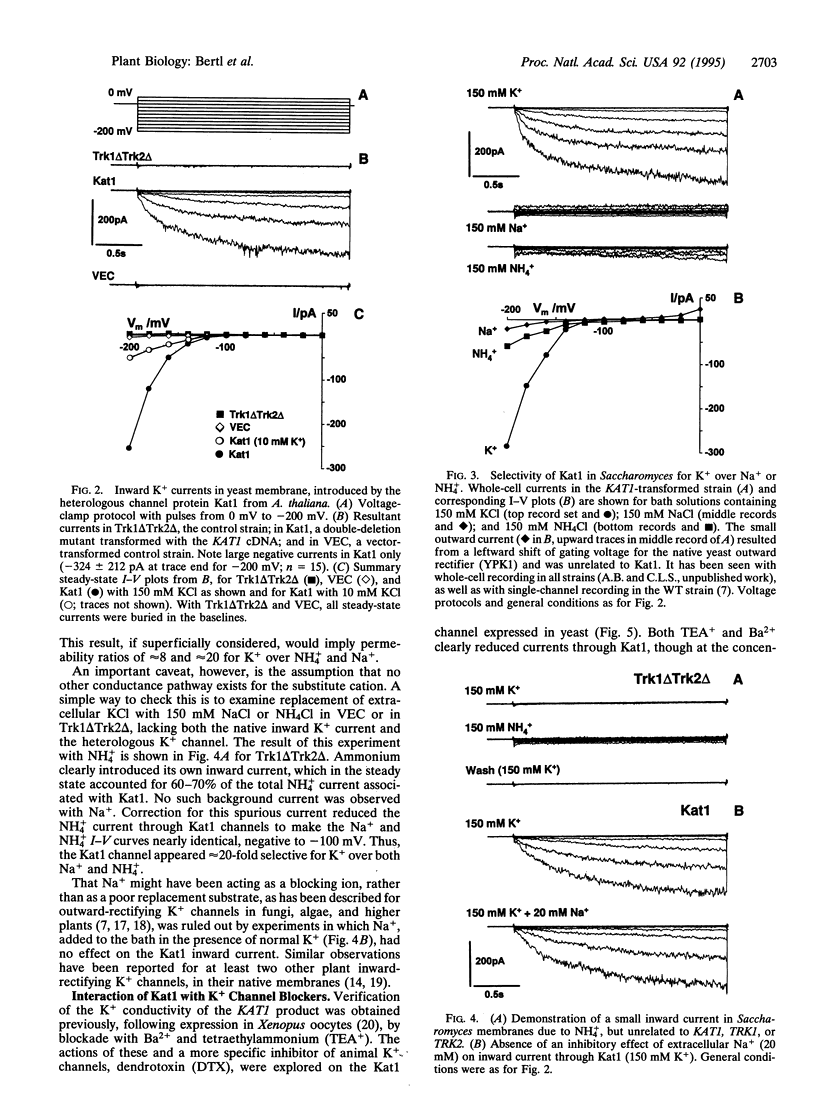
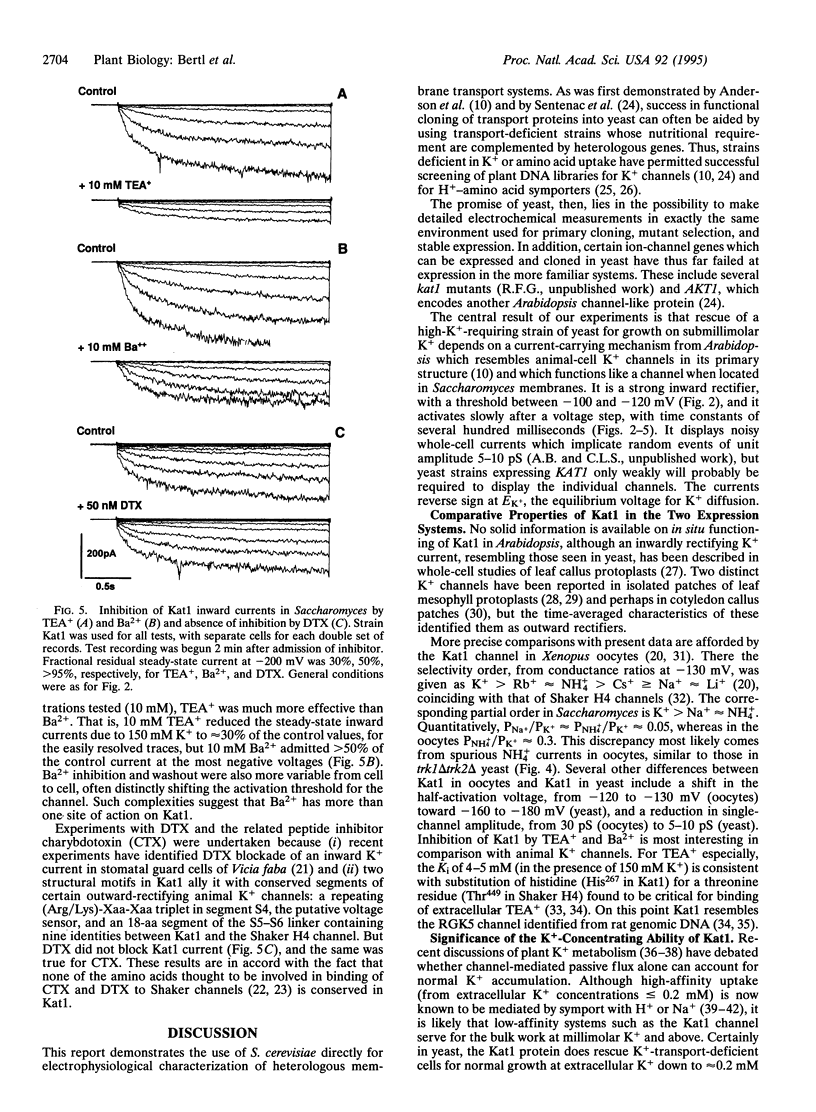
Abstract
Transport-deficient strains of the yeast Saccharomyces cerevisiae have recently proven useful for cloning, by functional complementation, of cDNAs encoding heterologous membrane transporters: specifically, H(+)-amino acid symporters and K+ channels from the higher plant Arabidopsis thaliana. The present study uses whole-cell patch-clamp experiments to show that yeast strains which grow poorly on submillimolar K+ due to the deletion of two K(+)-transporter genes (TRK1 and TRK2) are in fact missing a prominent K+ inward current present in wild-type cells. Rescue of such strains for growth on low K+ by transformation with a gene (KAT1) encoding an inward-rectifying K+ channel from Arabidopsis is accompanied by the appearance of an inward current whose characteristics are in qualitative agreement with previous studies in the Xenopus oocyte system, but differ in quantitative details. The ability to make such measurements directly on Saccharomyces should facilitate structure-function studies of any electrogenic or electrophoretic ion transporters which can be expressed in the plasma membrane (or tonoplast) of that organism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Huprikar S. S., Kochian L. V., Lucas W. J., Gaber R. F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., Slayman C. L. Cation-selective channels in the vacuolar membrane of Saccharomyces: dependence on calcium, redox state, and voltage. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7824–7828. doi: 10.1073/pnas.87.20.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., Slayman C. L. Complex modulation of cation channels in the tonoplast and plasma membrane of Saccharomyces cerevisiae: single-channel studies. J Exp Biol. 1992 Nov;172:271–287. doi: 10.1242/jeb.172.1.271. [DOI] [PubMed] [Google Scholar]
- Bertl A., Slayman C. L., Gradmann D. Gating and conductance in an outward-rectifying K+ channel from the plasma membrane of Saccharomyces cerevisiae. J Membr Biol. 1993 Mar;132(3):183–199. doi: 10.1007/BF00235737. [DOI] [PubMed] [Google Scholar]
- Blatt M. R. Ion channel gating in plants: physiological implications and integration for stomatal function. J Membr Biol. 1991 Nov;124(2):95–112. doi: 10.1007/BF01870455. [DOI] [PubMed] [Google Scholar]
