Abstract
We present a real-time multimodal near-infrared imaging technology that tracks externally induced axial motion of magnetic microbeads in single cells in culture. The integrated multimodal imaging technique consists of phase-sensitive magnetomotive optical coherence microscopy (MM-OCM) and multiphoton microscopy (MPM).MPMis utilized for the visualization of multifunctional fluorescent and magnetic microbeads, while MM-OCM detects, with nanometer-scale sensitivity, periodic displacements of the microbeads induced by the modulation of an external magnetic field. Magnetomotive signals are measured from mouse macrophages, human breast primary ductal carcinoma cells, and human breast epithelial cells in culture, and validated with full-field phase-sensitive microscopy. This methodology demonstrates the capability for imaging controlled cell dynamics and has the potential for measuring cell biomechanical properties, which are important in assessing the health and pathological state of cells.
Index Terms: Cellular biomechanics, magnetic tweezers, timodal microscopy, multiphoton microscopy (MPM), optical coherence tomography (OCT)
I. Introduction
Cellular mechanics play an important role in normal cell function, and numerous processes at the cellular level result in changes in the elastic properties of different cell components, along with biochemical changes [1]–[4]. Therefore, it is of great interest to develop technologies that enable measurements of these dynamic mechanical changes, as they would offer new fundamental insight into the inner workings of cells, and further our understanding of both normal and pathological processes. These biomechanical measurements at the cellular level have the potential to lead to new diagnostic paradigms or biomarkers for detecting and treating disease.
Magnetic tweezers are a well-established platform for probing at the single-molecule level (particularly for DNA measurements) [5] and can also be implemented in the study of cell functions and processes [6]–[11]. While the use of magnetic tweezers is preferred for ease of use and robustness, magnetic tweezers suffer from limitations in time resolution and spatial resolution, inherent in the imaging systems utilized to monitor their motion [5]. White-light bright-field video microscopy is the widely used imaging technique for magnetic tweezer experiments; however, its main drawback is the fact that it can only measure a 2-D plane in real time, while inference of displacement in the third dimension is usually done in postprocessing and suffers from lower precision and resolution compared to the real-time data [5].
Our group has developed magnetomotive optical coherence tomography (MM-OCT) and magnetomotive optical coherence elastography (MM-OCE), two imaging techniques that provide a new type of contrast and detect dynamic mechanical changes in biological samples probed with magnetic nanoparticles or microspheres [12]–[14]. These particles or microspheres are set into motion by an external magnetic field delivered by a custom-made solenoid coil. Our systems enable real-time measurements of axial displacements in tissues and cells with nanometer-scale sensitivity [12]–[15], enabling minute interrogation of their biomechanical properties. Previously, we have shown that MM-OCE can accurately measure the natural resonant frequencies of silicone samples that mimic the optomechanical properties of tissue [13]; however, acquiring magnetomotive signals from single cells presents new challenges, and many investigative opportunities. Our current goal is to explore the potential of our imaging system for measuring real-time cellular-level mechanics, which would potentially reveal mechanisms of important cell functions and processes.
We propose a new real-time, multimodal optical imaging technique that incorporates phase-sensitive MM-OCE with optical coherence microscopy (OCM). This technique provides fast high-resolution imaging of dynamic mechanical changes in cells probed with magnetic microbeads, which are similarly used in magnetic tweezer studies. This technique has the potential to greatly improve the existing methodology and enable new investigative studies in cellular biomechanics.
II. Methods
A. Cell Sample Preparation
Three types of cells were probed in this study: mouse macrophages, cell line TIB-67 (J774 A.1, ATCC), human breast epithelial primary ductal carcinoma cells, cell line CRL-2314 (HCC38, ATCC), and healthy human breast epithelial cells, cell line CRL-4010 (hTERT-HME1, ATCC). In the experiments using mouse macrophages, two types of magnetic microparticles were utilized. The first type was multifunctional microspheres custom-fabricated in our lab, which have an average diameter and standard deviation of 2.2 ± 1.3 µm. These microspheres consist of a liquid core containing a suspension of iron oxide nanoparticles in vegetable oil, and an encapsulating albumin protein shell [16]. These microspheres have previously been shown to provide good magnetomotive imaging contrast for OCT [16]. The second type of magnetic microparticles/microtransducers was fluorescent magnetic microbeads (ME04 F /9486, Bang Labs, Fishers, IN), with a diameter between 1 and 2 µm. These magnetic particles consist of iron oxide nanoparticles and a fluorescent dye embedded in a polystyrene matrix that allows for additional coregistered MPM imaging.
In the experiments involving cancer and normal human breast cells, magnetic microbeads (3-µm diameter, Invitrogen Dynabeads®) composed of iron oxide nanoparticles in a polystyrene matrix were used. These magnetic microbeads were either left uncoated or were functionalized with an RGD ligand to target the alpha-v-beta-3 integrin receptors overexpressed on cancer cells.
To facilitate targeting of the magnetic particles/beads to the cells, cultures of each cell type were incubated with magnetic microbeads for a period of 4 h at room temperature in a 5%CO2 environment. Prior to imaging, cell cultures were washed/rinsed with PBS in order to remove excess loose microbeads.
