CT perfusion parameter values are affected by the duration of acquisition of data.
Abstract
Purpose
To assess the effects of acquisition duration on computed tomographic (CT) perfusion parameter values in neuroendocrine liver metastases and normal liver tissue.
Materials and Methods
This retrospective study was institutional review board approved, with waiver of informed consent. CT perfusion studies in 16 patients (median age, 57.5 years; range, 42.0–69.7 years), including six men (median, 54.1 years; range, 42.0–69.7), and 10 women (median, 59.3 years; range 43.6–66.3), with neuroendocrine liver metastases were analyzed by means of distributed parametric modeling to determine tissue blood flow, blood volume, mean transit time, permeability, and hepatic arterial fraction for tumors and normal liver tissue. Analyses were undertaken with acquisition time of 12–590 seconds. Nonparameteric regression analyses were used to evaluate the functional relationships between CT perfusion parameters and acquisition duration. Evidence for time invariance was evaluated for each parameter at multiple time points by inferring the fitted derivative to assess its proximity to zero as a function of acquisition time by using equivalence tests with three levels of confidence (20%, 70%, and 90%).
Results
CT perfusion parameter values varied, approaching stable values with increasing acquisition duration. Acquisition duration greater than 160 seconds was required to obtain at least low confidence stability in any of the CT perfusion parameters. At 160 seconds of acquisition, all five CT perfusion parameters stabilized with low confidence in tumor and normal tissues, with the exception of hepatic arterial fraction in tumors. After 220 seconds of acquisition, there was stabilization with moderate confidence for blood flow, blood volume, and hepatic arterial fraction in tumors and normal tissue, and for mean transit time in tumors; however, permeability values did not satisfy the moderate stabilization criteria in both tumors and normal tissue until 360 seconds of acquisition. Blood flow, mean transit time, permeability, and hepatic arterial fraction were significantly different between tumor and normal tissue at 360 seconds (P < .001).
Conclusion
CT perfusion parameter values are affected by acquisition duration and approach progressively stable values with increasing acquisition times.
© RSNA, 2013
Introduction
Computed tomography (CT) perfusion is evolving as a clinically useful technique in oncology, with potential to assist in treatment monitoring, prognostication, and pathophysiologic understanding (1–4). A variety of perfusion parameters can be derived from CT perfusion studies, depending on the particular physiologic model that is used to describe the behavior of tissue perfusion. One such approach is based on an adiabatic approximation of the distributed parameter model, which models the distribution of contrast material in tissue. The approach is based on deconvolution of tissue and vascular (arterial) input time-attenuation curves obtained from continuous CT data during intravenous administration of contrast medium and results in estimates of blood flow, blood volume, mean transit time (MTT) and permeability-surface area product (PS) for a given tissue region of interest (ROI) (5).
The technique has been applied in several types of tumors, including those of the prostate (6), colon and rectum (7–12), head and neck (13–15), lung (16–18), and liver (19–22), and also in normal tissue for control purposes and in diffuse parenchymal diseases (eg, cirrhosis). A variety of imaging protocols have been used, and in particular, the acquisition durations in these studies have varied from 30 to 480 seconds.
There is clear motivation to reduce the overall duration of CT acquisition to the shortest time possible to reduce radiation exposure without compromising quantification of the CT perfusion parameter values. There have been relatively few studies that have systematically assessed the effects of acquisition duration on CT parameter values by using deconvolution modeling (8,12,23,24). These studies have suggested that acquisition times of 30–60 seconds might be satisfactory for some of the CT perfusion parameters.
Our purpose was to assess the effects of acquisition time on CT perfusion parameter values in neuroendocrine liver metastases and normal liver tissue.
Materials and Methods
Patients
This retrospective study was approved by our institutional review board with waiver of informed consent and was Health Insurance Portability and Accountability Act compliant. The two prospective clinical trials that provided the patients for this study also had been approved by the institutional review board, and written informed consent had been obtained from all patients. These studies included patients with metastatic neuroendocrine tumors who were treated with bevacizumab, a vascular endothelial growth factor inhibitor; everolimus, a mammalian target of rapamycin inhibitor; or pazopanib, a vascular endothelial growth factor receptor inhibitor, between April 2007 and September 2009, and in whom CT perfusion was an optional study. Novartis and Genentech provided partial funding support and drugs used for the study, everolimus and bevacizumab, respectively. One of the authors (A.G.C.) is employed by General Electric; however, the other authors had full control of inclusion of data and information submitted for publication.
Our study focused on patients who underwent CT perfusion for a target lesion in the liver that was clinically or radiologically determined to be malignant on the basis of biopsy of other lesions, widespread metastatic disease, and/or increase in the size of the lesion. Other inclusion criteria included (a) patient age older than 18 years, (b) Eastern Cooperative Oncology Group performance status score of 0–2 (1), (c) serum creatinine concentration of less than 1.5 mg per deciliter, and absence of contraindications to CT (eg, severe allergy to contrast medium or pregnancy). A single target lesion, required to be a well-demarcated, contrast-enhanced solid mass larger than 2.5 cm in longest diameter, had been identified by a radiologist (C.S.N., with more than 10 years of experience in interpreting CT studies) at review of previous imaging studies in each patient.
