Abstract
Melasma is an acquired pigmentary disorder characterized by symmetrical hyperpigmented macules on the face. Its pathogenesis is complex and involves the interplay of various factors such as genetic predisposition, ultraviolet radiation, hormonal factors, and drugs. An insight into the pathogenesis is important to devise treatment modalities that accurately target the disease process and prevent relapses. Hydroquinone remains the gold standard of treatment though many newer drugs, especially plant extracts, have been developed in the last few years. In this article, we review the pathogenetic factors involved in melasma. We also describe the newer treatment options available and their efficacy. We carried out a PubMed search using the following terms “melasma, pathogenesis, etiology, diagnosis, treatment” and have included data of the last few years.
Keywords: Etiology, hydroquinone, lasers, melasma, pathogenesis, peeling, treatment
INTRODUCTION
Melasma is a common pigmentary disorder that manifests as symmetric hyperpigmented macules and patches on the face. It typically affects women of reproductive age with Fitzpatrick skin type IV-VI, though the condition can occur in men also. Genetic predisposition, ultraviolet (UV) radiation exposure, hormonal factors such as female sex hormones and thyroid disease, pregnancy and drugs like phenytoin are the known risk factors.
In recent times, there have been studies throwing light on other factors that could be involved in the pathogenesis of melasma. These include various vascular growth factors, genetic factors, and the role of H19, inducible nitric oxide synthase (iNOS), and Wnt pathway modulator genes. Identifying these factors could facilitate the development of newer treatment options for melasma.
Hydroquinone (HQ) and triple combination creams (TCCs) remain the gold standard of treatment. There have been concerns about the side effects and long term safety of HQ; hence the need to develop alternate treatment options. Current treatment modalities include kojic acid, azelaic acid, arbutin, ascorbic acid, chemical peels and lasers. Newer formulations that are being tried include tranexamic acid (TA), rucinol (4-n-butylresorcinol), oligopeptides silymarin and orchid extracts. Various botanical extracts that have been tried in melasma are grape seed extract, pycnogenol, aloesin, green tea extracts, coffee berry, soy, and licorice extract.
EPIDEMIOLOGY
The prevalence of melasma varies between 1.5% and 33.3% depending on the population.[1,2] Its prevalence in pregnancy is around 50-70%.[3,4] Melasma can also occur in men, though less common. Sarkar et al. conducted an etiological and histological study in Indian males with melasma and found that men represent 20.5-25.83% of the cases.[5,6] In men, the malar pattern is more common than the centrofacial and mandibular patterns [Figures 1 and 2].[5,6,7]
Figure 1.
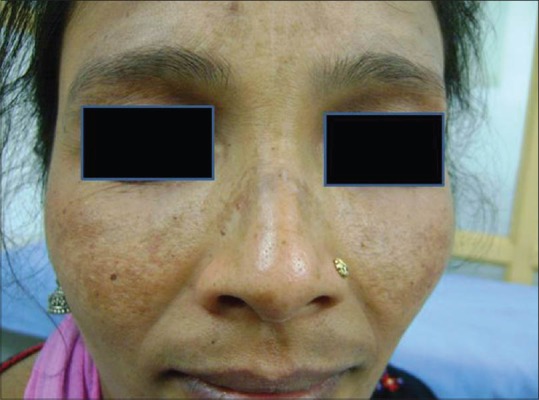
Centrofacial melasma
Figure 2.
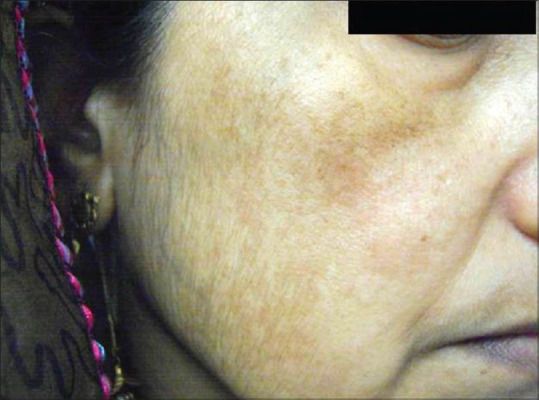
Malar melasma
A study conducted in male patients with melasma has shown that the levels of testosterone were low indicating a role of subtle testicular resistance in the pathogenesis of melasma.[8] Other etiological factors that have been described in men with melasma include the use of vegetable oil especially mustard oil on the face[5,7] and diethylstilboestrol therapy for prostate cancer.[8]
ETIOLOGY
Several factors have been implicated in the etiology of melasma. These are genetic predisposition, UV radiation, thyroid disease, pregnancy, oral contraceptive pills (OCPs) and drugs such as phenytoin.[9,10,11,12]
Hormonal influences
Various studies evaluating the hormonal profile in patients with melasma have found significantly increased levels of luteinizing hormone and lower levels of serum estradiol suggesting subclinical evidence of a mild ovarian dysfunction. Significant association has been reported between thyroid autoimmunity and melasma, mainly in women whose condition developed during pregnancy, or after ingestion of OCPs.
Ortonne et al. carried out a large survey in 12 centers of nine countries involving 324 patients to study the impact of UV radiation and hormonal influences in the development of melasma.[13] They found that only 20% of patients developed melasma in the peripregnancy period. The risk of onset during pregnancy was more with greater outdoor activity. An interesting finding in this study was the weak impact of OCPs on the evolution of melasma, especially in patients with a family history of melasma, supporting the view that hormonal contraceptives need not be changed in such patients.
Drugs
Pigmentation resembling chloasma develops in 10% of patients receiving phenytoin. The drug exerts direct action on melanocytes causing dispersion of melanin granules and also induces increased pigmentation in the basal epidermis. Pigmentation disappears in a few months after withdrawal of the drug.