B. Imaging System
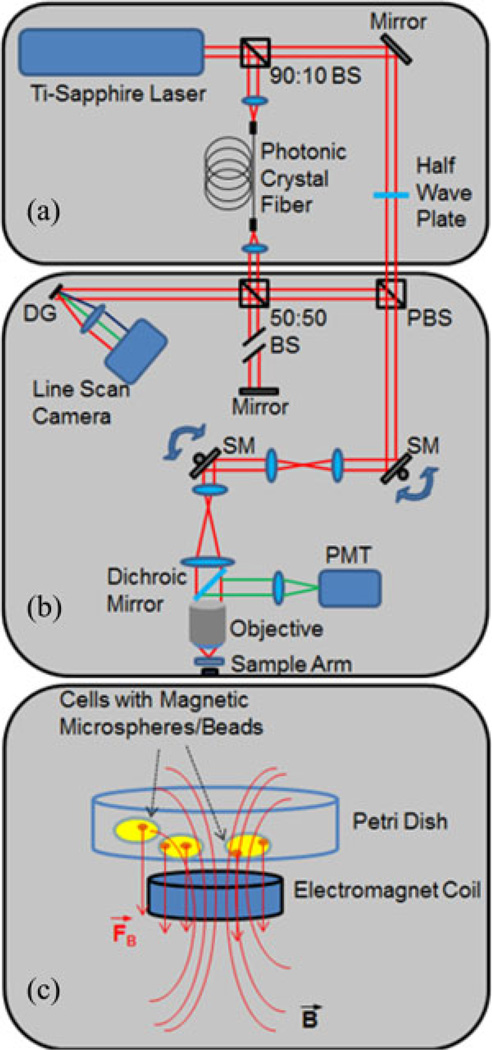
The imaging platform used for this experiment is an integrated optical coherence (OCM) and multiphoton microscope (MPM) [17]–[19]. OCM is a high resolution variation of optical coherence tomography (OCT) that uses a high numerical aperture (NA) beam to achieve high lateral spatial resolution. The high NA also restricts the depth-of-field, so images are typically acquired in an en face orientation, similar to confocal microscopy. Unlike a confocal microscope that relies only on spatial filtering, OCM produces optical sections of samples using coherence gating in addition to the confocal gating from the high NA. Images are based on optical scattering, which allows the microstructural features of cells or tissue to be visualized. MPM is a nonlinear imaging technique that can be used to excite two-photon fluorescence within the focal volume of a high NA beam. In this study, MPM was used to image multifunctional fluorescent and magnetic microspheres. The integrated OCM–MPM microscope allows simultaneous coregistered imaging with both modalities [20]. This allows the microspheres to be visualized and their location within the cells to be determined. For this study, a small, custom-fabricated magnetic solenoid was integrated below the sample plate to induce an alternating magnetomotive force on the magnetic beads in the cells. The magnetic field strength at the location of the sample was ~400 G, with a gradient of ~10 T/m. The modulation frequency of the coil was 5 Hz.
A schematic of the microscope is shown in Fig. 1(a). A dual spectrum laser source is implemented by splitting the output of a tunable Ti-sapphire laser into two beams, one for OCM and one for MPM. The details of this laser source have been previously described [17]. Briefly, the MPM beam is used directly for two-photon excited fluorescence, while the OCM beam is first coupled into a photonic crystal fiber (LMA-5, crystal fiber), where the spectrum is broadened through supercontinuum generation. The beams are recombined in the sample arm of the interferometer using a polarizing beam splitter. This laser source enables tuning of the center wavelength of the laser to optimally excite fluorescence in MPM while maintaining a broad spectrum for enhanced optical sectioning in OCM. The interference pattern between scattered light in the sample arm and the reference beam is detected by a linescan charge-coupled device camera operating at a linescan rate of 33 kHz. OCM processing consists of computational dispersion correction [18] and correction of coherence gate curvature [19] caused by scanning of the beam.
Fig. 1.
Schematic of integrated optical coherence and multiphoton microscope. (a) Dual-spectrum optical source. (b) Sample arm. The red beam lines represent light coming from the laser source as well as light backscattered from the sample, while the green beam lines represent the two photon-excited fluorescence. (c) Zoomed-in region showing the focused sample arm beam with the electromagnet coil, and the field lines generated at the culture of cells containing magnetic microbeads. Abbreviations: BS—beam splitter; DG—diffraction grating; PBS—polarizing beam splitter; SM—galvanometer scanning mirror).
A diagram of the sample arm is shown in Fig. 1(b). The dual spectrum laser beam passes through a pair of scanning galvanometers before entering a beam-expanding telescope. The beam is then focused by a 0.95 NA water immersion objective lens (XLUPLFL20XW, Olympus) onto the sample providing a transverse resolution of 2 µm. Fluorescence generated at the focal volume is reflected by a dichroic mirror and focused onto a PMT. Scattered light collected by the objective lens travels back along the beam path to the interferometer. The electromagnet situated below the sample is used to modulate the magnetic microspheres. Axial displacement of the particles and the cell are detected as phase shifts in the OCM signal, as a means for detecting the sample magnetomotive response. The phase sensitivity, determined from the standard deviation of the signal measured from a fixed mirror, was 290 mrad, corresponding to displacement sensitivity of 13 nm. The amplitude and phase of the oscillations relative to the driving waveform are determined by the local mechanical environment of the magnetic transducers.
