Abstract
Bone loss due to age and disuse contributes to osteoporosis and increases fracture risk. It has been hypothesized that such bone loss can be attenuated by modulation of the C-C chemokine receptor 2 (CCR2) and/or its ligands. The objectives of this study were to examine the effects of genetic elimination of CCR2 on cortical and trabecular bones in the mouse tibia and how bone loss was impacted following disuse and estrogen loss. Female CCR2 knockout (CCR2−/−) and wildtype mice underwent ovariectomy (OVX) or denervation of musculature adjacent to the tibia (DEN) to induce bone loss. Cortical and trabecular structural properties as well as mechanical properties (i.e., strength) of tibial bones were measured. Compared to wildtype mice, CCR2−/− mice had tibiae that were up to 9% larger and stronger; these differences could be explained mainly by the 17% greater body mass (p<0.001) of CCR2−/− mice. The majority of the tibia’s structural and functional responses to OVX and DEN were similar regardless of the lack or presence of CCR2, indicating that CCR2 is not protective against bone loss per se. These findings indicate that while CCR2−/− mice do have larger and stronger bones than do wildtype mice, there is minimal evidence that CCR2 elimination provides protection against bone loss during disuse and estrogen loss.
Keywords: Chemokine receptor, estrogen, disuse, bone remodeling, Monocyte Chemoattractant Protein-1 (MCP-1)
Introduction
Bone loss occurs throughout life and is exacerbated with advanced age, as well as following periods of decreased physical activity, injury, or disease [1]. In each of these circumstances, the rate of bone resorption by osteoclasts exceeds the rate of bone formation by osteoblasts [1, 2]. Many pharmacological agents, such as bisphosphonates, act directly on osteoclasts to impede bone loss by reducing their activity thereby decreasing bone resorption and the rate of bone remodeling [3]; however, the long-term efficacy of such treatments is controversial [2, 4, 5]. As a result, additional approaches are warranted to minimize the loss of bone.
C-C chemokine receptor 2 (CCR2), which is highly expressed on inflammatory cells and in particular macrophages, has been recently implicated as a potential mediator of bone loss [6]. CCR2 is expressed on preosteoclasts and osteoclasts [7, 8] and functions as a receptor for ligands such as Monocyte Chemoattractant Protein-1 (MCP-1) [9], which is also known as Chemokine (C-C Motif) Ligand 2 (CCL2). The interaction of this ligand with CCR2 has been associated with driving osteoclast fusion and maturation [7, 8, 10, 11]. MCP-1 has also been shown to play a critical role in osteoclast recruitment and differentiation required to initiate bone resorption [12]. Thus, the binding of MCP-1 to CCR2 (referred to as the MCP-1/CCR2 axis) may play a role in the regulation of bone resorption. In support of this function for the MCP-1/CCR2 axis, several studies demonstrated that elimination or inhibition of MCP-1 [11] or CCR2 [8, 13] resulted in a decreased number and function of mature osteoclasts and a decrease in bone resorption. CCR2 knockout (CCR2−/−) mice also have low serum concentrations of deoxypyridinoline (i.e., a marker of bone resorption), with minimal effect on dynamic bone formation markers such as serum osteocalcin [6]. The converse also supports a role for the MCP-1/CCR2 axis in regulating bone resorption. That is, when the MCP-1/CCR2 axis has increased activation the result is a greater number of osteoclasts with enhanced function [11, 12]. This translates to increased bone resorption that was not matched with a change in bone formation, and therefore caused a negative bone remodeling balance. Thus, manipulation of the MCP-1/CCR2 axis may be one potential mechanism to therapeutically limit bone resorption and blunt bone loss.
There are many conditions that disrupt bone remodeling and induce bone loss. One such condition is estrogen deficiency, which occurs in females with age or following surgical removal of the ovaries (ovariectomy) [10, 14, 15]. Estrogen regulates bone remodeling through directly affecting bone cells [10] and may act indirectly through the MCP-1/CCR2 axis as well [16]. Estrogen reduces inflammation which also can result in decreased activity of the axis and may aid in preservation of existing bone mass. To our knowledge, the only other study to have investigated the effect of CCR2 elimination on bone and bone loss was that by Binder and co-workers [6]. This study demonstrated that bone mass was increased in tibial and vertebral bones of CCR2−/− mice as assessed by morphometry and peripheral quantitative computed tomography. However, with the exception of the assessment of tibial bone cortical thickness, this research focused on trabecular bone and therefore much remains unknown as to the effect that CCR2 elimination has on mechanical and structural properties of cortical bone. It is particularly important to understand those effects because these properties more directly relate to whole bone strength and fracture risk in long bones [17, 18].
While ovariectomy (OVX) in rodents is known to induce trabecular bone loss, the impact on cortical bone is less [19, 20]. Therefore, to investigate the efficacy of CCR2 deficiency on the preservation of cortical bone, a more severe model of bone loss is desirable. One such model is denervation (DEN) of a bone’s adjacent musculature. This can cause simultaneous loss of cortical and trabecular bone because of reduced bone loading [21]. As such, the objective of the present study was to extend the study of Binder and colleagues [6] by: 1) using both OVX and DEN interventions in a mouse model to examine the effects of genetic elimination of CCR2 on preventing cortical and trabecular bone loss in the tibia and 2) performing a more rigorous structural and mechanical analysis focusing on cortical bone. We hypothesized that structural and mechanical properties in tibial cortical and trabecular bone would be less detrimentally affected by OVX and DEN in CCR2−/− mice than in wildtype mice.
Methods
Animals
Female CCR2−/− mice (n=27) that were backcrossed to C57BL/6 × strain were used in this study [22, 23] and compared to female C57BL/6 mice (wildtype; n=30). For one month prior to the study and for the entire study, all mice were housed in groups of 4–5 per cage, maintained on a 12–12 hour light-dark cycle, and provided phytoestrogen-free rodent chow and water ad libitum. All mice were 20 weeks old at the start of the study.