Role of visible light
Mahmoud et al. studied the impact of long-wavelength ultraviolet A (UVA) and visible light on melanocompetent skin.[14] They found that both UVA and visible light were able to increase pigmentation especially in patients with dark skin (skin type IV-VI). Furthermore, pigmentation was more intense and stable after visible light compared to UVA. The study shows that visible light can also induce skin hyperpigmentation, emphasizing the need to use physical sunscreens to prevent melasma relapses.
Pathogenesis
Studies have confirmed that both melanocytosis (increased number of melanocytes) as well as increased melanogenesis (increased production of melanin) are responsible for the hyperpigmentation in melasma. Kim et al. carried out a histological study to evaluate the vascular characteristics of melasma.[15] They found that vascular endothelial growth factor was increased in lesional skin in melasma signifying increased vascularization. This has clinical implications and indicates the need to devise treatment options to target the vascular component in melasma. In a prospective comparative split-face randomized study carried out by Passeron et al., it was found that combination of TCC and pulsed dye laser (PDL) was more effective than TCC alone.[16] The combination treatment also prevented the relapses.
Kang et al. performed a transcriptional analysis in melasma skin samples and found that 279 genes were stimulated and 152 were found to be downregulated. Many melanin biosynthesis related genes as well as melanocyte markers such as TYR, MITF, SILV, and TYRP1 were found to be upregulated in melasma skin.[17]
Several other genes involved in other biological pathways were found to be affected. These include Wnt pathway modulation genes, genes involved in prostaglandin synthesis and fatty acid metabolism.[17]
Another interesting finding was the role of H19 gene in melasma pathogenesis as studied by Kim et al.[18] H19 gene transcribes a noncoding ribonucleic acid (RNA) and is found to be downregulated in melasma lesions. This induces stimulation of melanogenesis and increased transfer of melanin from melanocytes to keratinocytes.
iNOS and nuclear factor-kappa B pathways have also been found to be implicated in melasma pathogenesis. In a study by Jo et al., iNOS expression was found to be increased in melasma lesions.[19]
The most affected biological process in melasma is lipid metabolism.[17] Lipid metabolism genes, such as peroxisome proliferator-activated receptor alpha (PPAR), arachidonate 15- lipoxygenase, PPAR gamma coactivator 1 alpha, type B (ALXO 15B), diacylglycerol o-acyltransferase 2-like 3 were found to be downregulated. This downregulation is caused by chronic UV exposure.[20] Another change seen in melasma skin is thinning of the stratum corneum (SC) as found in skin biopsies that show rete ridge flattening and epidermal thinning. SC thinning coupled with disturbed lipid synthesis is responsible for the impaired SC integrity and a delayed barrier recovery rate seen in melasma skin.[21]
In a study by Lee et al., melanin index, erythema index, and SC hydration were found to be significantly higher in lesional melasma skin as compared to perilesional normal skin. This proves that prominent vascularization accompanies hyperpigmentation.[21]
Prolonged UV exposure-induced dermal inflammation and fibroblast activation may upregulate stem cell factors in the melasma dermis, causing increased melanogenesis.
The above findings emphasize the need for devising treatment options targeting these pathogenetic factors in melasma.
Classification of melasma
Table 1 shows the traditional classification[22] being used for melasma. It is based on the depth of melanin pigment and helps in predicting the therapeutic outcome. However, Wood's light examination may not be accurate in determining the depth of pigment.[23,24] There is poor correlation between the classification based on Wood's light examination and histopathology [Figure 3].
Table 1.
Classification of melasma based on the depth of melanin pigment
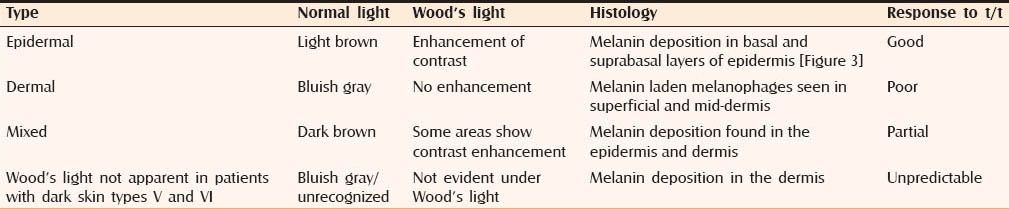
Figure 3.
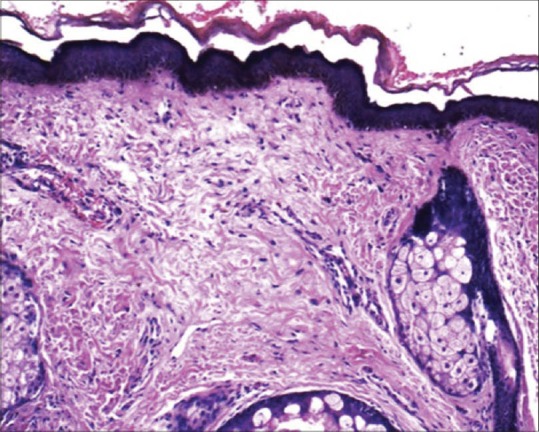
H and E, ×200 epidermis shows increased melanin concentration in basal keratinocytes and underlying solar elastosis along with dermal melanophages (Courtesy of Dr. Uday Khopkar, Mumbai, India)
Dermoscopy can be used for the diagnosis of melasma. Lesions of melasma show diffuse reticular pigmentation in various shades of brown with sparing of follicular openings. Figures 4–9 depict the dermoscopic appearance of a patient with epidermal melasma. Dermal melasma shows diffuse dark brown to grayish pseudoreticular pigmentation. Annular, honeycomb and arcuate structures may be seen.
Figure 4.
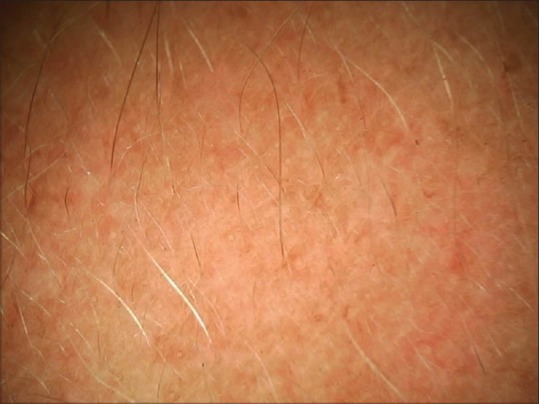
Dermoscopy from left cheek melasma showing dispersed brownish spots. White hairs are due to bleaching
Figure 9.