III. Results
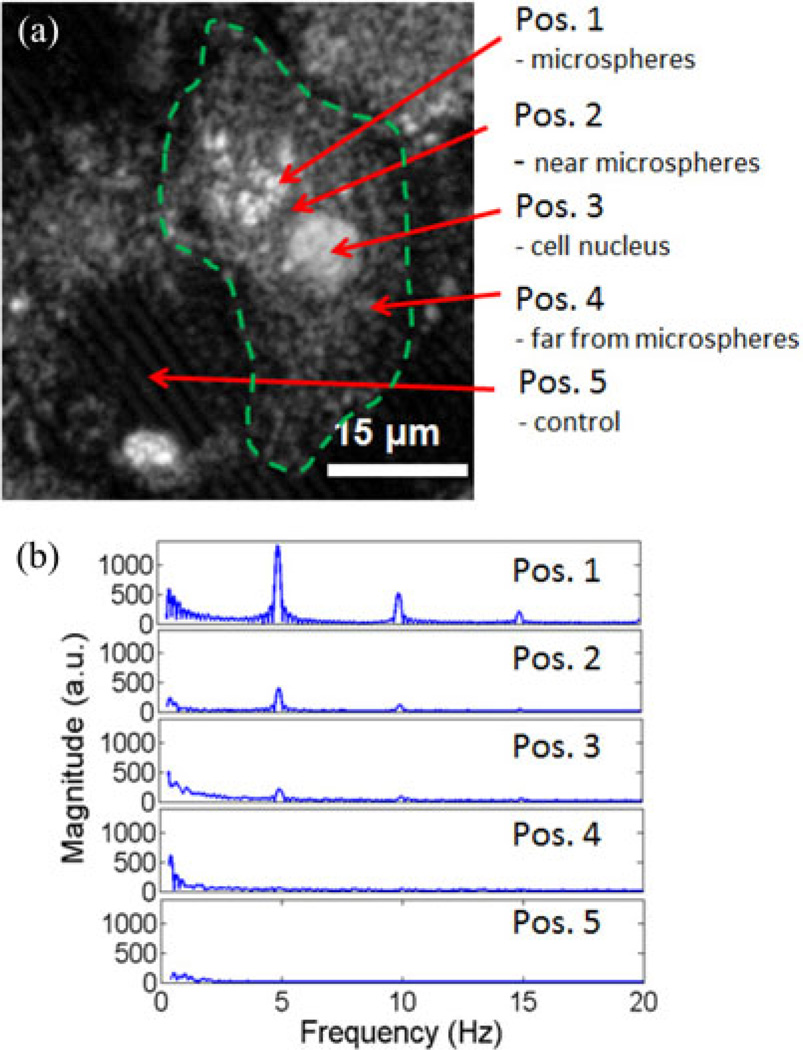
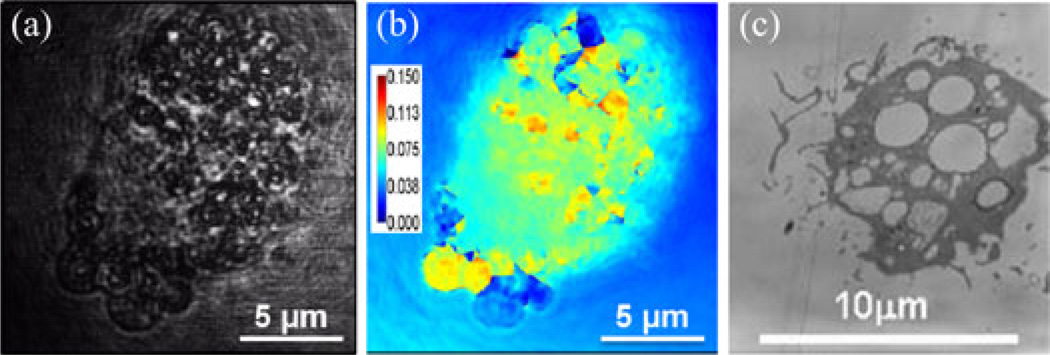
In the first set of experiments, mouse macrophages engulfed the magnetic microspheres that were produced in our lab. Fig. 2 shows an OCM image of a representative macrophage that has engulfed microspheres, clustered together at position (1), close to the cell nucleus, as indicated in the figure. The modulation frequency of the magnetic field was 5 Hz, and M-mode OCM data were acquired while the magnetic field was being modulated. The spectral analysis of the displacements measured at the cluster of microspheres, in their immediate vicinity, at the nucleus, away from the cluster of microspheres but still inside the cell, and outside the cell, shows that the signal is strongest at the location of the microspheres and decreases gradually as we probe locations further away from them. The phase amplitude data for positions (1), (2), and (3) were 10 rad, 5 rad, and 2 rad, respectively, corresponding to displacements of 448, 224, and 90 nm. Positions (4) and (5) did not show a response to the magnetic field. This is to be expected and confirms the fact that the microspheres are the source for the mechanical dynamics measured in and around the cell. The spectral data also show the presence of harmonics of the main 5-Hz mode, with a lower intensity compared to the former. The cell response decreases with increasing distance from the microspheres, and clearly indicates that the measured signal is localized and not a bulk sample response. Validation measurements taken with diffraction phase microscopy [21] and transmission electron microscopy (TEM), shown in Fig. 3, confirm that the microspheres were engulfed by the macrophages.
Fig. 2.
Phase-resolved MM-OCE from a single macrophage with phagocytosed microspheres. (a) OCM image of a single macrophage. Arrows indicate locations where M-mode magnetomotive measurements were made in and around the cell (dotted line approximates the contour of the cell). Protein-shell microspheres with a core suspension of magnetic nanoparticles in oil were custom-made in our lab for this experiment. (b) Frequency spectra of the cell displacement data show a response at the magnetic field modulation frequency, 5 Hz, as well as at weaker harmonic modes at 10 and 15 Hz. Positions correspond to those indicated in (a). The M-mode signal strength was strongest at the cluster of microspheres.
Fig. 3.
Validation of magnetomotive microspheres engulfed by macrophages. (a) Diffraction phase microscopy image of a macrophage exposed to magnetic microspheres, showing phagocytic inclusions of microspheres. (b) Spatial map of phase variance from a video-sequence of diffraction phase microscopy images collected during modulation of an applied magnetic field. Regions of high variance correspond to locations of microspheres. Color bar units are radians. (c) Transmission electron microscopy image of macrophages with engulfed microspheres.
