Abstract
The introduction of topical steroids (TS) of varying potency have rendered the therapy of inflammatory cutaneous disorders more effective and less time-consuming. However the usefulness of these has become a double edged sword with constantly rising instances of abuse and misuse leading to serious local, systemic and psychological side effects. These side effects occur more with TS of higher potency and on particular areas of the body like face and genitalia. The article reviews the side effects of TS with special mention about peadiatric age group, also includes the measures for preventing the side effects.
Keywords: Local systemic, side effects, topical steroids
INTRODUCTION
The introduction of topical corticosteroids (TC) by Sulzberger and Witten in 1952 is considered to be the most significant landmark in the history of therapy of dermatological disorders.[1] This historical event was gradually, followed by the introduction of a large number of newer TC molecules of varying potency rendering the therapy of various inflammatory cutaneous disorders more effective and less time consuming. Although, it is this very usefulness of the drug which has become a double edged sword and made it vulnerable to now an alarming proportion with constantly rising instances of abuse and misuse leading to serious local, systemic, and psychological side effects. Such misuse occurs more with TC of higher potency and on softer areas of the body particularly the face and genitalia. The end-users of TC are hapless patients. They tend to overuse TCs beyond the time limit set by clinicians by repeating prescriptions. Of more concern is the mass use of TCs as fairness creams. Vast sections of the Indian society have willingly or unknowingly become victims to the craze of beautification leading to a virtual epidemic of monomorphic acne, steroid atrophy, steroid rosacea, telangiectasia, perioral dermatitis, striae and other manifestations of a condition which has been collectively described as topical steroid damaged facies (TSDF).[2] Children are particularly prone to develop systemic side effects when potent TCs are used on their softer skin with enhanced capacity for absorption as also the issue of weight versus body surface.[3] TCs are the choice of therapy in atopic children however, steroid-phobia among parents of such children is now a well-documented phenomenon. At the opposite end of the spectrum lies the danger of steroid addiction. While TC addiction can manifest with features of TSDF, its withdrawal is also accompanied by repeated flares of photosensitivity, erythema, papules and pustules accompanied by intense itching and burning, features of the so called “TSDF.” TC misuse has thus become almost an epidemic needing immediate attention from all quarters.
Side effects due to topical steroids (TS) are more prevalent than systemic reactions.
The most common side effects are localized to sites of application.[4] The mechanisms responsible for their effectiveness are also responsible for their adverse effects.[5]
SIDE EFFECTS CAN BE DIVIDED INTO
Local side effects
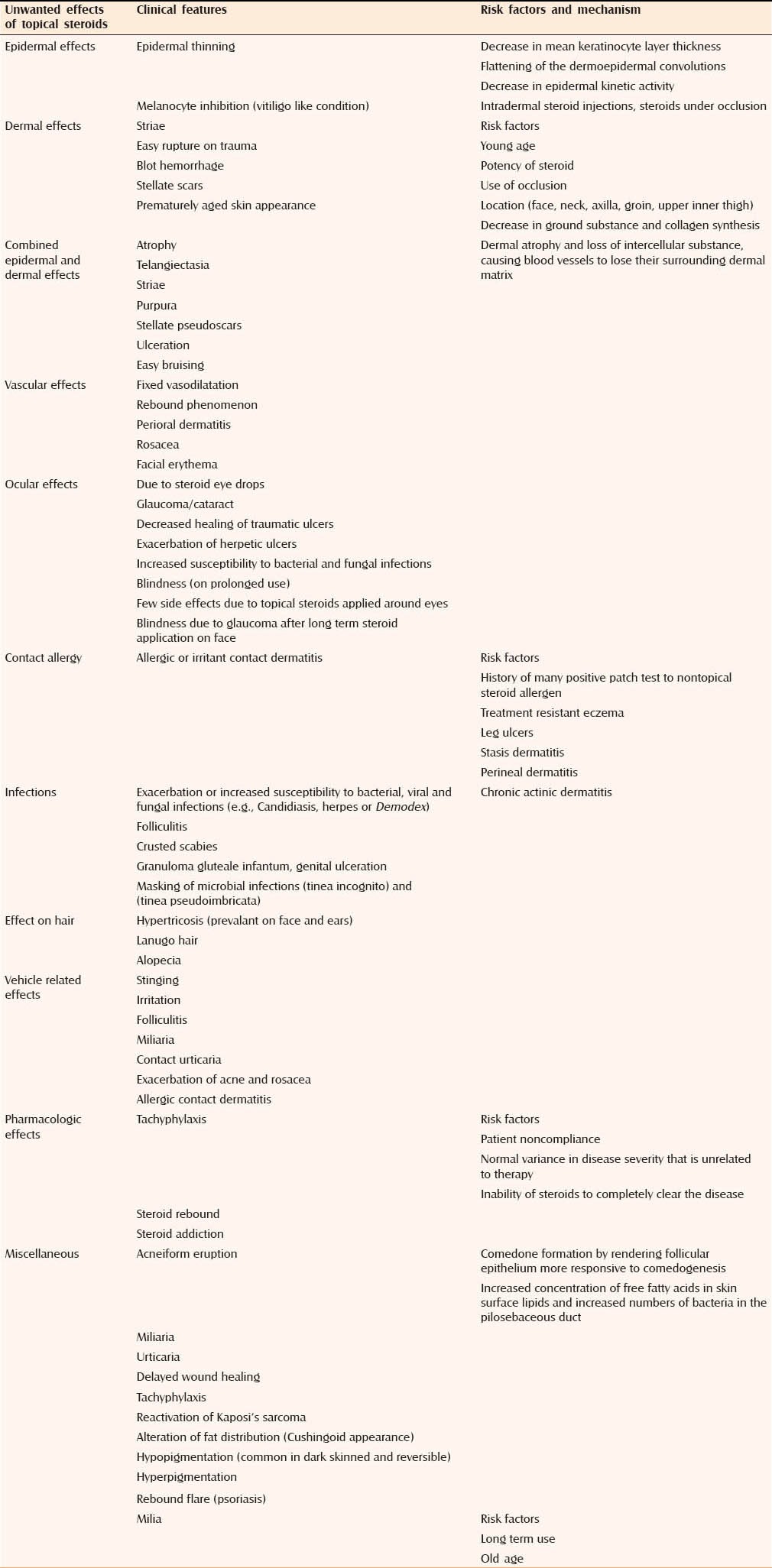