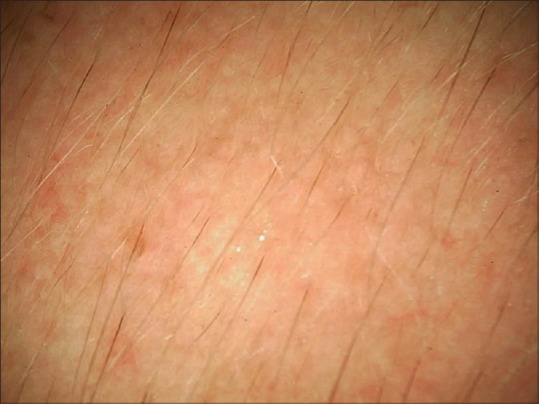
Dermoscopy from surrounding normal skin revealing mild steroid abuse-induced telangiectasias
Figure 5.
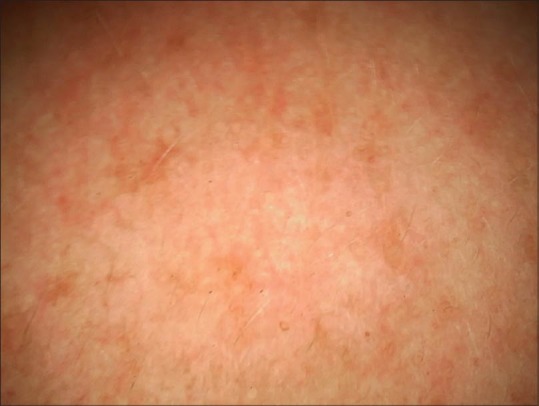
Dermoscopy from right cheek melasma showing dispersed brownish spots
Figure 6.
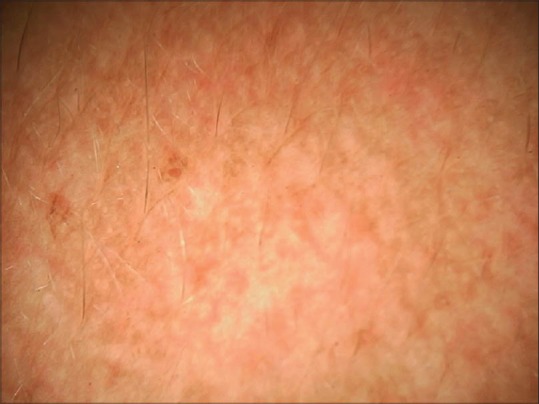
Dermoscopy from right cheek melasma showing dispersed pigment and depigmentation
Figure 7.
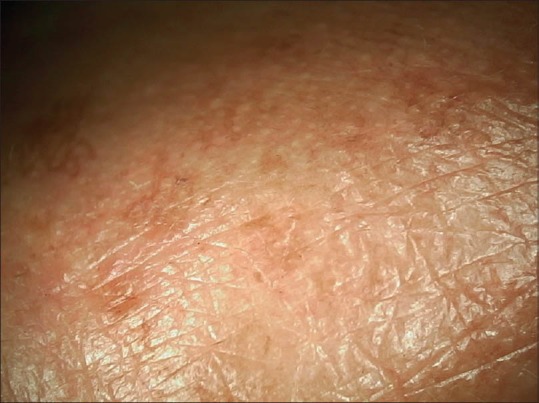
Dermoscopy from nasal melasma showing larger dispersed brownish spots
Figure 8.
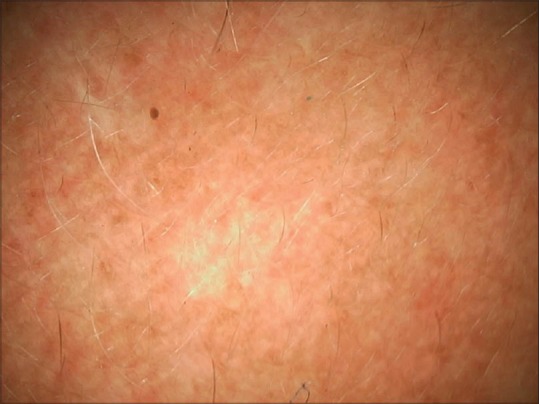
Dermoscopy from the surrounding skin revealing more steroid induced telangiectasias
Reflectance confocal microscopy (RCM) is a noninvasive tool for the evaluation of the skin up to the papillary dermis.[25] This technique can provide real-time en face images that have a resolution that matches that of histopathological examination. Kang et al. carried out a study to investigate the role of RCM in melasma and provide a set of morphological criteria with histological correlations.[26] These are depicted in Figures 10 and 11. These are shown in Table 2.
Figure 10.
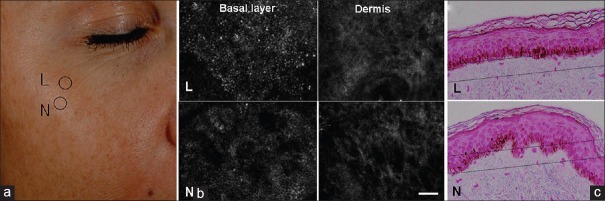
Confocal microscope images of melasma showing epidermal pigmentation: (a) Melasma on the cheek. L is for lesional skin and N is for normal perilesional skin. (b) Confocal images depict cobblestoning and loss of dermal papillary rings at the basal layer of the melasma lesion (L) compared to perlesional normal skin (N). Scale bar: 50 um. (c) Histopathology from same lesion showing greater epidermal hyperpigmentation and flattened rete ridges in lesion compared to perilesional normal skin Fontana-Masson staining, horizontal line indicates where reflectance confocal microscopy image is taken from (source acknowledged: Kang HY, Bahadoran P, Ortonne JP. Reflectance confocal microscopy for pigmentary disorders. Exp Dermatol 2010;19:233-9)
Figure 11.