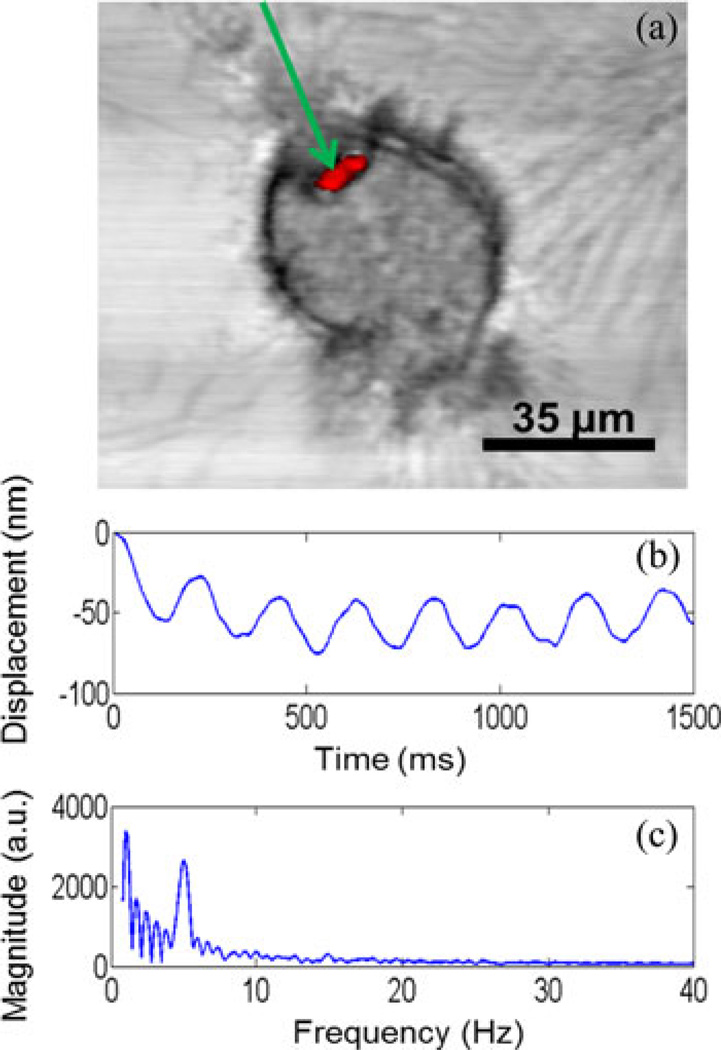
In a second experiment, mouse macrophages engulfed fluorescent magnetic microbeads (Bang Labs). Fig. 4 shows a fluorescence image of a cluster of microbeads overlaid on the OCM image of a macrophage containing the microbeads. These image data illustrate one advantage of this multimodal optical imaging system, where coregistered images can be used to identify where fluorescently labeled particles are spatially localized within cells that are structurally imaged with OCM. The dynamic nanometer-scale displacement of the microbeads and cell at the location corresponding to the microbeads is also shown in Fig. 4. The measured displacements are sinusoidal, with the same frequency as that of the magnetic field modulation, 5 Hz, as evident by the spectrum in Fig. 4.
Fig. 4.
Coregistered multimodal imaging and MM-OCE. (a) Simultaneously acquired and coregistered OCM/MPM images of a mouse macrophage that has phagocytosed two fluorescent microspheres (Bangs Labs). The OCM image data are shown in gray-scale, while the two-photon excited fluorescence MPM image data are shown in red. The location of the optical beam for recording MM-OCE displacements is indicated by the green arrow. (b) Plot of sinusoidal axial displacement of the microspheres as calculated from phase data. (c) Frequency spectrum of the magnetomotive signal obtained by taking the Fourier transform of the displacement signal during 5-Hz modulation by the external magnetic field.
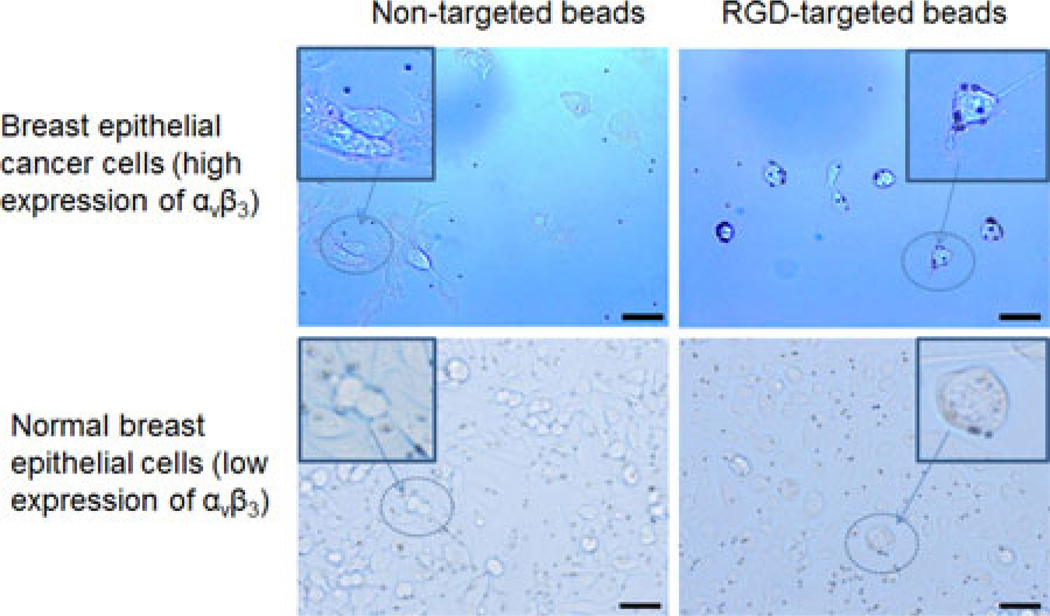
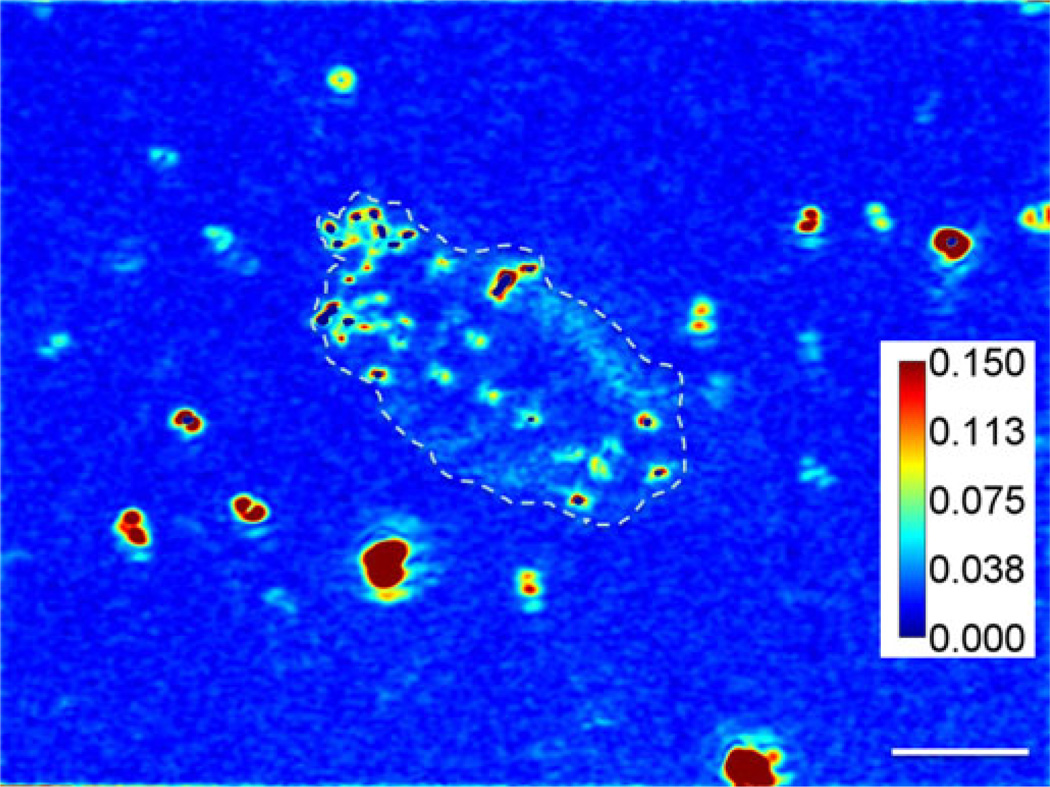
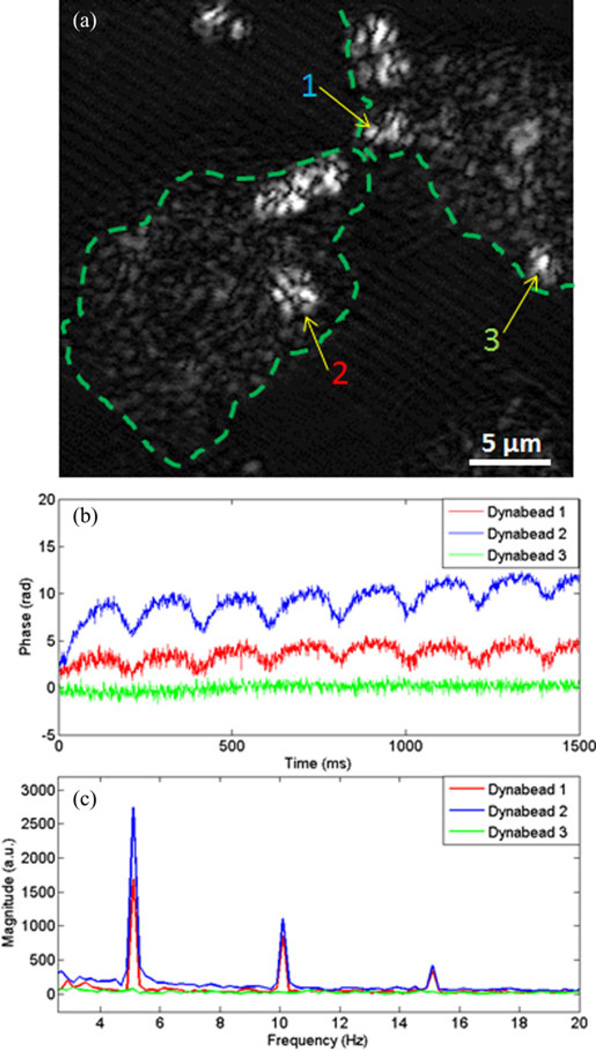