For surgical interventions, mice were anesthetized using an induction chamber with isoflurane and then maintained using inhalation of 1.75% isoflurane mixed with O2 at a flow rate of 200 ml/min. Aseptic techniques were used during all surgeries. Immediately following surgery, mice were given a subcutaneous injection of 0.3 µg buprenorphine for analgesia. At the time of sacrifice, mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (100 mg/kg). A deep level of anesthesia was used and all animals were checked for sufficient level of anesthetic depth during dissection. Following excision of tissues, mice were killed by exsanguination. The animal use protocol was approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Study Design
Wildtype and CCR2−/− mice were randomly assigned to one of three intervention groups: non-operated control (n=10 and 9, respectively), OVX (n=10 and 8, respectively), or DEN (n=10 for both). Body mass was measured at initiation of intervention and at time of sacrifice. Eight weeks post-intervention, mice were sacrificed and blood was collected via cardiac puncture to assess the circulating levels of osteocalcin (i.e., a dynamic marker of bone turnover that is present during the initial phase of bone formation) and carboxy-terminal collagen crosslinks (CTX) (i.e., a marker of collagen-1 degradation). Blood was collected in heparinized capillary tubes. Plasma was separated, frozen in liquid nitrogen, and stored at −80°C until the time of analysis. To assess bone mechanical and structural properties, tibial bones were excised and stored in phosphate-buffered saline at −20°C until micro-computed tomography (µCT) and mechanical tests were performed.
Surgical Interventions
The bilateral OVX surgery procedure has been described in detail previously [24, 25]. Briefly, a dorsal skin incision was made to access the ovaries through two small incisions in the dorsal abdominal muscle wall. The ovarian duct and artery were cauterized prior to removal of the ovary. The abdominal wall incisions were then closed using 6-0 silk suture while the skin incision was closed with 7-mm wound clips. Successful OVX intervention was confirmed by assessing uterine mass at the time of sacrifice; uterine masses differ by >5-fold in intact and ovariectomized mice.
For the DEN intervention, the tibial nerve, which innervates the posterior crural muscles, was transected on the left hindlimb as described previously [25]. In brief, a skin incision was made on the lateral surface of the thigh enabling access to the tibial nerve through an incision made in the biceps femoris muscle. A ~3.5-mm length section of the exposed tibial nerve was removed. The muscle and skin were then closed using 6-0 silk suture. Successful DEN intervention is difficult to visually confirm; however, we have not found evidence for nerve regeneration in the 2 month time period.
Bone Analyses
Bone geometry at the tibial mid-diaphysis and trabecular morphometry in the metaphysis of the left tibia were assessed by µCT. The tibiae were scanned with a µCT 40 (Scanco Medical, Brüttisellen, Switzerland) at a voltage of 55 kV, current of 145 µA, integration time of 200 ms, and voxel size of 12 µm. Global constant thresholding values to segment bone from background were chosen based on histogram analysis and visual inspection. These segmentation values were applied to generate 3D binarized images, which were used to quantify bone morphometry and density. Outcome measures obtained at the mid-diaphysis include: cross-sectional moment of inertia about the anterior-posterior axis (CSMI), cross-sectional area (CSA), cortical thickness, volumetric bone mineral density (vBMD), and periosteal diameter of cortical bone. In the proximal tibia, trabecular bone at the metaphysis was assessed 60 µm distal to the growth plate for bone volume fraction, average trabecular number, average trabecular thickness, trabecular spacing and vBMD. Structure Model Index (SMI) was calculated using a three-dimensional image analysis algorithm to assess the shape of trabeculae based on changes in surface are and volume by categorizing them as more plate-like (SMI=0), rod-like (SMI=3) or sphere-like (SMI=4) [26]. Morphometric measures were computed using direct distance transformation methods [27]. Tibial bones were stored in phosphate-buffered saline at −20°C following scanning until the time of mechanical testing.
Three-point bending was used to determine mechanical properties of the tibial bone at the mid-diaphysis. This was done using a Mecmesin MultiTest 1-D test machine and a Mecmesin AFG-25 load cell (Mecmesin, West Sussex, UK) as previously described [28–30]. In brief, the tibia was placed on its lateral side on the two supports (1 cm separation between supports) and a quasi-static load was applied to the mid-diaphysis of the bone until failure; the rate of displacement for the load was 2 mm/min. The load-displacement relationship for each bone was analyzed using a custom-written TestPoint program (TestPoint version 7; Measurement Computing Corp., Morton, MA) to quantify the mechanical properties of t ibia including ultimate load and stiffness, as previously described [28–30], toughness, defined as the amount of energy absorbed until fracture occurs, failure load, defined as the load immediately before fractures occurs (i.e., when the load is reduced rapidly to zero), and post-yield displacement, defined as the difference between displacement to ultimate load and displacement at which fracture occurs [31]. Intrinsic material properties, including ultimate stress and modulus of elasticity, were calculated from the three-point bending and µCT outcome measures using classical beam theory [28–30]. Intrinsic material properties are approximated values that provide additional insight into the relative contributions of the mechanical and geometric properties measured.
Markers of bone growth and collagen content
Plasma levels of osteocalcin, a marker of bone turnover, were measured by ELISA (Biomedical Technologies Inc., Stoughton, MA). Plasma levels of CTX, a marker of collagen-1 degradation, were also measured by ELISA (Immuno Diagnostic Systems Inc., Fountain Hills, AZ). ELISA assays were performed as per the manufacturer’s instructions; sample values were determined using absorbance data from the standards fit to a 4-parameter logistic curve. Hydroxyproline content, which can be used as an indicator of bone collagen content, was assessed using the methods of Woessner [32]. Prior to the hydroxyproline assay, wet and dry masses of the tibiae were measured; dry masses were obtained after drying the bones at 113 °C for 20 hours [33].