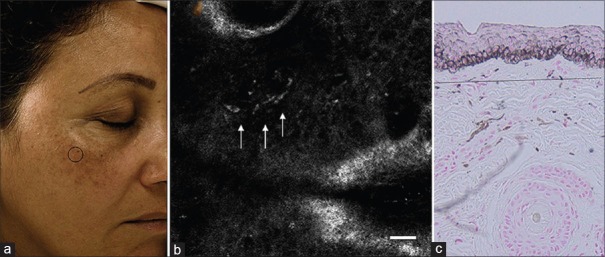
Confocal microscopy image of melasma showing dermal pigmentation: (a) Clinical picture of melasma. (b) Confocal microscopy showing bright plump cells in dermis. (c) Histopathology picture showing melanophages in the dermis (source acknowledged: Kang HY, Bahadoran P, Ortonne JP. Reflectance confocal microscopy for pigmentary disorders. Exp Dermatol 2010;19:233-9)
Table 2.
Morphological criteria along with histological correlation seen in patients with melasma using RCM
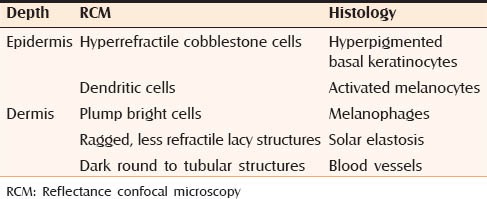
RCM showed significant solar elastosis and increase of blood vessels in the dermis. It was also seen that the topographic distribution of melanophages was heterogenous within and among different regions. RCM offers an innovative way of classifying melasma by pigment changes. This can help us in refining the prognosis of melasma. It can also help in monitoring response to therapy.
THERAPEUTIC MODALITIES
Current role of HQ
HQ is a dihydric phenol that inhibits the conversion of dopa to melanin by inhibiting the tyrosinase enzyme. Other proposed mechanisms of action include the inhibition of RNA and deoxyribonucleic acid synthesis and destruction of melanocytes.
HQ is considered the gold standard of treatment for melasma. It is used at a concentration of 2-4%. Its efficacy is enhanced when used in combination with other agents. These combinations include the Kligman formula (5% HQ, 0.1% tretinoin, and 0.1% dexamethasone), modified Kligman's formula (4% HQ, 0.05% tretinoin and 1% hydrocortisone acetate), Pathak's (2% HQ and 0.05-0.1% tretinoin) and Westerhof's formula (4.7% N-acetylcysteine, 2% HQ and 0.1% triamcinolone acetonide).[27] Combination of microencapsulated HQ 4% with retinol 0.15% and antioxidants has also been tried in melasma with studies showing improvement in disease severity, pigmentation intensity, lesion area and colorimetry assessments.[28]
In a randomized, split-face study, Monheit and Dreher compared the safety and efficacy of skin lightening cream (containing HQ 4% and four skin brightening actives) with a TCC (containing 4% HQ, 0.05% tretinoin, and 0.01% fluocinolone acetonide) in 20 Caucasian female patients with melasma.[29] They found that after 4 weeks once daily treatment, the skin lightening cream SLC was comparable in both efficacy and safety to the well-established TCC treatment for melasma. One-third of patients experienced local side effects in the form of erythema, peeling of skin and dryness with both the creams.
In a randomized, controlled study Arellano et al. compared the clinical efficacy and safety of two 6-month triple combination (containing HQ, fluocinolone acetonide and tretinoin) maintenance regimens in 242 Brazilian and Mexican patients with moderate-to-severe melasma.[30] Global severity score, melasma area severity index (MASI), and quality of life questionnaire were used to assess the efficacy of treatment. After eight weeks of daily TCC application, subjects were randomized to receive TCC in a twice weekly or tapering regimen (3/week [1st month], 2/week [2nd month], 1/week [4th month]) for 6 months. They found that twice-weekly regimen showed better effectiveness on postponing relapse in patients with severe melasma. About 53% of patients were relapse-free after 6 months.
Tranexamic acid
Tranexamic Acid (TA) is trans-4-(aminomethyl) cyclohexane carboxylic acid, a lysine analog that is in use as an antifibrinolytic agent for over 30 years. It inhibits UV-induced plasmin activity in keratinocytes by preventing the binding of plasminogen to the keratinocytes. This results in less free arachidonic acid and a diminished ability to produce prostaglandins, and this decreases melanocyte tyrosinase activity. Zhang et al. demonstrated that TA can interfere with the catalytic reaction of tyrosinase and inhibit melanogenesis.[31] In the treatment of melasma, TA can be used orally, topically or by intradermal microinjection. As a hemostatic, TA is prescribed in a dose of 1000 mg 3 times daily, whereas in the treatment of melasma, it is used in a dose of 250 mg twice daily.[32]
In a study conducted by Wu et al., 74 Chinese patients with melasma were treated with TA 250 mg twice daily for 6 months. The effects of treatment were evaluated by two physicians and by the patients based on the reduction in melasma size and improvement of pigmentation. The results were excellent in 10.8%, good in 54%, fair in 31.1% and poor in 4.1%. Side effects seen were gastrointestinal discomfort and hypomenorrhea. Recurrence was seen in 9.5% patients.[33]
TA can also be used topically. In a recent double-blind randomized prospective study by Kanechorn et al., 5% TA incorporated in a liposome gel formulation was used in 23 Asian women with bilateral epidermal melasma. The treatment was given for 12 weeks and compared with the vehicle in a split-face trial.[34] 78.2% of patients showed a decrease in the melanin index. However, results were not significant as compared with vehicle. Another finding seen in this study was the erythema induced by topical TA.