These tend to occur with prolonged treatment and depend on potency of TS, its vehicle and site of application. The most common include atrophy, striae, rosacea, perioral dermatitis, acne and purpura. Hypertrichosis, pigment alteration, delayed wound healing and exacerbation of skin infections are less frequent.[5] Table 1 lists the local side effects of TS with associated risk factors and mechanism.
Table 1.
In the infection column, put comma between Granuloma gluteale infantum and genital ulceration
Systemic adverse effects
Systemic adverse effects from TS have also been described and they are more likely to develop when highly potent TS are used for prolonged periods on thin skin (e.g. face) or on raw/inflamed surfaces.[4,5]
SUPPRESSION OF THE HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
Hypothalamic-pituitary adrenal axis suppression is known to occur with all TS. Factors responsible for HPA axis suppression include:
Increased application of moderately potent TS
Modest use of more potent derivatives
Increase in the steroid penetration (increases HPA suppression potential, especially in atopic children)
Use of TS under occlusion
Use of higher concentrations of TS
Application of TS over large surface areas.
Betamethasone dipropionate and diflorasone diacetate have an increased ability to suppress adrenal function. Around 14 g/week of clobetasol propionate ointment may induce suppression in children, while 49 g/week of betamethasone dipropionate reduces plasma cortisol levels. Temporary reversible suppression is seen with 49 g of superpotent TS used for 2 weeks. Children and babies have a high ratio of surface area to body volume hence are more likely develop HPA axis due to systemic absorption. Iatrogenic Cushing syndrome, corticosteroid-related Addison crises, growth retardation and death are also reported. The reactivity of the HPA axis can be assessed with the adrenocorticotropin hormone test. Recovery is time-dependent and occurs spontaneously.[4,5,8]
Hyperglycemia and diabetes mellitus
Hypergylcemia and the unmasking of latent diabetes mellitus can occur after prolonged application and high percutaneous absorption of TS; also systemically absorbed TS may precipitate or exacerbate hyperglycemia, especially in patients with preexisting hepatic disease.[8]
Mineralocorticoid effects
Topical steroids have minimal or no mineralocorticoid activities, but hydrocortisone and 9-a-fluoroprednisolone, have measurable mineralocorticoid activity. Prolonged treatment may lead to edema and hypocalcemia.[8] Table 2 lists the rare systemic adverse effects of TS.
Table 2.
Rare systemic adverse effects of topical steroids[8]

Side effects due to intralesional steroids
Hypopigmentation and skin atrophy can occur when TS are applied topically or injected locally. However, the mechanism by which hyopigmentation occurs is not clear. Linear extension of the hypopigmentation is due to lymphatic uptake of steroid crystals. Venkatesan and Fangman demonstrated that melanocytes are intact in steroid-induced hypopigmentation, which indicates that TS may impair melanocyte function. Triamcinolone is more likely to cause depigmentation due to its larger size, the higher tendency to aggregate and higher density. Hence for lesions close to the skin surface, especially in hyperpigmented patient triamcinolone should be avoided, instead TS with smaller particles and less tendency to aggregate should be preferred.[10] Various side effects of topical steroids are depicted in Figures 1–18.
Figure 1.