Statistical Analyses
Power analyses were performed to determine groups sizes necessary to detect significant ANOVA main effects and interactions. These analyses determined that at an -level of 0.05 our group sizes (n=8–10) yielded >85% power to detect a medium effect (0.5) [34]. Two-way ANOVAs (genotype × intervention) were performed to analyze the effects of genetic elimination of CCR2 and surgical interventions on the bone measures, plasma markers, and body mass; there were two levels of the genotype factor (i.e., CCR2−/− vs. wildtype) and three levels of the intervention factor (i.e., control vs. OVX vs. DEN). Significant interactions were further explored using a Holms-Sidak post-hoc analysis. Because of significant genotype differences in body mass, multiple regression analyses were performed for bone measures where significant main effects of genotype were detected in order to determine if the effect could be explained by the genotype difference in body mass rather than a genotype difference not associated with body mass. In these situations, the multiple regression analysis was done including both genotype (factor) and body mass (covariate) with and without an interaction term. All statistical analyses were performed using SigmaStat version 3.5 (Systat Software, Chicago, IL). Statistical significance was accepted at an -level of 0.05.
Results
Body mass, bone masses, and bone length
Body mass can influence bone size and strength. To determine if body mass differed between wildtype and CCR2−/− mice or following surgical interventions (i.e., control, OVX, and DEN), body masses at baseline and 8 weeks later at sacrifice were compared. There was a main effect of genotype on body mass at both baseline and following eight weeks of intervention. CCR2−/− mice, on average, were 9% heavier than wildtype mice at baseline and 17% heavier following intervention (Table 1). There was also a main effect of intervention following the treatment period. OVX mice weighed 19% more than control and DEN mice (Table 1).
Table 1.
Lack of C-C chemokine receptor 2 (CCR2−/−) and interventions affect body and tissue masses, tibial length, and serum markers
| wildtype | CCR2−/− | Two-way ANOVA P-values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=10) |
OVX (n=10) |
DEN (n=10) |
Control (n=9) |
OVX (n=8) |
DEN (n=10) |
Genotype | Intervention | Interaction | |
| Body mass: | |||||||||
| Pre-intervention mass (g) | 20.99 (0.47) | 21.17 (0.47) | 20.67 (0.47) | 23.17 (0.50) | 23.40 (0.53) | 22.18 (0.47) | <0.001 | 0.183 | 0.704 |
| Mass at sacrifice (g) | 23.34 (0.88) | 27.57 (0.88) | 23.02 (0.88) | 27.34 (0.93) | 32.59 (0.99) | 26.63 (0.88) | <0.001 | <0.001 | 0.733 |
| Tibial bone mass and length: | |||||||||
| Wet mass (mg) | 43.64 (0.80) | 43.26 (0.76) | 40.69 (0.76) | 46.08 (0.80) | 45.75 (0.85) | 43.34 (0.88) | <0.001 | <0.001 | 0.990 |
| Dry mass (mg) | 26.35 (0.45) | 26.19 (0.43) | 23.74 (0.43) | 28.46 (0.45) | 27.93 (0.48) | 26.97 (0.43) | <0.001 | <0.001 | 0.850 |
| Length (mm) | 17.91 (0.08) | 18.37 (0.08) | 18.04 (0.08) | 18.43 (0.08) | 18.48 (0.09) | 18.33 (0.08) | <0.001 | 0.005 | 0.051 |
| Markers of bone turnover and collagen content: | |||||||||
| Osteocalcin (ng/mL) | 25.24 (2.0) | 30.72 (2.0) | 30.94 (2.0) | 29.64 (2.1) | 28.47 (2.2) | 27.86 (2.0) | 0.853 | 0.506 | 0.137 |
| CTX (ng/mL) | 15.76 (1.41) | 18.90 (1.41) | 16.94 (1.49) | 15.43 (1.49) | 17.59 (1.58) | 18.60 (1.41) | 0.165 | 0.996 | 0.590 |
| Hydroxyproline content (µg) | 35.65 (0.71) | 35.32 (0.68) | 35.83 (0.68) | 36.29 (0.71) | 36.52 (0.76) | 34.72 (0.68) | 0.672 | 0.535 | 0.227 |
| Mechanical properties: | |||||||||
| Failure load (N) | 6.59 (0.61) | 5.61 (0.64) | 5.68 (0.61) | 7.63 (0.65) | 5.84 (0.68) | 7.62 (0.61) | 0.045 | 0.105 | 0.412 |
| Post yield displacement (mm) | 0.437 (0.084) | 0.475 (0.089) | 0.383 (0.084) | 0.326 (0.089) | 0.452 (0.094) | 0.228 (0.084) | 0.184 | 0.209 | 0.750 |
Values are given as mean (SEM)
There were independent main effects of genotype and intervention on tibial bone wet and dry masses. The mean mass of tibial bones from CCR2−/− mice was 6–8% greater than that from wildtype mice and DEN mice tibial bones had a mean mass that was 6–9% less compared to the control condition (Table 1). Independent main effects of genotype and intervention were also identified for tibia length. That is, CCR2−/− mice had, on average, 2% longer tibia than did wildtype mice and OVX mice had 2% longer tibia than control and DEN mice (Table 1).