- Blatt M. R. K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH. J Gen Physiol. 1992 Apr;99(4):615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt M. R., Rodriguez-Navarro A., Slayman C. L. Potassium-proton symport in Neurospora: kinetic control by pH and membrane potential. J Membr Biol. 1987;98(2):169–189. doi: 10.1007/BF01872129. [DOI] [PubMed] [Google Scholar]
- Colombo R., Cerana R. Inward Rectifying K Channels in the Plasma Membrane of Arabidopsis thaliana. Plant Physiol. 1991 Nov;97(3):1130–1135. doi: 10.1104/pp.97.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Osborne P. B., Cai Y. C., Wilkinson M., Christie M. J., Adelman J. P. Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel. J Immunol. 1990 Jun 15;144(12):4841–4850. [PubMed] [Google Scholar]
- Frommer W. B., Hummel S., Riesmeier J. W. Expression cloning in yeast of a cDNA encoding a broad specificity amino acid permease from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5944–5948. doi: 10.1073/pnas.90.13.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W., Ward J. M., Schroeder J. I. Physiological Roles of Inward-Rectifying K+ Channels. Plant Cell. 1993 Nov;5(11):1491–1493. doi: 10.1105/tpc.5.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. Mechanism of charybdotoxin block of a voltage-gated K+ channel. Biophys J. 1993 Oct;65(4):1613–1619. doi: 10.1016/S0006-3495(93)81200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Martinac B., Saimi Y., Culbertson M. R., Kung C. Ion channels in yeast. Science. 1986 Sep 12;233(4769):1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. Conduction properties of the cloned Shaker K+ channel. Biophys J. 1993 Nov;65(5):2089–2096. doi: 10.1016/S0006-3495(93)81244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hsu L. C., Chiou T. J., Chen L., Bush D. R. Cloning a plant amino acid transporter by functional complementation of a yeast amino acid transport mutant. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7441–7445. doi: 10.1073/pnas.90.16.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. B., Rothstein A., Sherman F., Stannard J. N. Variation K+ and Na+ content during the growth cycle of yeast. Biochim Biophys Acta. 1965 Jun 15;104(1):310–312. doi: 10.1016/0304-4165(65)90257-6. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Ko C. H., Gaber R. F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Aug;11(8):4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Can K+ Channels Do It All? Plant Cell. 1993 Jul;5(7):720–721. doi: 10.1105/tpc.5.7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lew R. R. Substrate regulation of single potassium and chloride ion channels in Arabidopsis plasma membrane. Plant Physiol. 