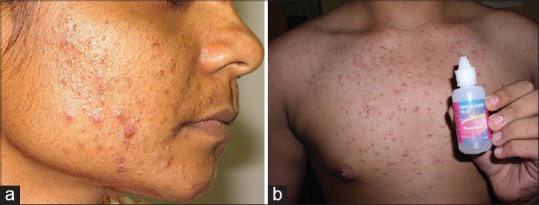
(a) Comedonal acne Courtesy Dr. Koushik Lahiri, (b) Steroid acne
Figure 18.
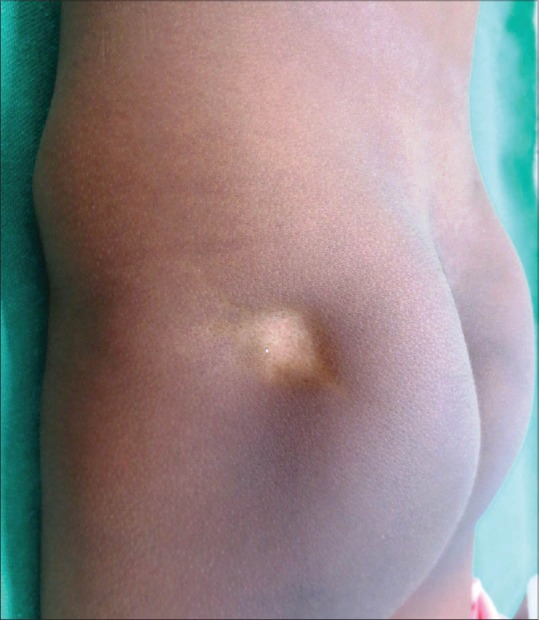
Atrophy and hypopigmentation secondary to IM steroids
Figure 2.
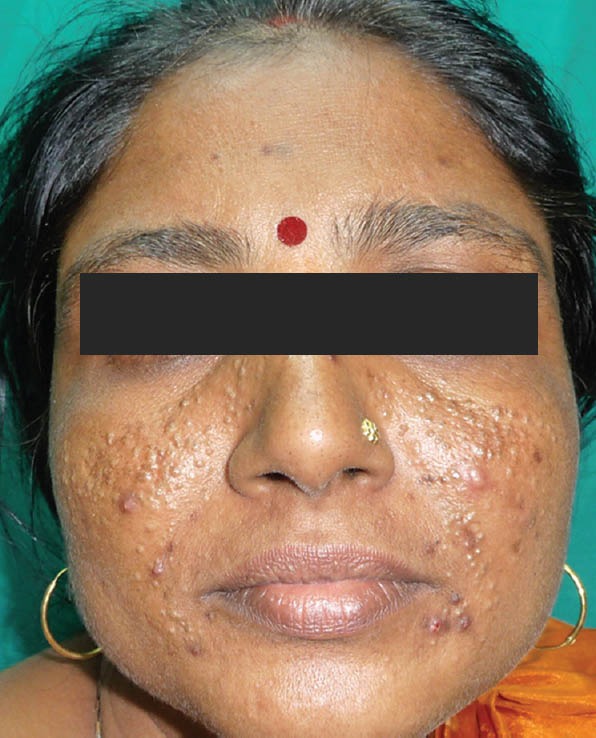
Comedonal acne Courtesy
Figure 3.
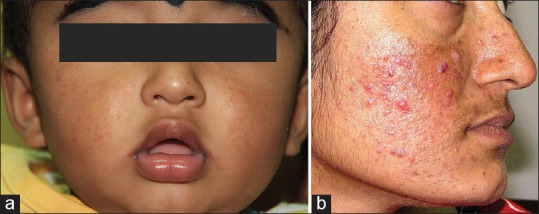
(a) Mild infantile acne Courtesy Dr. Shyam Verma, (b) Comedonal and papular acne
Figure 4.
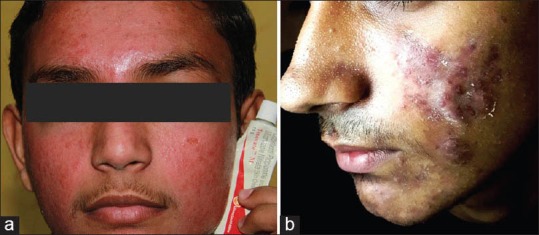
(a) Marked facial erythema on a background of acne (b) Nodular acne Courtesy
Figure 5.
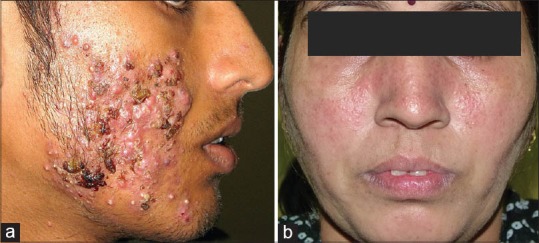
(a) Nodular ance with crusting (b) Rosacea
Figure 6.