Tibial bone mechanical and intrinsic material properties
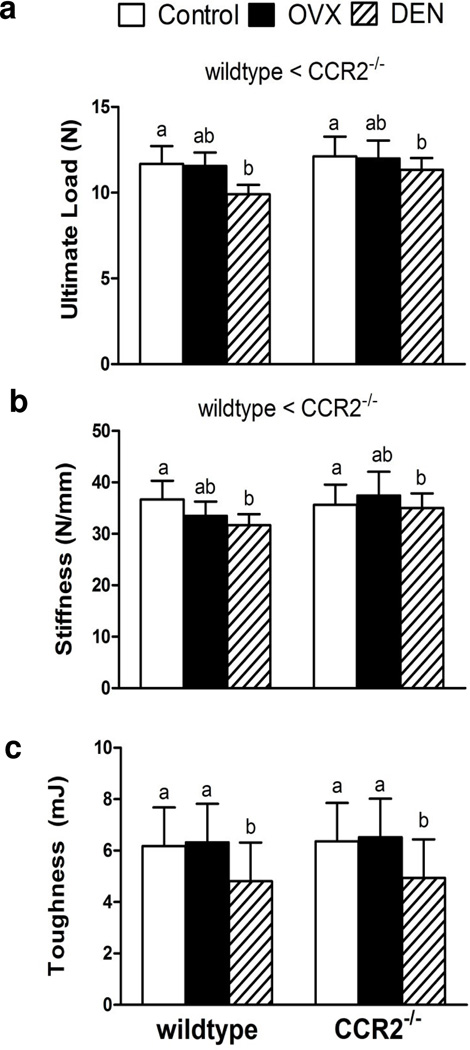
Using two-way ANOVAs, it was determined that tibial bone mechanical properties were affected independently by genotype and intervention. Specifically, bones from CCR2−/− mice had 6% greater ultimate loads than bones from wildtype mice (Figure 1A). However, when body mass was entered as a covariate in the multiple regression analysis predicting ultimate load from genotype, only the covariate was statistically significant (p<0.001). This result indicated that the greater bone strength in CCR2−/− mice was more related to those mice being bigger as opposed to a genotype difference independent of body mass. For intervention effects, tibial bone ultimate load was 11% lower in DEN mice compared to control and OVX mice (Figure 1A), indicating that the DEN intervention was more detrimental to bone strength than OVX.
Fig. 1.
Lack of C-C-chemokine receptor 2 (CCR2−/−) and intervention affect tibial mechanical properties: a) Tibial bone ultimate load, b) Tibial bone stiffness and c) Tibial bone toughness. Values are expressed as mean ± SEM. The main effect of genotype is indicated in text above the bars. For the main effect of intervention, differing lowercase letters above the bars are used to indicate significant differences between interventions. Two interventions with the same letter are not significantly different. Lowercase letters are only used for comparisons within a given genotype.
Independent main effects of genotype and intervention (p≤0.032) were also found for tibial bone stiffness with a trend towards a significant interaction (p=0.056; Figure 1B). On average, CCR2−/− tibia were 6% stiffer than wildtype tibia, but multiple regression analysis determined that this result could be explained by the genotype difference in body mass (p=0.003) rather than a genotype difference independent of body mass. Similar to the findings for ultimate load, DEN reduced tibial bone stiffness by 8% compared to control mice, but the stiffness value for DEN mice was not different from that for OVX mice (Figure 1B). Combined, these results indicate that the genetic elimination of CCR2 resulted in tibial bones that were stronger and stiffer; however, it appeared that these differences were more associated with the genotype difference in body mass rather than a direct effect of CCR2 deficiency on bone.
Toughness exhibited a main effect of intervention but not genotype. Specifically, toughness was 22–24% less in DEN mice compared to control and OVX mice (p= 0.004, Figure 1C). The calculated post-yield displacement to failure was not affected by genotype or intervention (Table 1). Tibial bone failure load exhibited a main effect of genotype but no effect of intervention. Specifically, CCR2−/− mice had approximately 18% greater failure loads than wildtype mice (Table 1). The results of a multiple regression indicated that this genotype main effect was not attributed to a genotype difference in body mass (p=0.790).
Tibial bone cortical geometry
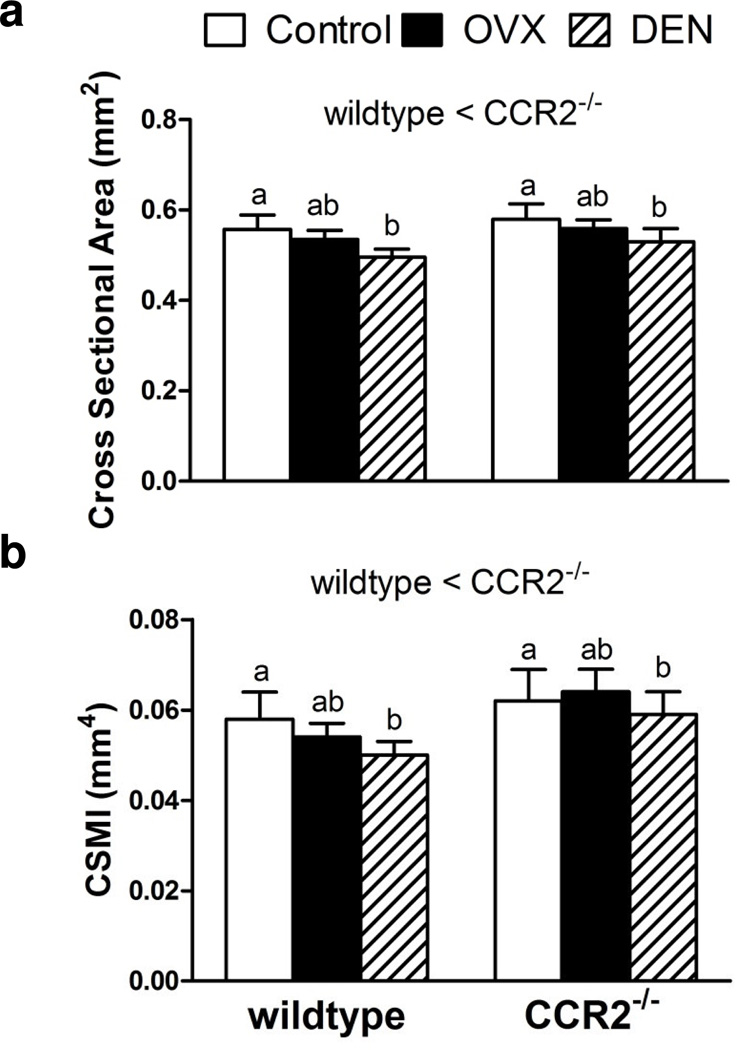
To determine if the observed differences in bone mechanical properties could be explained by differences in tibial bone cortical geometry, the effects of genotype and intervention were assessed on cortical bone CSA, CSMI, thickness, and periosteal diameter. From the 2-way ANOVAs, independent main effects of both genotype (p<0.001) and intervention (p≤0.002) were found for cortical bone CSA and CSMI (Figure 2). CCR2−/− mice had 6% larger cortical bone CSA (Figure 2A) and 15% larger CSMI than wildtype mice (Figure 2B). Multiple regression analysis for both measures indicated that the genotype main effect could be explained at least partially by the genotype difference in body mass (p≤0.001). On average, DEN mice had 11% smaller cortical bone CSA (Figure 2A) and 10% lower CSMI than both control and OVX mice (Figure 2B).