CT Perfusion Scanning Technique
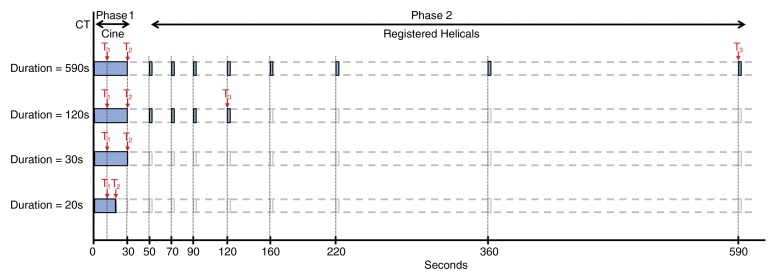
CT perfusion images were obtained with a 64-row multidetector CT scanner (VCT; GE Healthcare, Waukesha, Wis), and were acquired in two phases. Phase 1 was a cine acquisition during a breath hold, and phase 2 consisted of eight intermittent short breath-hold helical scans. The phase 1 and phase 2 scanning approach outlined in Figure 1 is similar to that previously described (18,25). Further details appear in Appendix E1 (online).
Figure 1:
Schematic of overall study evaluating effects of acquisition duration and pre-enhancement set points. First row is reference dataset: Phase 1 cine and eight anatomically registered phase 2 images. T1 = pre-enhancement set point; T2 = last first phase (postenhancement) set point; T3 = last second phase set point. Second row shows reduction in acquisition duration affecting phase 2 (T3). Third row shows reduction in acquisition duration: T2 = 30 seconds. Fourth row: Reduction in acquisition duration affecting phase 1 (T2).
CT Perfusion Analyses
Before the CT perfusion analyses were undertaken, the phase 2 images of each patient dataset were anatomically registered with the phase 1 images. To have the most robust data for our analysis, we limited our datasets to those in which there was negligible motion in the phase 1 images. This step removed the need to register the phase 1 images anatomically. Of the 90 patients enrolled in the two clinical trials, 47 underwent CT perfusion studies of metastases in the liver, of which 16 met the phase 1 requirements. The median age of the patients was 57.5 years (range, 42.0–69.7 years), and included six men (median, 54.1 years; range, 42.0–69.7), and 10 women (median, 59.3 years; range, 43.6–66.3). Semiautomated rigid registration of phase 2 images to those of phase 1 was undertaken in a manner similar to that previously described (18,26). This resulted in CT perfusion datasets for each patient that consisted of 59 eight-section cine images temporarily sampled at 0.5 second from the phase 1 acquisition, together with eight anatomically matched eight-section images from the phase 2 acquisition. These formed the reference dataset for subsequent analyses.
The reference datasets were analyzed by using commercially available CT perfusion software (CT Perfusion 4, version 4.3.1, Advantage Windows 4.4; GE Healthcare). We used the liver protocol of the vendor software, which uses a dual vascular input algorithm. Circular or oval ROIs were placed in the abdominal aorta and in the portal vein on the source images to provide these vascular inputs (C.S.N.) (Fig 2a, 2b). Three set points were then determined: a pre-enhancement set point (T1), which corresponded to the time when the arterial signal first began to rise; a postenhancement set point (T2), which corresponded to the final time point of the phase 1 data acquisition; and the last second phase set point (T3), which corresponded to the final phase 2 image (Figs 1, 2a).
Figure 2a:
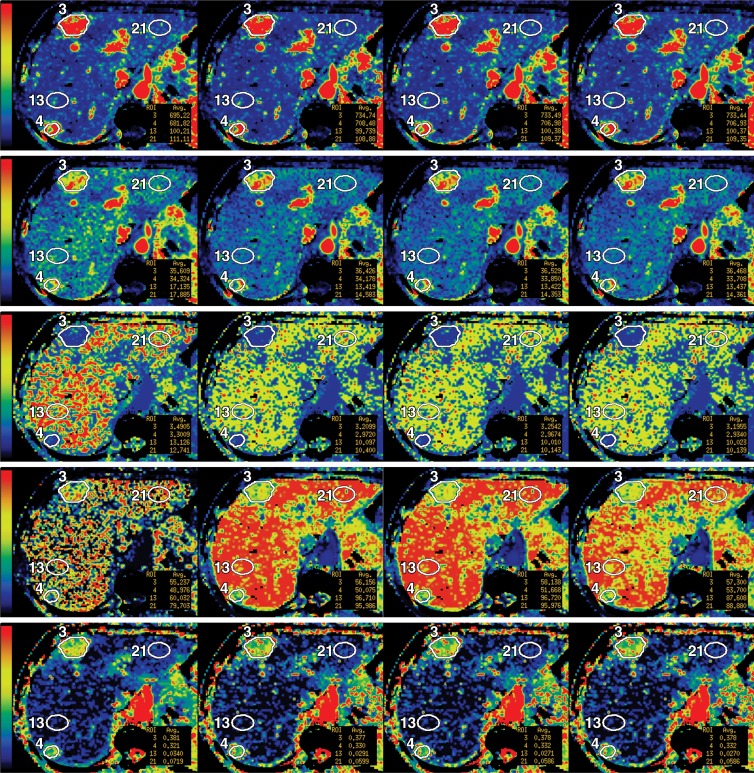
(a) Time-attenuation curves and (b) reference CT perfusion image in a 61-year-old woman with metastases to liver from neuroendocrine tumor with corresponding ROIs: aortic (light blue) and portal vein (dark blue), tumors (purple), and normal liver tissue (green). (c) Parametric maps for blood flow (first row), blood volume (second row), MTT (third row), PS (fourth row), and HAF (fifth row), with illustrative examples at acquisition durations of 30, 160, 220, and 590 seconds (left to right columns, respectively). Blood flow is expressed in mL/min per 100 g; blood volume, in mL/100 g; MTT, in seconds; and PS, in mL/min per 100 g. The color scales are identical for each row of parametric maps.