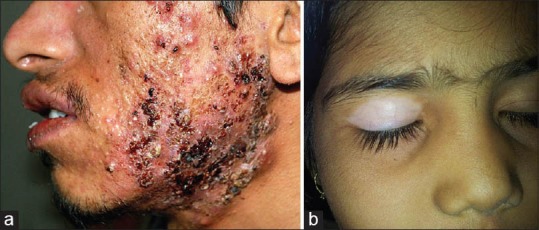
(a) Nodular acne with hemorrhagic crusting and pustulation Courtesy Dr. Koushik Lahiri, (b) Telangiectasia overlying vitiligo patch on eyelid
Figure 7.
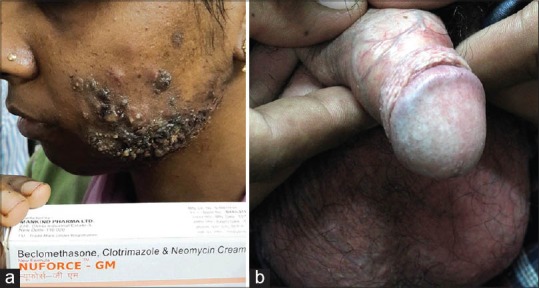
(a) Nodular acne with overlying hyperpigmented keratotoc papules (b) Telangectasia with hypopigmentation over genitals
Figure 8.
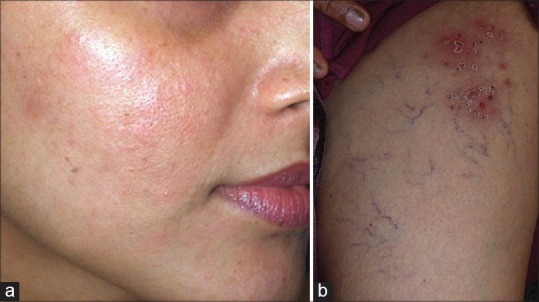
(a) Facial erythema Courtesy Dr. Kaushik Lahiri, (b) Telangiectasia with bacterial infection
Figure 9.
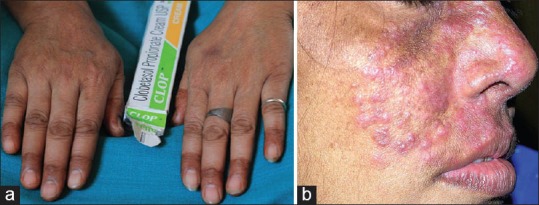
(a) Thinning of skin on dorsal aspect of hands with visibility a vessels, (b) Tinea incognito
Figure 10.
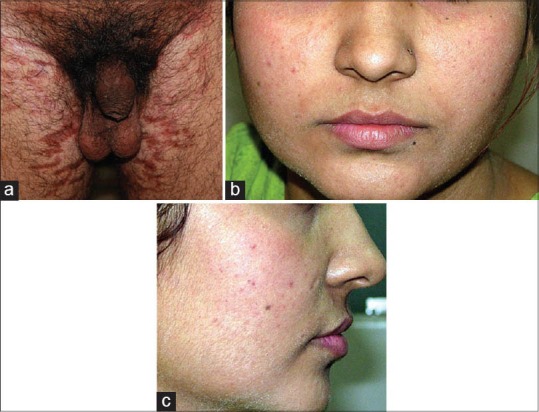
(a) Striae rubra with associated hypopigmentation (b) Perioral dermatitis with background erythema and mild acne (c) Perioral dermatitis with background erythema and mild acne
Figure 11.
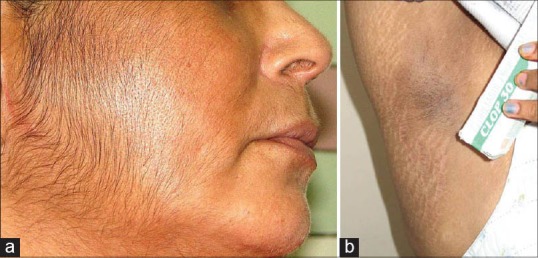
(a) Facial hypertrichosis (b) Striae alba
Figure 12.
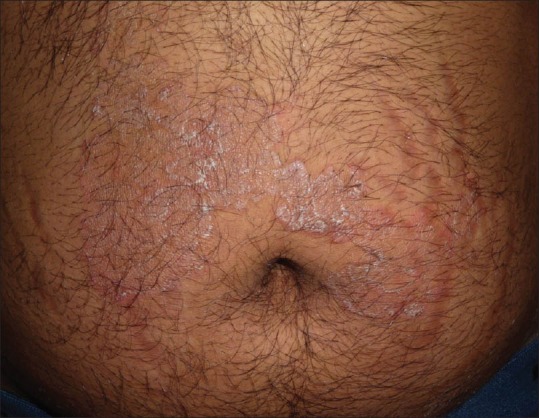
Striae secondary to steroid application for tinea corporis
Figure 13.