1991 Feb;95(2):642–647. doi: 10.1104/pp.95.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis F. J., Sanders D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9272–9276. doi: 10.1073/pnas.91.20.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Heginbotham L., Abramson T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron. 1990 Dec;5(6):767–771. doi: 10.1016/0896-6273(90)90335-d. [DOI] [PubMed] [Google Scholar]
- Mirzayan C., Copeland C. S., Snyder M. The NUF1 gene encodes an essential coiled-coil related protein that is a potential component of the yeast nucleoskeleton. J Cell Biol. 1992 Mar;116(6):1319–1332. doi: 10.1083/jcb.116.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer G., Armstrong F., Blatt M. R. Selective block by alpha-dendrotoxin of the K+ inward rectifier at the Vicia guard cell plasma membrane. J Membr Biol. 1994 Feb;137(3):249–259. doi: 10.1007/BF00232593. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Blatt M. R., Slayman C. L. A potassium-proton symport in Neurospora crassa. J Gen Physiol. 1986 May;87(5):649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman D. P., Schroeder J. I., Lucas W. J., Anderson J. A., Gaber R. F. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992 Dec 4;258(5088):1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Schroeder J. I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994 Aug 25;370(6491):655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Ward J. M., Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J. M., Gaymard F., Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992 May 1;256(5057):663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Respiration and the intracellular potential. J Gen Physiol. 1965 Sep;49(1):93–116. doi: 10.1085/jgp.49.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding E. P., Goldsmith MHM. Activation of K+ Channels in the Plasma Membrane of Arabidopsis by ATP Produced Photosynthetically. Plant Cell. 1993 Apr;5(4):477–484. doi: 10.1105/tpc.5.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding E. P., Slayman C. L., Goldsmith M. H., Gradmann D., Bertl A. Ion channels in Arabidopsis plasma membrane : transport characteristics and involvement in light-induced voltage changes. Plant Physiol. 1992 May;99(1):96–102. doi: 10.1104/pp.99.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]