Fig. 2.
Lack of C-C-chemokine receptor 2 (CCR2−/−) and intervention affect tibial cortical properties: a) Cross sectional area and b) Cross sectional moment of inertia (CSMI). Values are expressed as mean ± SEM. Statistical differences are indicated as described in Figure 1.
Other measures of cortical bone geometry including cortical thickness and periosteal diameter were differentially affected by genotype and intervention. Cortical thickness at the mid-diaphysis exhibited independent main effects of genotype and intervention in the 2-way ANOVA (Table 2). Cortical thickness was 3% larger in CCR2−/− than wildtype mice. The results of a multiple regression indicated that this genotype main effect was not attributed to a genotype difference in body mass (p=0.073). Compared to control mice, cortical thickness was 6% smaller in OVX mice and 11% smaller in DEN mice (Table 2). A significant interaction (p=0.024) between genotype and intervention was found in the 2-way ANOVA analyzing periosteal diameter. Compared to the control condition, wildtype mice had 3% smaller diameters following OVX and DEN, whereas there was no difference in periosteal diameter among the three intervention groups in CCR2−/− mice (Table 2). CCR2−/− mice that were ovariectomized and denervated had greater periosteal diameters than wildtype mice that underwent the same intervention (Table 2).
Table 2.
Lack of C-C chemokine receptor 2 (CCR2−/−) and interventions affect several tibial bone cortical and trabecular properties
| wildtype | CCR2−/− | Two-way ANOVA P-values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=10) |
OVX (n=10) |
DEN (n=10) |
Control (n=9) |
OVX (n=8) |
DEN (n=10) |
Genotype | Intervention | Interaction | |
| Cortical bone geometry: | |||||||||
| Cortical thickness (mm) | 0.184 (0.002) | 0.176 (0.002) | 0.165 (0.002) | 0.191 (0.003) | 0.177 (0.003) | 0.171 (0.002) | 0.033 | <0.001 | 0.414 |
| Periosteal diameter (mm) | 1.19 (0.01) | 1.15+ (0.01) | 1.15+ (0.01) | 1.20 (0.01) | 1.23* (0.01) | 1.20* (0.01) | 0.024 | ||
| Cortical intrinsic properties: | |||||||||
| Ultimate stress (MPa) | 273 (5.8) | 290 (5.8) | 267 (5.8) | 276 (6.1) | 267* (6.5) | 272 (5.8) | 0.037 | ||
| Modulus of elasticity (GPa) | 13.1 (0.3) | 12.8 (0.3) | 13.3 (0.3) | 12.0 (0.4) | 12.3 (0.4) | 12.4 (0.3) | 0.005 | 0.653 | 0.705 |
| Cortical bone mineral density (mg/cm3) | 1440 (7) | 1450 (7) | 1440 (7) | 1460 (7) | 1470 (7) | 1460 (7) | <0.001 | 0.268 | 0.966 |
| Trabecular morphology at the metaphysis: | |||||||||
| Trabecular number (number/mm) | 3.13 (0.07) | 3.06 (0.07) | 2.88 (0.07) | 3.10 (0.07) | 2.98 (0.07) | 2.99 (0.07) | 0.993 | 0.041 | 0.317 |
| Trabecular spacing (mm) | 0.33 (0.01) | 0.34 (0.01) | 0.36 (0.01) | 0.33 (0.01) | 0.34 (0.01) | 0.35 (0.01) | 0.850 | 0.015 | 0.827 |
| Structure model index | 2.70 (0.08) | 2.91 (0.08) | 3.35+ (0.08) | 2.46* (0.08) | 2.84+ (0.09) | 2.75*+ (0.08) | 0.005 | ||
| Bone mineral density (mg/cm3) | 1220 (5) | 1200+ (5) | 1230 (5) | 1220 (5) | 1230* (6) | 1240 (5) | 0.009 | ||
Values are given as mean (SEM). Cortical bone geometry was measured mid-shaft. When a statistical interaction is present, an asterisk (*) indicates different from wildtype for the same intervention and a plus sign (+) indicates different from control of the same genotype.
Cortical bone intrinsic material properties
The intrinsic material properties of tibial cortical bone were assessed using ultimate stress, modulus of elasticity, and cortical vBMD. Multiple regressions for main effects of genotype were not performed on mechanical intrinsic properties (ultimate stress and modulus of elasticity) because these are expected to be independent of body mass. There was a significant genotype × intervention interaction for ultimate stress. Specifically, compared to OVX wildtype mice, ultimate stress was 8% lower in CCR2−/− OVX mice (Table 2). A significant main effect of genotype but not intervention was identified in the analysis of modulus of elasticity (Table 2). CCR2−/− mice had a 6% lower modulus of elasticity. Analysis of cortical vBMD identified a significant genotype main effect. On average, vBMD was 1% greater in CCR2−/− mice than in wildtype mice (Table 2), and the effect was not associated with the genotype difference in body mass (p=0.347).