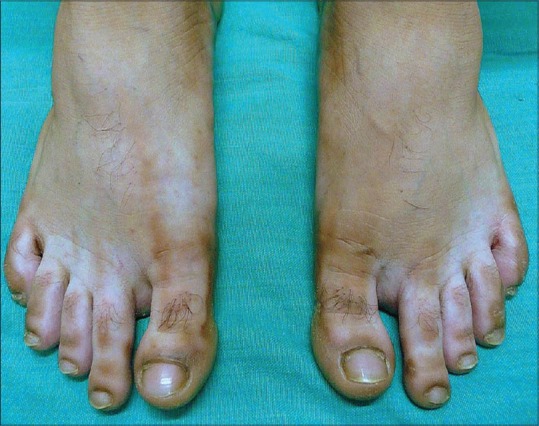
Diffuse Hypopigmentation
Figure 14.
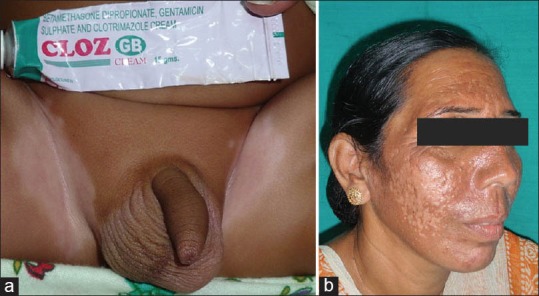
(a) Bilateral hypopigmentation (b) Facial patchy hypopigmentation
Figure 15.
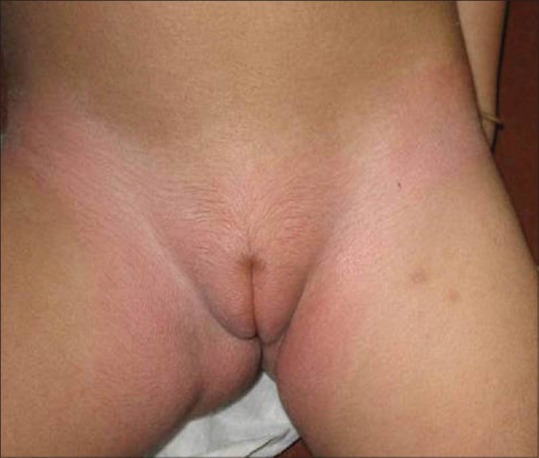
Bilateral mild hypopigmentation with erythema and labial hypertrichosis
Figure 16.
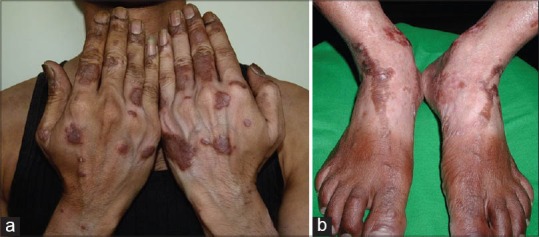
(a) Perilesional diffuse hypopigmentation Courtesy Dr. Shyam Verma, (b) Perilesional diffuse hypopigmentation Courtesy Dr. Shyam Verma
Figure 17.
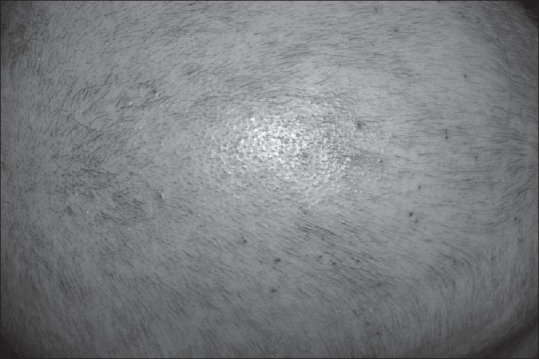
Diffuse scalp hypopigmentation
ATROPHY-THE COMMONEST SIDE EFFECT
Skin atrophy is the commonest side effect, reported to be caused by all topical TS. Epstein et al. first reported it from use of topical triamcinolone acetonide.[8] Atrophic changes can affect both epidermis and dermis. Microscopic degenerative changes in epidermis are evident following 3-14 days of treatment. Initially epidermis becomes thin due to reduction in epidermal cell size, which reflects a decreased metabolic activity. After prolonged exposure there is a reduction in cell layers, that is, stratum granulosum disappears and stratum corneum becomes thin. Synthesis of stratum corneum lipids and keratohyalin granules and formation of corneodesmosomes (required for structural integrity of stratum corneum) are suppressed. Inhibition of the function of melanocytes may occur, giving rise to localized hypopigmentation.[4,5]
Topical steroids induce resorption of mucopolysaccharide ground substance in the dermis. Repeated use in the same area causes epidermal thinning and changes in connective tissue of dermis leading to lax, transparent, wrinkled and shiny skin along with striae, fragility, hypopigmentation and prominence of underlying veins. The loss of connective tissue support for dermal vasculature results in erythema, telangiectasia and purpura.[4]
Degree of skin atrophy is influenced by age, body site, potency and presence of occlusion. It is caused by suppressive action on cell proliferation and inhibition of collagen synthesis. Dermal atrophy is caused by decreased fibroblast growth and reduced synthesis of collagen, acid mucopolysaccharides and stimulation of human dermal microvascular endothelial cells. Intertriginous areas are particularly susceptible due to thinner skin, increased moisture, elevated temperature and partial occlusion provided by the skin in these sites.[8] The atrophy is reversible on stoppage of TS, but the normalization may take months.[11]
Striae
Striae due to TS use need to be distinguished from those that occur due to excessive weight gain and pregnancy.[8]
Contact allergy
Contact hypersensitivity to TS may cause persistence or worsening of skin diseases. It is rare, but its risk increases with prolonged exposure. Nonfluorinated TS (e.g, hydrocortisone hydrocortisone-17-butyrate, and budesonide) result in a higher prevalence of contact allergy in comparison with fluorinated compounds. Binding to the amino acid arginine is probably required for development of contact allergy.[8]