In a study conducted by Lee et al. in 100 Korean women with melasma, TA was used intradermally (4 mg/ml) every week for 12 weeks.[35] The treatment caused a significant decrease in MASI score (13.22 vs. 9.02 at week 8 and vs. 7.57 at week 12, P < 0.05 for both) with minimal side effects.
The advantage with TA is its good safety profile. Furthermore, it is temperature stable, UV insensitive and does not get oxidized easily, hence it can be used in SLCs. More studies are needed to evaluate its anti-melasma potential.
4-n-Butylresorcinol
It is a derivative of resorcinol that inhibits tyrosinase and tyrosinase related protein, enzymes in the melanin biosynthetic pathway.[36] Studies have shown good efficacy and safety with 4-n-butylresorcinol in patients with melasma.[37]
Huh et al. conducted a randomized controlled split-face trial in 23 patients with melasma to evaluate the efficacy and safety of liposome-encapsulated 4-n-butylresorcinol 0.1% cream. They found a statistically significant reduction (P < 0.043) in the melanin index on the treatment side.[38]
Oligopeptides
Oligopeptides have emerged as a new class of tyrosinase inhibitors with a good efficacy and cytotoxicity profile. In a study carried out by Ubeid et al., the inhibitory activity of octapeptides and HQ was assessed using mushroom and human tyrosinase and melanin content through human primary melanocytes.[39] It was seen that octapeptides P16-18 outperformed HQ in all categories tested. They showed minimal toxicity toward major human skin cell types.
Hantash and Jimenez carried out a split-face, double-blind, randomized and placebo controlled pilot study to evaluate the efficacy of an emulsion containing 0.01% decapeptide-12 (Lumixyl cream) in five patients with Fitzpatrick phototype IV and moderate, recalcitrant melasma.[40] The preparation was used twice daily for 16 weeks. Ten- and five-point grading scales were used to assess the improvement in melasma and volunteer satisfaction. All patients showed statistically significant improvement with minimal side effects. The same authors evaluated the efficacy of the Lumixyl Topical Brightening system (0.01% oligopeptide cream, an antioxidant cleanser, 20% glycolic acid [GA] lotion and physical sunscreen) in patients with mild to moderate melasma and found improvement in all patients with no side effects.[41]
Silymarin
Silymarin, is derived from the of milk thistle plant silybum marianum. It is a polypeptide flavonoid whose main component is silybin (silibinin) that has been found to have antioxidant properties. It reduces the harmful effects of UV radiation and also inhibits melanin production in a dose-dependent manner.
In a study performed by Altaei, 96 adults with melasma were divided into three groups receiving: (1) silymarin cream 0.1%, (2) silymarin cream 0.2%, and (3) placebo.[42] The treatment was applied twice daily for 4 weeks. Response was assessed using lesion size, MASI score, physician global assessment and subjective assessment. All patients treated with silymarin cream showed excellent improvement and lesion size reduction from the first week. No adverse effects were seen.
Orchid extracts
Orchid extracts contain various flavonoids that act as antioxidant. Tadokoro et al. performed a study to assess the efficacy of a cosmetic formulation containing plant extracts including orchid extracts, compared to topical 3% vitamin C derivative in Japanese female patients with melasma.[43] The efficacy was evaluated by colorimetric measurements and subjectively using a questionnaire. They found that after eight weeks of treatment, the plant extract preparation was significantly effective in improving the pigmentation and lesion size and was similar in efficacy to vitamin C derivative.
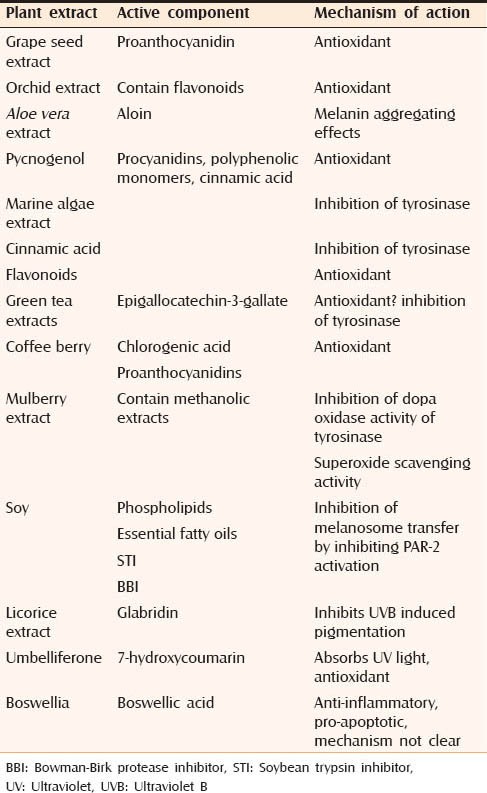
Table 3 highlights the botanical/plant extracts that are being used for the treatment of melasma.[44]
Table 3.
Botanical extracts being used for the treatment of melasma
NEWER CHEMICAL PEELS
A number of new peeling agents are being developed for the treatment of melasma out of which the following deserve mention.
Tretinoin peel
Tretinoin is widely used for the treatment of melasma as an over-the-counter lightening agent. Considering its efficacy, peeling with tretinoin may improve pigmentation in melasma. Faghihi et al. performed a randomized, double-blinded clinical trial to compare the efficacy of 1% tretinoin peel versus 70% GA peel in 63 female patients with bilateral melasma.[45] One side of the face was treated with 70% GA and the opposite side with 1% tretinoin peel for four sessions at two week intervals. It was found that the efficacy of 1% tretinoin peel was similar to 70% GA. Postprocedure discomfort as expressed by the patients was significantly lower with 1% tretinoin.
Tretinoin can also be used as a peeling mask. Ghersetich et al. evaluated the efficacy of 10% tretinoin peeling mask using standardized digital photos, mexameter measurement and MASI evaluation in 20 female patients with Fitzpatrick skin type II-VI.[46] They found moderate or marked improvement in all patients using these three parameters without any adverse events. At 10 weeks, the difference in the average MASI calculated at baseline and after 10 weeks was 2.9.