Lastly, cancer and normal human breast epithelial cells were probed with RGD-targeted and nontargeted magnetic Dynabeads. Fig. 5 shows bright field microscopy images of the four different combinations of cells and Dynabeads. It is evident that the targeted beads attach strongly to the cancer cells, and less so to the normal cells, while the nontargeted beads do not adhere in any predictable or preferential manner to either of the two cell lines. In these experiments, the magnetomotive signals were weaker than in previous experiments, with less than 20% of the Dynabeads that attached to cells responding to the magnetic field. Video data captured by instantaneous spatial light interference microscopy (iSLIM) [22], a quantitative phase imaging technique, of cancer cells with targeted Dynabeads show only two out of 16 Dynabeads in the field of view responding to the magnetic field modulation. A representative image of the signal standard deviation in time of the cell culture and Dynabeads is shown in Fig. 6. High values are registered at the location of the beads. No significant differences in magnetomotive signal amplitude or oscillation patterns were observed between the cancer cells with targeted Dynabeads and the normal cells with targeted Dynabeads, suggesting that the biomechanical properties of the targeted alpha-v-beta-3 integrin receptors are likely similar. The cell cultures containing nontargeted beads that attached nonspecifically to some of the cells also produced magnetomotive signals from a low percentage of cells. Fig. 7 is representative of some relative displacements and frequency spectra of the magnetomotive signals from three neighboring nontargeted Dynabeads attached to cancer cells. It is evident that bead number 3 does not produce a signal, while beads 1 and 2 do. These different displacement oscillation patterns, and in some cases, no displacement, highlight the observed variability.