Contact sensitivity can develop not only to constituents (e.g. lanolin, preservatives such as parabens and antibiotics) of the preparation, but also to the steroid molecule. Sensitivity to more than one TS is common. Risk factors for development of contact sensitivity include history of multiple patch test positivity to non-TS allergen, treatment resistant eczema, leg ulcers, stasis dermatitis, perineal dermatitis and chronic actinic dermatitis. It presents as chronic dermatitis not responding to locally applied steroids or rarely, as acute eczema, urticaria, acute local edema, immediate-type reaction, or id eruption like spread over the body.[4,5,6]
Infections
Mucocutaneous infections (tinea versicolor, onychomycosis due to Trichophyton and Candida species, dermatophytosis) are common during treatment with TS, occurring early in the therapy. The incidence varies between 16% and 43%. When dermatophyte infections are treated with TS, the symptoms and signs improve transiently, giving rise to tinea incognito. TS suppress the normal cutaneous immune response to dermatophytes leading to enchantment of fungal infections. An immune mediated phenomenon called “tinea pseudoimbricata” is a particular type of tinea incognito which has been described by one of the authors.[12] Pruritus in scabies improves by TS but infestation persists unless scabicidal treatment is given. Granuloma gluteale infantum a persistent reddish-purple, granulomatous, papulonodular eruption seen on buttocks, thighs or inguinal fold in children, is a well-known consequence of diaper dermatitis being treated with TS, caused by impairment of immune response to Candida by TS.[4]
Similar effects on mitigation or prolongation of herpes simplex, molluscum contagiosum and scabies infection have also been reported; hence TS should not be used in presence of these infections. TS also facilitate proliferation of Propionibacterium acnes and Demodex folliculoroum leading to acne-rosacea like condition. Reactivation of Kaposi sarcoma has also been reported.[8,9]
Acneiform eruptions
Systemic corticosteroid therapy, in some cases intravenous or inhaled TS are known to induce acneiform lesions. The eruption consists of small and uniformly sized (monomorphic) inflammatory papules and pustules with few or no comedones, located predominantly on trunk and extremities, with less involvement of the face. In the case of inhaled steroids, lesions occur in and around nose or mouth. Anti-inflammatory effects of TS may initially suppress inflammatory lesions and erythema, but flare-ups occur on stopping TS. The eruption subsequently resolves after discontinuation of the TS.