Obagi blue peel
It is composed of a fixed concentration of trichloroacetic acid (TCA) with a blue peel base. The latter contains glycerine, saponins and blue color base that is non-ionic. It ensures a slow and uniform penetration of TCA by reducing the surface tension of TCA, water and glycerine.
In a split-face comparative study, 18 Korean women with moderate-to-severe melasma were treated with a single session of 1550-nm fractional photothermolysis (FP) on one cheek, and with a 15% TCA peel on the other cheek. MASI score and subjective patient-rated overall improvement were used to assess treatment efficacy at 4 and 12 weeks. Both treatment groups showed significant improvement of melasma after 4 weeks of therapy, but recurrence was observed at 12 weeks in the TCA peel group.[47]
Another new peeling agent being used is the amino fruit acid peel. It is a potent antioxidant and also acts against photopigmentation. Ilknur et al. performed a single blind randomized right-left comparison study in patients with melasma using GA and amino fruit acid peels. Modified MASI score was performed at baseline and at 3 and 6 months. It decreased significantly in both groups; adverse effects were less with amino fruit acid peels.[48]
Lasers for melasma
Various lasers that have been used for melasma include the following:[49]
Green light: Flashlamp-pumped PDL (510 nm), frequency doubled Q switched neodymium: Yttrium aluminium garnet-532 nm (QS Nd: YAG)
Red light: Q switched ruby (694 nm), Q switched alexandrite (755 nm)
Near-infrared: QS Nd: YAG (1064 nm).
The concept of FP has been applied for the treatment of melasma as well. Fractional lasers have been used for the treatment of pigmented lesions, including melasma.
The overview of lasers being currently used for melasma is given in Table 4.
Table 4.
Overview of lasers being currently used in the treatment of melasma
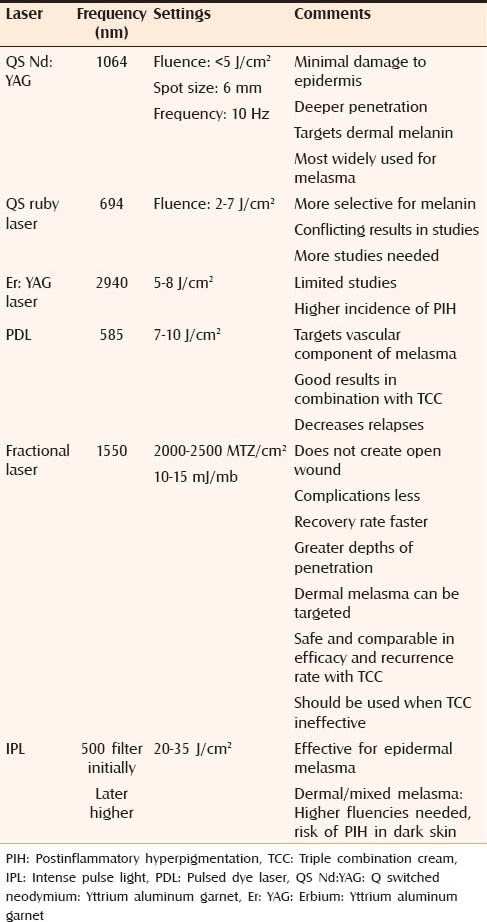
QS Nd:YAG is the most widely used laser for the treatment of melasma. The fluence used is less than 5 J/cm2, spot size 6 mm, and frequency of 10 Hz. The number of treatment sessions varies from 5 to 10 at 1-week intervals.
Nowadays, technique called “laser toning” or “laser facial” has become increasingly popular for the treatment of melasma. This involves the use of a large spot size (6-8 mm), low fluence (1.6-3.5 J/cm2), multiple passed QS 1064 nm Nd: YAG laser performed every 1-2 weeks for several weeks.[50] Although good efficacy has been seen with this technique, side effects have also been reported. These include hypopigmentation, depigmentation, rebound hyperpigmentation, physical urticaria, acneiform eruption, petechiae, and herpes simplex reactivation.
Combination of ablative and pigment selective lasers has also been tried in melasma. Ablative lasers remove the epidermis containing excess melanin; this is followed by the use of Q switched pigment selective laser that can target the dermal melanophages.
Angsuwarangsee and Polnikorn studied the efficacy of combined ultrapulse CO2 laser and Q switched alexandrite laser (QSAL) alone in six patients with melasma. The site that received combination treatment showed significant response compared with the site that was treated with QSAL alone.[51] However, side effects in the form of contact dermatitis and hyperpigmentation were observed in few patients, especially in those with dark skin. Hence, the use of combination of lasers should only be used for refractory melasma.