Fig. 5.
Molecular targeting of Dynabeads for MM-OCE measurements. Bright field microscopy images of cell cultures (human breast epithelial and primary ductal carcinoma cells) that were incubated with targeted and nontargeted magnetic Dynabeads (dark point-like objects). The targeted beads show preferential attachment to the cancer cells that overexpress the alpha-v-beta-3 integrin receptor. Inset images are zoomed-in regions to highlight the spatial location the Dynabeads relative to the cells. Scale bar indicates 25 µm.
Fig. 6.
Validation of magnetic Dynabead response from cancer cells. Instantaneous spatial light interference microscopy (iSLIM) image of cancer cells with targeted Dynabeads show high standard deviations in time at the location of the Dynabeads. The data were taken using iSLIM with a 40×/0.75NA objective. The time lapse was taken at 13 Hz for 2 s, and then processed to map the standard deviation of each pixel. The red and blue colors indicate areas with high and low standard deviations of the optical path length, respectively. Scale bar indicates 30 µm. Color bar units are radians. Dotted line approximates the contour of the cell.
Fig. 7.
Magnetomotive response from cancer cells. (a) Representative OCM image of cancer cells (dotted line approximates cell contours) showing nontargeted Dynabeads (indicated by arrows) attached to the cells. M-mode magnetomotive signal data were taken at locations 1, 2, and 3. (b) Representative phase displacement responses of the cells at the locations of the Dynabeads indicated in part (a). The different amplitudes exemplify the variability of the signals from varying spatial positions on the cells. (c) The frequency spectra (via the Fourier transform) of the displacement signals plotted in (b) during 5-Hz modulation by the external magnetic field.
IV. Discussion
We have demonstrated a new real-time multimodal high-resolution imaging technique that combines OCM and MPM with magnetic actuation for dynamic nanoscale magnetomotive displacement measurements at the cellular level. Magnetic microparticles act as transducers by attaching to or being engulfed by cells, thus enabling probing of the molecular receptor or cellular mechanical environment. Microspheres and beads were chosen for this study because single or small aggregates of magnetic nanoparticles were found insufficient to generate a measurable magnetomotive signal, either because the magnetic force generated by our custom solenoid was too small to set a nanoparticle bound to the surrounding cellular medium in motion, or due to the fact that, even if the nanoparticle moved, its displacement would be too small to have a measurable effect.
We initially chose macrophages for the proof-of-principle demonstration of this technique due to their versatile function that ensures phagocytosis of magnetic microspheres and localization in the interior of the cell. This platform constitutes a reliable model with a well-understood mechanism of microparticle phagocytosis. When probed with an external magnetic field, the microspheres experience a force that sets them in motion, which engages the cellular architecture in a similar dynamic response. Our imaging system is capable of detecting this motion with nanometer-scale sensitivity, as shown in Fig. 3. Because the cyto-architecture and cellular membrane are elastic, we expected the largest displacements to be found at the location of the microspheres, and show gradually less displacement at points further away from the microsphere location. Our data confirmed this prediction.
Two-photon excited fluorescence imaging (see Fig. 4) enhances the structural imaging obtained with OCM by spatially locating the microparticles inside cells with high precision. These results also demonstrate the possibility for more sophisticated, versatile, probing of cell mechanics with an emphasis on certain functional groups, based on specialized dyes that may be employed to reveal specific organelles or processes inside a cell.
Our technique is also appropriate for the study of the mechanical responses of molecular membrane-bound receptors on cells, using magnetic agents that are functionalized and targeted to these external membrane receptors, such as the alpha-v-beta-3 integrin receptors found on normal human breast epithelial cells and overexpressed on the human breast cancer cells (see Fig. 5). However, the measured magnetomotive displacement signals from these experiments were found to be more variable compared to the macrophage experiments. This finding may be due to several different factors, such as the strength of the molecular bond between the RGD-functionalized magnetic Dynabeads and the outer membrane-expressed integrin receptors of the cell (compared to the phagocytosed microspheres residing inside the macrophages in the first set of experiments), the stiffness of the breast epithelial cell membrane, possibly higher than that of macrophage membrane, and the potential in homogeneity of the mechanical properties of the biological cell culture microenvironment. We note that the oscillatory displacement patterns observed for the samples of breast cells with the Dynabeads have a rectified profile, different from the sinusoidal-type of displacement measured from macrophages. The difference in the mechanism of binding between the microspheres or beads and the host cell may account for this. Further investigations are needed to elucidate the details of the physical biochemistry at play in these systems. Our technique, however, is a novel platform for these future studies.
The inherent variability of the biological samples results in variable signal strengths, a fact previously observed in similar cellular studies that employ magnetic tweezers [5], [6]. However, responsive microspheres are reliable in that repeated measurements under the same conditions render similar magnetomotive signals that are always modulated at the same frequency as the solenoid coil driving frequency. This demonstrates that our technique is robust and appropriate for studies that would focus on characterizing the biological variability of well-controlled systems, while taking full advantage of the high sensitivity to nanometer-scale displacements. These results will also lead to future research to determine the sources of the variability, which will greatly advance our understanding of cellular processes and support the further development of novel methodologies for manipulating and interrogating the biomechanical properties of molecules and cells.