Topical steroids induce comedone formation by rendering follicular epithelium more responsive to comedogenesis. They also lead to increased concentration of free fatty acids in skin surface lipids and increased numbers of bacteria in the pilosebaceous duct. Free fatty acids, formed in pilosebaceous ducts by breakdown of triglycerides in the sebaceous secretion, may contribute to comedogenesis.[13,14]
Rosacea
Topical steroids induced rosacea is seen in middle-aged woman, presenting with papules and pustules. These are initially controlled with low potency TS, but lesions may reappear and require continued use of higher potency TS.[8]
Perioral dermatitis
Perioral dermatitis occurs in females on the face and is caused by long term use of potent TS on face. It presents as follicular papules and pustules on an erythematous base seen in a perioral distribution, with sparing of skin adjacent to the vermilion border. It is also seen in men and children.[8]
Hypertrichosis
Steroids promote vellus hair growth by unknown mechanism. Local and disseminated hypertrichosis due to TS is rare, seen commonly with systemic steroids. Even months after withdrawal of TS the darker hairs may persist.[8]
Hyper/hypopigmentation
Hypopigmentation after topical use is quite common, but not noticed frequently in very light skinned individuals. People with Type IV to VI are particularly affected. TS probably interfere with the melanin synthesis by smaller melanocytes, causing patchy areas of hypopigmentation which are reversible after discontinuation of steroids. Hyperpigmentation after intralesional steroids has been well-documented.[8]
Purpura, stellate pseudoscars, and ulcerations
These develop after severe steroid induced dermal atrophy and loss of intercellular substance, causing blood vessels to lose their dermal matrix support. The resulting fragility of dermal vessels leads to purpuric, irregularly shaped, hypopigmented, depressed pseudoscars over extremities. Continued misuse of TS can also lead to ulceration.[8]
Tachyphylaxis
Tachyphylaxis is characterized by decreasing efficacy of TS during continued treatment. It occurs commonly in psoriasis patients.[9] It may reflect patient noncompliance, normal variance in disease severity unrelated to therapy, or inability of TS to completely clear the disease. Withdrawal of TS is followed by a disease flare. As the tissue becomes less sensitive (tachyphylaxis), increasingly potent preparations are required to achieve comparable effects, leading to more severe side effects.[8] Tachyphylaxis can be quantified by vasoconstrictor assay and inhibition of fibroblast proliferation.[4,5]
Rebound phenomenon
Withdrawal of potent TS applied to the extensive area of psoriasis for a prolonged period may result in a relapse or a papulopustular flare and may even precipitate unstable or severe generalized pustular psoriasis. This is especially likely when steroids are used in large quantities or applied under occlusion. vascular effect of TS is vasoconstriction of superficial small vessels, followed by rebound vasodilatation which may become fixed after prolonged treatment and may be more conspicuous, as a result, of dermal and epidermal atrophy. Similarly, abrupt withdrawal can cause eczema flares.[4,5]
Steroid addiction
Steroid addiction is known to occur after inadvertent application of potent TS usually on the face.[8] Patients with steroid addiction have acne, rosacea, perioral dermatitis, or telangiectasia and continue its use, fearing that there may be flare of their condition on steroid withdrawal. Three phases have been described: (1) Initial treatment improves pustulation, pruritus, erythema and scaling; (2) with continued use, local immunosuppression increases microbial growth and (3) on treatment withdrawal, rebound flares of itching, redness, postulation and scaling are seen.[5] “Red burning skin syndrome” may be the presentation in some cases.[8]
TOPICAL STEROID-DEPENDENT FACE
Misuse of TS on the face is seen all over India and its incidence appears to be increasing rapidly. TSDF has also been described under various names like steroid addiction, dermatitis rosaceaformis steroidica and red face syndrome. In this condition after long term application of TS on the face, there is severe rebound erythema, burning and scaling on the face on attempting to stop the application of TS.[2]
Ocular side effects
Side effects caused by steroid eye drops are listed in Table 1. But there are few reports of such ocular complications due to TS.[5,6] Use of TS around eyes can rarely lead to glaucoma as penetration of TS is 300 times greater through the eyelid as compared to other body sites. Blindness due to glaucoma following extended TS use on the face is reported.[8]
Effect on wound healing
Topical steroids have demonstrated to impair wound healing and re-epithelialization in animal and human models.[5] The effects are on keratinocytes (epidermal atrophy, delayed reepithelialization), fibroblasts (reduced collagen and ground substance), vascular connective tissue support and angiogenesis (delayed granulation tissue formation).[8]
Alterations in skin elasticity and mechanical properties
Topical steroids are known to decrease skin elasticity. This can be assessed by pulling the skin and observing incomplete retraction on mechanical stress cessation.[8]
Influence of sun and aging
Skin aging pathophysiology is similar to the one that follows TS application. Marked skin thickness decrease, especially in light-exposed areas and delayed skin recovery are reported.[8]
Epidermal barrier disturbance
Topical steroids application can lead to subtle changes in the epidermal barrier as observed by decreased formation of lipid lamellar bodies and delayed barrier recovery. This effect, theoretically may worsen barrier impairment in atopic dermatitis and psoriasis, but it seems to be outweighed by reducing inflammation and permitting barrier repair.[8]
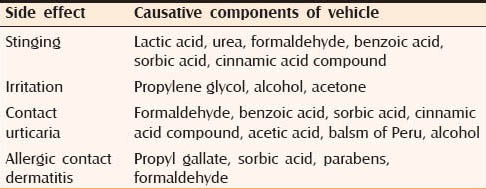
Vehicle related effects
Vehicle of TS can potentiate the side effects of TS and cause local side effects of its own. Table 3 lists the components of the vehicle that cause side effect.[6]
Table 3.
Components of vehicles of topical steroids that cause side effect
EFFECTS OF TOPICAL STEROIDS IN PEDIATRIC PATIENTs
British National Formulary states that skin of children is sensitive so they are likely to be susceptible to side effects of TS, hence they should be avoided in children or, if necessary, used with care and for short periods. Food and Drug Administrations center for drug evaluations and research have reported TS side effects similar to those seen in adults. Some side effects not reported in adults but seen in children include local irritation, mood change, gynecomastia, genital hypertrichosis and staphylococcal infection.