CONCLUSION
Melasma is a complex disorder and various factors are involved in its pathogenesis, identification of which will help us in developing better treatment options with more efficacy, less side effects and longer periods of remission. Newer compounds, especially botanical extracts and device-based treatments are being developed and add to the list of options available for treatment. However, more randomized controlled trials are needed to evaluate their efficacy compared to the well-known treatments available. There is also need to define the role of combination therapy and design protocols to provide optimum results and prevent relapses.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pichardo R, Vallejos Q, Feldman SR, Schulz MR, Verma A, Quandt SA, et al. The prevalence of melasma and its association with quality of life in adult male Latino migrant workers. Int J Dermatol. 2009;48:22–6. doi: 10.1111/j.1365-4632.2009.03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werlinger KD, Guevara IL, González CM, Rincón ET, Caetano R, Haley RW, et al. Prevalence of self-diagnosed melasma among premenopausal Latino women in Dallas and Fort Worth, Tex. Arch Dermatol. 2007;143:424–5. doi: 10.1001/archderm.143.3.424. [DOI] [PubMed] [Google Scholar]
- 3.Rathore SP, Gupta S, Gupta V. Pattern and prevalence of physiological cutaneous changes in pregnancy: A study of 2000 antenatal women. Indian J Dermatol Venereol Leprol. 2011;77:402. doi: 10.4103/0378-6323.79741. [DOI] [PubMed] [Google Scholar]
- 4.Wong RC, Ellis CN. Physiologic skin changes in pregnancy. J Am Acad Dermatol. 1984;10:929–40. doi: 10.1016/s0190-9622(84)80305-9. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar R, Jain RK, Puri P. Melasma in Indian males. Dermatol Surg. 2003;29:204. [PubMed] [Google Scholar]
- 6.Sarkar R, Puri P, Jain RK, Singh A, Desai A. Melasma in men: A clinical, aetiological and histological study. J Eur Acad Dermatol Venereol. 2010;24:768–72. doi: 10.1111/j.1468-3083.2009.03524.x. [DOI] [PubMed] [Google Scholar]
- 7.Vázquez M, Maldonado H, Benmamán C, Sánchez JL. Melasma in men. A clinical and histologic study. Int J Dermatol. 1988;27:25–7. doi: 10.1111/j.1365-4362.1988.tb02329.x. [DOI] [PubMed] [Google Scholar]
- 8.Sialy R, Hassan I, Kaur I, Dash RJ. Melasma in men: A hormonal profile. J Dermatol. 2000;27:64–5. doi: 10.1111/j.1346-8138.2000.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 9.Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601–5. [PubMed] [Google Scholar]
- 10.Lutfi RJ, Fridmanis M, Misiunas AL, Pafume O, Gonzalez EA, Villemur JA, et al. Association of melasma with thyroid autoimmunity and other thyroidal abnormalities and their relationship to the origin of the melasma. J Clin Endocrinol Metab. 1985;61:28–31. doi: 10.1210/jcem-61-1-28. [DOI] [PubMed] [Google Scholar]
- 11.Moin A, Jabery Z, Fallah N. Prevalence and awareness of melasma during pregnancy. Int J Dermatol. 2006;45:285–8. doi: 10.1111/j.1365-4632.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- 12.Grimes PE. Melasma. Etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453–7. doi: 10.1001/archderm.131.12.1453. [DOI] [PubMed] [Google Scholar]
- 13.Ortonne JP, Arellano I, Berneburg M, Cestari T, Chan H, Grimes P, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009;23:1254–62. doi: 10.1111/j.1468-3083.2009.03295.x. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092–7. doi: 10.1038/jid.2010.95. [DOI] [PubMed] [Google Scholar]
- 15.Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci. 2007;46:111–6. doi: 10.1016/j.jdermsci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Passeron T, Fontas E, Kang HY, Bahadoran P, Lacour JP, Ortonne JP. Melasma treatment with pulsed-dye laser and triple combination cream: A prospective, randomized, single-blind, split-face study. Arch Dermatol. 2011;147:1106–8. doi: 10.1001/archdermatol.2011.255. [DOI] [PubMed] [Google Scholar]
- 17.Kang HY, Suzuki I, Lee DJ, Ha J, Reiniche P, Aubert J, et al. Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Invest Dermatol. 2011;131:1692–700. doi: 10.1038/jid.2011.109. [DOI] [PubMed] [Google Scholar]
- 18.Kim NH, Lee CH, Lee AY. H19 RNA downregulation stimulated melanogenesis in melasma. Pigment Cell Melanoma Res. 2010;23:84–92. doi: 10.1111/j.1755-148X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 19.Jo HY, Kim CK, Suh IB, Ryu SW, Ha KS, Kwon YG, et al. Co-localization of inducible nitric oxide synthase and phosphorylated Akt in the lesional skins of patients with melasma. J Dermatol. 2009;36:10–6. doi: 10.1111/j.1346-8138.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: Histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146:228–37. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee DJ, Lee J, Ha J, Park KC, Ortonne JP, Kang HY. Defective barrier function in melasma skin. J Eur Acad Dermatol Venereol. 2012;26:1533–7. doi: 10.1111/j.1468-3083.2011.04337.x. [DOI] [PubMed] [Google Scholar]
- 22.Victor FC, Gelber J, Rao B. Melasma: A review. J Cutan Med Surg. 2004;8:97–102. doi: 10.1007/s10227-004-0158-9. [DOI] [PubMed] [Google Scholar]
- 23.Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96–101. doi: 10.1097/01.dad.0000154419.18653.2e. [DOI] [PubMed] [Google Scholar]
- 24.Sarvjot V, Sharma S, Mishra S, Singh A. Melasma: A clinicopathological study of 43 cases. Indian J Pathol Microbiol. 2009;52:357–9. doi: 10.4103/0377-4929.54993. [DOI] [PubMed] [Google Scholar]
- 25.Rito C, Pineiro-Maceira J. Reflectance confocal microscopy in the diagnosis of cutaneous melanoma. An Bras Dermatol. 2009;84:636–42. doi: 10.1590/s0365-05962009000600009. [DOI] [PubMed] [Google Scholar]
- 26.Kang HY, Bahadoran P, Suzuki I, Zugaj D, Khemis A, Passeron T, et al. In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp Dermatol. 2010;19:e228–33. doi: 10.1111/j.1600-0625.2009.01057.x. [DOI] [PubMed] [Google Scholar]