Trabecular bone properties at the tibial metaphysis
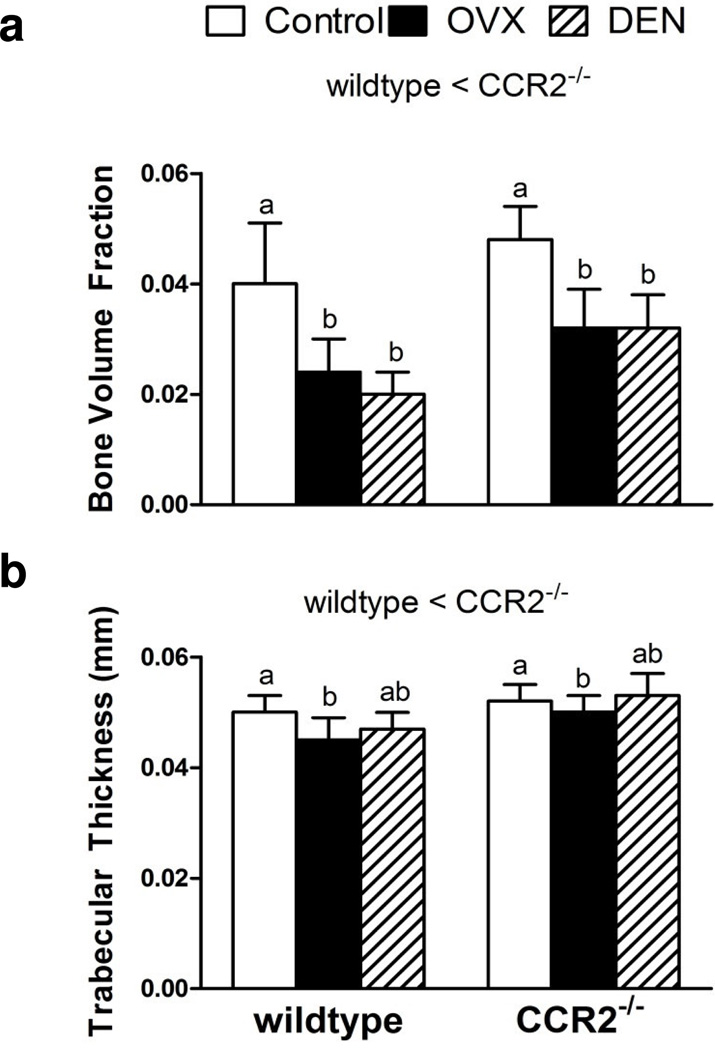
There were independent main effects of genotype and intervention on trabecular bone volume fraction and trabecular thickness, but only a main effect of intervention on trabecular number. Bone volume fraction was 32% greater in CCR2−/− mice than in wildtype mice (Figure 3A). This greater proportion of bone volume in CCR2−/− mice was primarily attributed to the 11% greater trabecular thickness observed in those mice (Figure 3B), as trabecular number was not different between genotypes (Table 2). The effect of eliminating CCR2 on bone volume fraction was attributed to a genotype effect not related to the difference in body mass (p=0.364). This was not the case for trabecular thickness where the body mass covariate in the multiple regression analysis was significant (p=0.030). As expected, up to a 41% decrease in bone volume fraction was observed following the two interventions (p<0.001; Figure 3A). The decline in bone volume fraction resulted from 7% thinner trabeculae (p=0.002, Figure 3B) and 3% fewer trabeculae following OVX whereas DEN resulted in 6% fewer trabeculae compared to control (Table 2). There was no effect of genotype on trabecular spacing; however, DEN resulted in a 7% greater spacing compared to control (Table 2).
Fig. 3.
Lack of C-C-chemokine receptor 2 (CCR2−/−) and intervention affect tibial trabecular properties at the metaphysis: a) Bone volume fraction and b) Trabecular thickness. Values are expressed as mean ± SEM. Statistical differences are indicated as described in Figure 1.
The effect of genotype on trabecular structure as assessed by the structure model index (SMI) was dependent on intervention (i.e., interaction between genotype and intervention, Table 2). In wildtype mice, SMI was not affected by OVX but was greater following DEN. In CCR2−/− mice, both OVX and DEN resulted in a slightly greater SMI than control (Table 2). The effect of genotype on trabecular vBMD was also dependent on intervention (Table 2). Wildtype OVX mice had 2% less trabecular vBMD than controls; however, vBMD was preserved in CCR2−/− OVX mice compared to control CCR2−/− mice (Table 2). This potential bone-conserving role was also apparent in CCR2−/− DEN mice that had a significant, yet slightly greater (i.e., 1%) trabecular vBMD than did control mice, although no difference was detected between DEN and control wildtype mice (Table 2).
Markers of bone turnover and collagen content
There was no significant effect of genotype or intervention on plasma osteocalcin level, a marker of bone turnover, and plasma CTX level, a marker of collagen-1 degradation (Table 1). There was also no significant effect of genotype or intervention on hydroxyproline content normalized to dry bone mass (Table 1).
Discussion
This study addressed the hypothesis that, compared to wildtype mice, the structural and mechanical properties of cortical bone as well as trabecular bone structure in tibiae from CCR2−/− mice would be less detrimentally affected by OVX and DEN. Overall, the results did not support our hypothesis. Our results showed that genetic elimination of CCR2 resulted in tibiae that were larger and stronger, but for the most part the responses of these bones to OVX and DEN were very similar to those for wildtype mice.