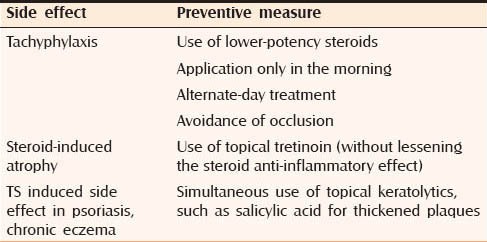
PREVENTION OF ADVERSE EFFECTS
Guidelines regarding TS use are available to prevent their misuse. General measures to prevent TS induced side effects are mentioned in Table 4 while Table 5 mentions measures to prevent site specific side effects.[8]
Table 4.
General measures to prevent topical steroid induced side effects

Table 5.
Measures to prevent side specific side effects
CONCLUSION
Topical corticosteroids misuse occurs at various levels. The pharmaceuticals manufacture and promote unethical combinations (e.g. “modified” Kligman's regime). Of further concern is the unrestricted over the counter (OTC) sale of TCs of all potencies though many of them are Schedule H drugs. The official IMS Health figures for sale of TCs in 2013 stands at a staggering annual figure of Rs. 1400 crore accounting for 82% among sale of all topical dermatological molecules.[6] This may only be the tip of the iceberg. Since a large number of TCs are sold as OTC product, the actual sales figure might be much more.[15] However, the main onus to curb this menace is on Government of India with its laxity in formulation, interpretation and implementation of laws regarding TC manufacture and sales, particularly the rampant OTC sale.[15] TC misuse benefits pharmaceutical industry but the ultimate victims are the unaware populace. The onus of countering this menace behoves on Indian dermatologists. A continuing chain of intensive awareness campaigns on their part with relentless interaction with the policy makers may halt the relentless progress of TC abuse.[15]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sulzberger MB, Witten VH. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol. 1952;19:101–2. doi: 10.1038/jid.1952.72. [DOI] [PubMed] [Google Scholar]
- 2.Saraswat A, Lahiri K, Chatterjee M, Barua S, Coondoo A, Mittal A, et al. Topical corticosteroid abuse on the face: A prospective, multicenter study of dermatology outpatients. Indian J Dermatol Venereol Leprol. 2011;77:160–6. doi: 10.4103/0378-6323.77455. [DOI] [PubMed] [Google Scholar]
- 3.Coondoo A, Chattopadhyay C. Use and abuse of topical corticosteroids in children. Indian J Paediatr Dermatol. 2014;15:1–4. [Google Scholar]
- 4.Berth-Jones J. Topical therapy. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rooks Textbook of Dermatology. 8th ed. 73. Vol. 1. UK: Blackwell Science Ltd; 2010. pp. 1–73. [Google Scholar]
- 5.Srinivas CR, Lakshmi C. Principles of topical therapy in dermatology. In: Valia RG, Valia AR, editors. IADVL Textbook of Dermatology. 3rd ed. Vol. 2. Mumbai, India: Bhalani Publishing House; 2008. pp. 1591–618. [Google Scholar]
- 6.Wolverton SE. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia USA: Saunders Elsevier; 2007. Topical Corticosteroids; pp. 595–624. [Google Scholar]
- 7.Hsu SP. Formulary. In: Arndt KA, Hsu JT, editors. Manual of Dermatologic Therapeutics. 7th ed. USA: Lippincott Williams and Wilkins; 2007. pp. 273–359. [Google Scholar]
- 8.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Fisher DA. Adverse effects of topical corticosteroid use. West J Med. 1995;162:123–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Liang J, McElroy K. Hypopigmentation after triamcinolone injection for de Quervain tenosynovitis. Am J Phys Med Rehabil. 2013;92:639. doi: 10.1097/PHM.0b013e318269ebdc. [DOI] [PubMed] [Google Scholar]
- 11.Dermatology. [Last accessed on 2014 May 27]. Available from: http://www.about.com/cs/medications/a/steroideffects.htm .
- 12.Verma SB, Hay R. Topical steroid induced tinea pseudoimbricata: A striking form of tinea incognito. Int J Dermatol. 2014 doi: 10.1111/ijd.12734. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 13.Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97–100. [PubMed] [Google Scholar]
- 14.Momin S, Peterson A, Del Rosso JQ. Drug-induced acneform eruptions: Definitions and causes. Cosmet Dermatol. 2009;22:28–37. [Google Scholar]
- 15.Verma SB. Sales, status, prescriptions and regulatory problems with topical steroids in India. Indian J Dermatol Venereol Leprol. 2014;80:201–3. doi: 10.4103/0378-6323.132246. [DOI] [PubMed] [Google Scholar]