- 27.Cestari TF, Hexsel D, Viegas ML, Azulay L, Hassun K, Almeida AR, et al. Validation of a melasma quality of life questionnaire for Brazilian Portuguese language: The MelasQoL-BP study and improvement of QoL of melasma patients after triple combination therapy. Br J Dermatol. 2006;156(Suppl 1):13–20. doi: 10.1111/j.1365-2133.2006.07591.x. [DOI] [PubMed] [Google Scholar]
- 28.Cook-Bolden FE, Hamilton SF. An open-label study of the efficacy and tolerability of microencapsulated hydroquinone 4% and retinol 0.15% with antioxidants for the treatment of hyperpigmentation. Cutis. 2008;81:365–71. [PubMed] [Google Scholar]
- 29.Monheit GD, Dreher F. Comparison of a skin-lightening cream targeting melanogenesis on multiple levels to triple combination cream for melasma. J Drugs Dermatol. 2013;12:270–4. [PubMed] [Google Scholar]
- 30.Arellano I, Cestari T, Ocampo-Candiani J, Azulay-Abulafia L, Bezerra Trindade Neto P, Hexsel D, et al. Preventing melasma recurrence: Prescribing a maintenance regimen with an effective triple combination cream based on long-standing clinical severity. J Eur Acad Dermatol Venereol. 2012;26:611–8. doi: 10.1111/j.1468-3083.2011.04135.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Yang X, Yang H, Yang Y. Study of inhibitory effect of acidum tranexamicum on melanin synthesis. Chin J Dermatovenerol Integr Tradit West Med. 2003;2:227–9. [Google Scholar]
- 32.Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999;57:1005–32. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Shi H, Wu H, Yan S, Guo J, Sun Y, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964–70. doi: 10.1007/s00266-012-9899-9. [DOI] [PubMed] [Google Scholar]
- 34.Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, Nakakes A. Topical 5% tranexamic acid for the treatment of melasma in Asians: A double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150–4. doi: 10.3109/14764172.2012.685478. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Park JG, Lim SH, Kim JY, Ahn KY, Kim MY, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: A preliminary clinical trial. Dermatol Surg. 2006;32:626–31. doi: 10.1111/j.1524-4725.2006.32133.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim DS, Kim SY, Park SH, Choi YG, Kwon SB, Kim MK, et al. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol Pharm Bull. 2005;28:2216–9. doi: 10.1248/bpb.28.2216. [DOI] [PubMed] [Google Scholar]
- 37.Khemis A, Kaiafa A, Queille-Roussel C, Duteil L, Ortonne JP. Evaluation of efficacy and safety of rucinol serum in patients with melasma: A randomized controlled trial. Br J Dermatol. 2007;156:997–1004. doi: 10.1111/j.1365-2133.2007.07814.x. [DOI] [PubMed] [Google Scholar]
- 38.Huh SY, Shin JW, Na JI, Huh CH, Youn SW, Park KC. Efficacy and safety of liposome-encapsulated 4-n-butylresorcinol 0.1% cream for the treatment of melasma: A randomized controlled split-face trial. J Dermatol. 2010;37:311–5. doi: 10.1111/j.1346-8138.2010.00787.x. [DOI] [PubMed] [Google Scholar]
- 39.Ubeid AA, Do S, Nye C, Hantash BM. Potent low toxicity inhibition of human melanogenesis by novel indole-containing octapeptides. Biochim Biophys Acta. 2012;1820:1481–9. doi: 10.1016/j.bbagen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Hantash BM, Jimenez F. A split-face, double-blind, randomized and placebo-controlled pilot evaluation of a novel oligopeptide for the treatment of recalcitrant melasma. J Drugs Dermatol. 2009;8:732–5. [PubMed] [Google Scholar]
- 41.Hantash BM, Jimenez F. Treatment of mild to moderate facial melasma with the Lumixyl topical brightening system. J Drugs Dermatol. 2012;11:660–2. [PubMed] [Google Scholar]
- 42.Altaei T. The treatment of melasma by silymarin cream. BMC Dermatol. 2012;12:18. doi: 10.1186/1471-5945-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadokoro T, Bonté F, Archambault JC, Cauchard JH, Neveu M, Ozawa K, et al. Whitening efficacy of plant extracts including orchid extracts on Japanese female skin with melasma and lentigo senilis. J Dermatol. 2010;37:522–30. doi: 10.1111/j.1346-8138.2010.00897.x. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar R, Arora P, Garg KV. Cosmeceuticals for hyperpigmentation: What is available? J Cutan Aesthet Surg. 2013;6:4–11. doi: 10.4103/0974-2077.110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faghihi G, Shahingohar A, Siadat AH. Comparison between 1% tretinoin peeling versus 70% glycolic acid peeling in the treatment of female patients with melasma. J Drugs Dermatol. 2011;10:1439–42. [PubMed] [Google Scholar]
- 46.Ghersetich I, Troiano M, Brazzini B, Arunachalam M, Lotti T. Melasma: Treatment with 10% tretinoin peeling mask. J Cosmet Dermatol. 2010;9:117–21. doi: 10.1111/j.1473-2165.2010.00488.x. [DOI] [PubMed] [Google Scholar]
- 47.Hong SP, Han SS, Choi SJ, Kim MS, Won CH, Lee MW, et al. Split-face comparative study of 1550 nm fractional photothermolysis and trichloroacetic acid 15% chemical peeling for facial melasma in Asian skin. J Cosmet Laser Ther. 2012;14:81–6. doi: 10.3109/14764172.2012.655287. [DOI] [PubMed] [Google Scholar]
- 48.Ilknur T, Biçak MU, Demirtaşoğlu M, Ozkan S. Glycolic acid peels versus amino fruit acid peels in the treatment of melasma. Dermatol Surg. 2010;36:490–5. doi: 10.1111/j.1524-4725.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg DJ. Laser treatment of pigmented lesions. Dermatol Clin. 1997;15:397–407. doi: 10.1016/s0733-8635(05)70449-6. [DOI] [PubMed] [Google Scholar]
- 50.Chan NP, Ho SG, Shek SY, Yeung CK, Chan HH. A case series of facial depigmentation associated with low fluence Q-switched 1,064 nm Nd: YAG laser for skin rejuvenation and melasma. Lasers Surg Med. 2010;42:712–9. doi: 10.1002/lsm.20956. [DOI] [PubMed] [Google Scholar]
- 51.Angsuwarangsee S, Polnikorn N. Combined ultrapulse CO 2 laser and Q-switched alexandrite laser compared with Q-switched alexandrite laser alone for refractory melasma: Split-face design. Dermatol Surg. 2003;29:59–64. doi: 10.1046/j.1524-4725.2003.29009.x. [DOI] [PubMed] [Google Scholar]