Our finding that CCR2−/− mice had larger and stronger cortical bone and more trabecular bone than wildtype mice lends support for the previous studies’ findings that loss of CCR2, either through inhibition or genetic elimination, impairs the ability of osteoclasts to resorb bone due to altered osteoclast fusion and maturation [6–8, 13]. Similar to previous reports [6], we observed 32% greater trabecular bone volume fraction and 11% greater trabecular thickness in tibiae from CCR2-deficient mice. Additionally, our study found 6–15% greater cortical CSA and CSMI in tibiae from CCR2-deficient mice compared to wildtype. Our study also found that tibiae in CCR2−/− mice were up to 6% stronger and stiffer than those in wildtype mice (Figure 1). This modest improvement in bone strength contrasts that of a previous study in which compressive strength of the L5 vertebral body was 47% greater in CCR2−/− mice compared to wildtype [6]. Combined, these data suggest that CCR2 elimination increases bone size and strength.
The resulting larger and stronger bones in our CCR2−/− mice may not necessarily have been due to decreased resorption but rather that the CCR2−/− mice were 17% heavier than the wildtype mice at the time of sacrifice. Based on principles of allometry, one would expect larger and stronger bones in heavier mice. From the multiple regression analyses, the genotype difference in body mass could statistically account for all or part of the genotype main effect observed in the ANOVAs for eight measures of bone structural and mechanical properties (i.e., dry and wet bone masses, bone length, cortical CSA and CSMI, ultimate load, stiffness, and trabecular thickness). For three bone metrics, the genotype difference in body mass could not statistically explain any part of the significant genotype main effect observed in the ANOVAs (i.e., cortical vBMD, cortical thickness, and trabecular bone volume fraction). The previous study by Binder and colleagues [6] demonstrated differences in bone structural and mechanical properties between CCR2−/− and wildtype mice but they did not report body mass values. The only other study to report body mass on CCR2−/− mice found that body mass of the knockout mice was lower than that of wildtype mice [35]; however, methodological differences in the development of the CCR2−/− mouse strain could perhaps differentially affect body mass.
Because a genotype difference in body mass can statistically account for all or part of genotype difference in bone measures does not mean that the relationship is causal in nature. Other factors, such as the effects of altered osteoclast and osteoblast activity resulting in reduced resorption during development should also be considered. The current study investigated the circulating level of CTX as an indication of resorption activity by osteoclasts and found no effect of genotype on this marker, suggesting that bone resorption is not significantly altered. This conclusion is further substantiated by the circulating osteocalcin level, a marker of bone turnover (relative osteoclast and osteoblast activity), which was also not affected by elimination of CCR2. OVX and DEN interventions also had no effect on serum markers of bone resorption and turn-over, in agreement with a recent study by Miyagawa et al [21]. This is in contrast to previous studies which found increases in these markers with ovariectomy [6, 36]. The discrepancy between studies suggests that timing and marker-specificity may be important as these factors tend to vary from study to study [10, 37, 38]. A limitation of the current study is that histomorphometric analyses were not performed. Inclusion of such measurements would be helpful in determining the relative contribution of osteoclasts and osteoblasts to the alterations in bone that were observed. Future experiments are warranted to distinguish a possible body mass effect from a reduced resorption effect on bone properties in CCR2−/− mice. This could be accomplished by weight matching and pair feeding CCR2−/− and wildtype mice. Though the CCR2-deficient mouse has been referred to as a high-bone mass phenotype [6], this may be inappropriate based on the present study’s findings. The ratio of bone mass to body mass was actually greater in the wildtype mouse (Table 1). Thus, for CCR2−/− and wildtype mice of the same body mass, one would predict that the wet and dry bone masses for the tibia would be on average 8–10% greater for the wildtype mice.
There was little evidence for a protective effect of CCR2 deficiency against OVX and/or DEN based on the outcomes of this study’s genotype × intervention analyses. For us to have concluded that there was a protective effective of CCR2 deficiency against OVX and/or DEN on any given bone measure, a significant interaction between genotype and intervention would have been required. A significant interaction occurred for only four of the 19 bone measures (i.e., cortical periosteal diameter, ultimate stress, SMI, and trabecular BMD). For only two of those measures (i.e., cortical periosteal diameter and trabecular BMD) could the interaction be interpreted as the CCR2 deficiency providing protection against OVX and/or DEN. The interaction observed for SMI suggests that elimination of CCR2 results in a more rod-like lattice of trabeculae in CCR2−/− mice following OVX and DEN whereas the trabeculae are more sphere-like following DEN in wildtype mice. On the other hand, there were 10 bone measures (i.e., dry and wet bone masses, bone length, cortical CSA, cortical CSMI, cortical thickness, ultimate load, stiffness, trabecular thickness and trabecular bone volume fraction) for which there were significant independent effects of genotype and intervention indicating that the effects of OVX and/or DEN on the two mouse strains were the same. Prior to the present study, the evidence for a protective effect of CCR2 deficiency against any bone loss was based solely on measures of tibial trabecular and spinal BMD following OVX [6]. The findings of that study and ours are directionally similar for tibial trabecular BMD, and if this was the only bone measure being considered, we might also conclude that CCR2 deficiency was protective against bone loss. However, our findings on 15 other bone measures argue against such a protective effect.
In conclusion, CCR2-deficient mice have larger and stronger tibiae than wildtype mice, but it is unclear whether the effects were caused by reduced bone resorption and/or greater body mass in the CCR2-deficient mice. Additional experiments, as discussed above, are required to further tease apart these effects. Finally, based on the measures of mouse tibial bone structural and mechanical properties we made, there was minimal evidence that CCR2 deficiency provided protection against bone loss induced by ovariectomy or muscle denervation. As opposed to previous studies, our findings do not support the idea that CCR2 is a potential therapeutic target to reduce bone loss.
Acknowledgements
The authors thank Sarah Greising, Kristen Baltgalvis, and Greg Cochrane for their technical assistance. This work was supported by National Institutes of Health (NIH) grants R01 AG031743 (DL and GW), K02 AGA036827 (DL), T32-AR07612 (SN), and T32-AG029796 (TM).