V. Conclusion
Magnetic tweezers have become an established technique for measuring the mechanical properties of molecules and cells. However, this technique offers limited axial displacement resolution, hampering its use in many applications. The methodology of using phase-sensitive magnetomotive measurements in a multimodal microscope platform presented in this study has the potential for becoming a new paradigm for assessing molecular and cellular biomechanics. The high nanometer-scale sensitivity to axial displacements facilitates access to probing macromolecular bonds and could enable measurements of biomechanical properties at the cellular level. This novel approach for studying cellular processes and functions could offer new insight into how different mechanical processes, such as stretching of membranes or receptors that are attached to controllable magnetic beads, affect them. Rigorous modeling and simulations of cellular and extracellular microenvironments, coupled with statistically significant experimental studies using magnetomotive forces, are further needed to rigorously investigate and spatially map the biomechanical properties of single cells and their associated molecular receptors.
Acknowledgment
The authors thank Prof. K. Suslick for his assistance and expertise with microsphere fabrication. We also thank E. Chaney for biological support and D. Spillman for operations and information technology support.
This work was supported in part by the National Institutes of Health under grant R01 EB009073, S.A.B. and in part by the National Science Foundation under Grant CBET 10-33906, S.A.B.
Biographies
Vasilica Crecea was born in Piatra-Neamt, Romania, in 1979. She received the B.A. degree in physics from Bard College, Annandale-on-Hudson, NY, USA, in 2003, and the M.S. degree in physics from the University of Illinois at Urbana-Champaign, Urbana, IL, USA, in 2006, where she is currently working toward the Ph.D. degree in the Department of Physics.
Her current research interests include studying cell and tissue mechanics using magnetic particles in conjunction with optical imaging techniques.
Benedikt W. Graf (M’09)was born in Red Bank, NJ, USA, in 1985. He received the B.S., M.S., and Ph.D. degrees in electrical engineering from the University of Illinois at Urbana-Champaign, Urbana, IL, USA, in 2007, 2009, and 2012, respectively.
He was a Research Assistant in the Electro-Optics System Laboratory, the University of Illinois, from 2005 to 2007, and an Intern at HEP Prijenos, an electric power company in Croatia, in 2006. He received a Predoctoral Fellowship from the NIEHS Training Program in endocrine, developmental, and reproductive toxicology, the University of Illinois at Urbana-Champaign. He is a member of the Biophotonics Imaging Laboratory at Beckman Institute for Advanced Science and Technology. His research interests include the development of novel optical imaging techniques and image processing/analysis methods for clinical and research applications. Research applications include stem cell tracking in live skin and in vivo tracking of nanoparticles for studying mechanisms of transport and biodistribution.
Dr. Graf is a student member of the Optical Society of America. He received the E. C. Jordan Undergraduate Research Award from the University of Illinois, in 2007.
Taewoo Kim was born in Anyang, Korea in 1989. He received the B.S. degree in 2009 and M.S. degree in 2013, both in electrical and computer engineering from the University of Illinois at Urbana-Champaign, Urbana, IL, USA, where he is currently working toward his Ph.D. degree in electrical engineering.
He is the first author of three peer-reviewed journal articles including Optics Letters and Optics Express, and also a participating author of Handbook of Coherent-Domain Optical Methods: Biomedical Diagnostics (New York, NY, USA: Springer, 2013). His research interest includes biomedical imaging and microscopy, especially the development and applications of quantitative phase imaging instruments.
Gabriel Popescu received the B.S. and M.S. degrees in physics from the University of Bucharest, Bucharest, Romania, in 1995 and 1996, respectively, and the M.S. and Ph.D. degrees in optics from the School of Optics/CREOL (now the College of Optics and Photonics), University of Central Florida, Orlando, FL, USA, in 1999 and 2002, respectively.
He continued his training with the G. R. Harrison Spectroscopy Laboratory, Massachusetts Institute of Technology, Cambridge, MA, USA, as a Postdoctoral Associate. He joined the University of Illinois at Urbana-Champaign, Urbana, IL, USA, in August 2007, where he is an Assistant Professor in the Department of Electrical and Computer Engineering, and holds a full faculty appointment with the Beckman Institute for Advanced Science and Technology. He is also an affiliate faculty member in bioengineering.
Stephen A. Boppart (S’90–M’90–SM’06–F’11) was born in Harvard, IL, USA, in 1968. He received the B.S. degree in electrical and bioengineering and the M.S. degree in electrical engineering both from the University of Illinois at Urbana-Champaign, Urbana, IL, USA, in 1990 and 1991, respectively, the Ph.D. degree in electrical and medical engineering from the Massachusetts Institute of Technology, Cambridge, MA, USA, in 1998, and the M.D. degree from Harvard Medical School, Boston, MA, USA, in 2000.
He was a Research Scientist with the Air Force Laser Laboratory, Brooks Air Force Base, San Antonio, TX, USA, where he was engaged in research on developing national (ANSI) and Air Force laser safety standards. Since 2000, he has been with the University of Illinois at Urbana-Champaign from where he completed residency training in internal medicine in 2005. He is currently a Bliss Professor of Engineering in the Departments of Electrical and Computer Engineering, Bioengineering, and Medicine, and the Head of the Biophotonics Imaging Laboratory, Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, and also the Director of the Illinois Imaging Initiative. He has authored or coauthored more than 205 invited and contributed publications, and more than 470 invited and contributed presentations. He holds more than 30 patents, filed or pending. His research interests include the development of novel optical imaging technologies for biological and medical applications, with particular emphasis on translating these to clinical applications in cancer detection and diagnosis.
Dr. Boppart is a Fellow of the Optical Society of America and the International Society for Optical Engineering (SPIE). He is a member of the American Association for the Advancement of Science, the American Association for Cancer Research, and the American Medical Association. He was named one of the top 100 innovators in the world by the Technology Review Magazine for his research in medical technology, and received the IEEE Engineering in Medicine and Biology Society Early Career Achievement Award. He received the Paul F. Forman Engineering Excellence Award from the Optical Society of America for dedication and advancement in undergraduate research education, and recently, the international Hans Sigrist Prize for his work in laser medicine.
Footnotes
Color versions of one or more of the figures in this paper are available online at http://ieeexplore.ieee.org.
Contributor Information
Vasilica Crecea, Department of Physics, University of Illinois at Urbana-Champaign, Urbana, IL 61801 USA (crecea@illinois.edu).
Benedikt W. Graf, University of Illinois at Urbana-Champaign, Urbana, IL 61801 USA. He is now with NinePoint Medical, Cambridge, MA 02139 USA (bgraf@ninepointmedical.com).
Taewoo Kim, Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801 USA (tkim44@illinois.edu).
Gabriel Popescu, Departments of Electrical and Computer Engineering and Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801 USA (gpopescu@illinois.edu).
Stephen A. Boppart, Departments of Electrical and Computer Engineering, Bioengineering, and Internal Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61801 USA (boppart@illinois.edu).
References
- 1.Trepat X, Lenormanda G, Fredberga JJ. Universality in cell mechanics. Soft Matter. 2008;4:1750–1759. [Google Scholar]
- 2.Daniels B, Masi B, Wirtz D. Probing single-cell micromechanics in vivo: The microrheology of C. elegans developing embryos. Biophys. J. 2006;90:4712–4719. doi: 10.1529/biophysj.105.080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang X, et al. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys. J. 2010;99:2460–2469. doi: 10.1016/j.bpj.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert D, Nguyen T-H, Gallet F, Wilhelm C. In vivo determination of fluctuating forces during endosome trafficking using a combination of active and passive microrheology. PLoS ONE. 2010;5(no. 4):e10046. doi: 10.1371/journal.pone.0010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vlaminck I, Dekker C. Recent advances in magnetic tweezers. Annu. Rev. Biophys. 2012;41:453–472. doi: 10.1146/annurev-biophys-122311-100544. [DOI] [PubMed] [Google Scholar]
- 6.Ellerbee AK, McDowell EJ, Choma MA, Applegate BE, Izatt JA. Spectral domain phase microscopy for local measurements of cytoskeletal rheology in single cells. J. Biomed. Opt. 2007;12:044008. doi: 10.1117/1.2753755. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 8.Bausch AR, Möller W, Sackmann E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 1999;76(no. 1):573–579. doi: 10.1016/S0006-3495(99)77225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei C-W, Xia J, Pelivanov I, Jia C, Huang SW, Hu X, Gao X, O’Donnell M. Magnetomotive photoacoustic imaging: In vitro studies of magnetic trapping with simultaneous photoacoustic detection of rare circulating tumor cells. J. Biophoton. 2013;6(no. 6–7):513–522. doi: 10.1002/jbio.201200221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Wei C-W, Xia J, Pelivanov I, O’Donnell M, Gao X. Trapping and photoacoustic detection of CTCs at single cell per milliliter level with magneto-optical coupled nanoparticles. Small. 2013;9(no. 12):2046–2052. doi: 10.1002/smll.201202085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, Jia C, Huang S-W, O’Donnell M, Gao X. Multifunctional nanoparticles as coupled contrast agents. Nat. Commun. 2010;1(no. 41) doi: 10.1038/ncomms1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldenburg AL, Crecea V, Rinne SA, Boppart SA. Phase-resolved magnetomotive OCT for imaging nanomolar concentrations of magnetic nanoparticles in tissues. Opt. Exp. 2008;16(no. 15):11525–11539. [PMC free article] [PubMed] [Google Scholar]
- 13.Crecea V, Oldenburg AL, Liang X, Ralston TS, Boppart SA. Magnetomotive nanoparticle transducers for optical rheology of viscoelastic materials. Opt. Exp. 2009;17(no. 25):23114–23122. doi: 10.1364/OE.17.023114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John R, et al. In vivo magnetomotive optical molecular imaging using targeted magnetic nanoprobes. Proc. Nat. Acad. Sci. 2010;107:8085–8090. doi: 10.1073/pnas.0913679107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang X, et al. Magnetomotive optical coherence microscopy for cell dynamics and biomechanics. Proc. SPIE. 2011;7889:788926. [Google Scholar]
- 16.John R, et al. Targeted multifunctional multimodal protein-shell microspheres as cancer imaging contrast agents. Mol. Imag. Biol. 2011;14(no. 1):17–24. doi: 10.1007/s11307-011-0473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinegoni C, Ralston T, Tan W, Luo W, Marks DL, Boppart SA. Integrated structural and functional optical imaging combining spectral-domain optical coherence and multiphoton microscopy. Appl. Phys. Lett. 2006;88:053901. [Google Scholar]
- 18.Graf BW, Jiang Z, Tu H, Boppart SA. Dual-spectrum laser source based on fiber continuum generation for integrated optical coherence and multiphoton microscopy. J. Biomed. Opt. 2009;14:034019. doi: 10.1117/1.3147422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf BW, Adie SG, Boppart SA. Correction of coherence gate curvature in high numerical aperture optical coherence imaging. Opt. Lett. 2010;35:3120–3122. doi: 10.1364/OL.35.003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang X, Graf BW, Boppart SA. Multimodality microscopy for imaging three-dimensional engineered and natural tissues. J. Biophoton. 2009;2:643–655. doi: 10.1002/jbio.200910048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popescu G, Ikeda T, Dasari RR, Feld MS. Diffraction phase microscopy for quantifying cell structure and dynamics. Opt. Lett. 2006;31:775–777. doi: 10.1364/ol.31.000775. [DOI] [PubMed] [Google Scholar]
- 22.Ding H, Popescu G. Instantaneous spatial light interference microscopy (iSLIM) Opt. Exp. 2010;18:1569. doi: 10.1364/OE.18.001569. [DOI] [PubMed] [Google Scholar]