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Martin TJ. Therapeutic Approaches to Bone Diseases. Science. 2000;289(5484):1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 3.Baron R, Hesse E. Update on Bone Anabolics in Osteoporosis Treatment: Rationale, Current Status, and Perspectives. Journal of Clinical Endocrinology & Metabolism. 2012;97(2):311–325. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salari P AM. Long term bisphosphonate use in osteoporotic patients; a step forward, two steps back. J Pharm Pharm Sci. 2012;15(2):305–317. doi: 10.18433/j3rk5j. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, et al. Long-term treatment of osteoporosis in postmenopausal women: a review from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF) Current Medical Research and Opinion. 2012;28(3):475–491. doi: 10.1185/03007995.2012.663750. [DOI] [PubMed] [Google Scholar]
- 6.Binder NB, et al. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15(4):417–424. doi: 10.1038/nm.1945. [DOI] [PubMed] [Google Scholar]
- 7.Kim MS, et al. Induction of chemokines and chemokine receptors CCR2b and CCR4 in authentic human osteoclasts differentiated with RANKL and osteoclast like cells differentiated by MCP-1 and RANTES. J Cell Biochem. 2006;97(3):512–518. doi: 10.1002/jcb.20649. [DOI] [PubMed] [Google Scholar]
- 8.Xing Z, et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3(7–8):451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charo IF, et al. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proceedings of the National Academy of Sciences. 1994;91(7):2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sul O-J, et al. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. Journal of Cellular Physiology. 2012;227(4):1619–1627. doi: 10.1002/jcp.22879. [DOI] [PubMed] [Google Scholar]
- 12.Wu AC, et al. MCP-1 Expression Is Specifically Regulated During Activation of Skeletal Repair and Remodeling. Calcified Tissue International. 2013;92(6):566–575. doi: 10.1007/s00223-013-9718-6. [DOI] [PubMed] [Google Scholar]
- 13.Barros SP, et al. Therapeutic effect of a topical CCR2 antagonist on induced alveolar bone loss in mice. Journal of Periodontal Research. 2011;46(2):246–251. doi: 10.1111/j.1600-0765.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 14.Jilka R, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 15.Pacifici R. Editorial: Cytokines, Estrogen, and Postmenopausal Osteoporosis—The Second Decade. Endocrinology. 1998;139(6):2659–2661. doi: 10.1210/endo.139.6.6087. [DOI] [PubMed] [Google Scholar]
- 16.Janis K, et al. Estrogen decreases expression of chemokine receptors, and suppresses chemokine bioactivity in murine monocytes. Am J Reprod Immunol. 2004;51(1):22–31. doi: 10.1046/j.8755-8920.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 17.Faulkner KG, et al. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. Journal of Bone and Mineral Research. 1993;8(10):1211–1217. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 18.Augat P, Reeb H, Claes LE. Prediction of fracture load at different skeletal sites by geometric properties of the cortical shell. Journal of Bone and Mineral Research. 1996;11(9):1356–1363. doi: 10.1002/jbmr.5650110921. [DOI] [PubMed] [Google Scholar]
- 19.Brouwers JEM, et al. Comparison of bone loss induced by ovariectomy and neurectomy in rats analyzed by in vivo micro-CT. Journal of Orthopaedic Research. 2009;27(11):1521–1527. doi: 10.1002/jor.20913. [DOI] [PubMed] [Google Scholar]
- 20.Westerlind KC, et al. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proceedings of the National Academy of Sciences. 1997;94(8):4199–4204. doi: 10.1073/pnas.94.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyagawa K, et al. A novel underuse model shows that inactivity but not ovariectomy determines the deteriorated material properties and geometry of cortical bone in the tibia of adult rats. Journal of Bone and Mineral Metabolism. 2011;29(4):422–436. doi: 10.1007/s00774-010-0241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuziel WA, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proceedings of the National Academy of Sciences. 1997;94(22):12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuziel WA, et al. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis. 2003;167(1):25–32. doi: 10.1016/s0021-9150(02)00382-9. [DOI] [PubMed] [Google Scholar]
- 24.Moran A, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100(2):548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 25.Greising SM, et al. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110(1):109–115. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildebrand TR, Peter Quantification of Bone Microarchitecture with the Structure Model Index. Computer Methods in Biomechanics and Biomedical Engineering. 1997;1(1):15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand T, et al. Direct Three-Dimensional Morphometric Analysis of Human Cancellous Bone: Microstructural Data from Spine, Femur, Iliac Crest, and Calcaneus. Journal of Bone and Mineral Research. 1999;14(7):1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 28.Novotny SA, et al. Bone is functionally impaired in dystrophic mice but less so than skeletal muscle. Neuromuscul Disord. 2011;21(3):183–193. doi: 10.1016/j.nmd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren GL, et al. Voluntary run training but not estradiol deficiency alters the tibial bone-soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R2015–R2026. doi: 10.1152/ajpregu.00569.2007. [DOI] [PubMed] [Google Scholar]
- 30.Warren GL, et al. Estradiol effect on anterior crural muscles-tibial bone relationship and susceptibility to injury. Journal of Applied Physiology. 1996;80(5):1660–1665. doi: 10.1152/jappl.1996.80.5.1660. [DOI] [PubMed] [Google Scholar]
- 31.Turner CH, Burr DB. BASIC BIOMECHANICAL MEASUREMENTS OF BONE - A TUTORIAL. Bone. 1993;14(4):595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 32.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 33.Novotny SA, et al. Prednisolone treatment and restricted physical activity further compromise bone of mdx mice. J Musculoskelet Neuronal Interact. 2012;12(1):16–23. [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 35.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of Clinical Investigation. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen V, et al. Short-term changes in histomorphometric and biochemical turnover markers and bone mineral density in estrogen- and/or dietary calcium-deficient rats. Bone. 1995;16(1):149–156. [PubMed] [Google Scholar]
- 37.Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9(12):699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph C, et al. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Molecular Aspects of Medicine. 2005;26(3):181–201. doi: 10.1016/j.mam.2005.01.004. [DOI] [PubMed] [Google Scholar]