Figure 2b:
(a) Time-attenuation curves and (b) reference CT perfusion image in a 61-year-old woman with metastases to liver from neuroendocrine tumor with corresponding ROIs: aortic (light blue) and portal vein (dark blue), tumors (purple), and normal liver tissue (green). (c) Parametric maps for blood flow (first row), blood volume (second row), MTT (third row), PS (fourth row), and HAF (fifth row), with illustrative examples at acquisition durations of 30, 160, 220, and 590 seconds (left to right columns, respectively). Blood flow is expressed in mL/min per 100 g; blood volume, in mL/100 g; MTT, in seconds; and PS, in mL/min per 100 g. The color scales are identical for each row of parametric maps.
Figure 2c:
(a) Time-attenuation curves and (b) reference CT perfusion image in a 61-year-old woman with metastases to liver from neuroendocrine tumor with corresponding ROIs: aortic (light blue) and portal vein (dark blue), tumors (purple), and normal liver tissue (green). (c) Parametric maps for blood flow (first row), blood volume (second row), MTT (third row), PS (fourth row), and HAF (fifth row), with illustrative examples at acquisition durations of 30, 160, 220, and 590 seconds (left to right columns, respectively). Blood flow is expressed in mL/min per 100 g; blood volume, in mL/100 g; MTT, in seconds; and PS, in mL/min per 100 g. The color scales are identical for each row of parametric maps.
For each of the eight axial section locations of each dataset, a liver tumor ROI was drawn freehand around the periphery of the primary target lesion by using an electronic cursor and mouse, with reference to the source cine CT images and perfusion parametric maps, displaying the images at soft tissue windows (width = 350 HU, level = 40 HU). Wherever possible, a second tumor ROI was delineated, provided it was greater than 1.5 cm in diameter (E.F.A. and D.H.H. in consensus, each with more than 15 years of experience in CT perfusion analyses) (Fig 2).
Similar analyses were undertaken for normal liver parenchyma on associated CT sections. Circular or oval ROIs were delineated in normal liver regions, which were determined after review of the current perfusion images and before and after staging scans; these ROIs were as large as possible and placed to avoid vessels and artifacts. We delineated two normal liver ROIs on each of the eight sections; and wherever possible, separate ROIs were placed in the left and right liver lobes (C.S.N.).
Average tumor blood flow, blood volume, MTT, PS, and HAF values were obtained from each tumor ROI drawn on each CT level. The mean values throughout all CT levels in which each tumor ROI was drawn were computed. Similar analyses were undertaken for each normal liver ROI. All ROIs were saved in the software to enable identical placement in all the subsequent analyses.
Acquisition duration.—The effect of acquisition duration on CT perfusion parameter values was explored by repeating the analyses with systematic reductions in the postenhancement set points between 590 and 12 seconds by means of sequential reductions of T3 at the discrete time points in phase 2, and then by means of reductions of T2 in 2-second decrements in phase 1. All the datasets were analyzed in the CT perfusion software by using the same arterial, portal venous, and tissue ROIs as used in the corresponding reference analyses.
There were 25 separate tumor ROIs: Nine patients had two, and seven patients had one ROI. There were 30 separate normal liver tissue ROIs: 12 patients had one ROI in the right lobe and one in the left lobe; three patients had two ROIs in the right lobe (which were averaged); and one patient did not have delineable normal tissue, resulting in 27 lobe-specific normal liver ROIs.
The median maximum axial diameter of the liver metastases was 5.1 cm (range, 1.7–16.0 cm), with median cross-sectional area of tumor ROIs of 1525 mm2 (range, 50–16000 mm2). The median cross-sectional area of normal liver ROIs was 460 mm2 (range, 100–1300 mm2), aortic ROI was 55 mm2 (range, 20–95 mm2), and portal vein ROIs were 45 mm2 (range, 15–75 mm2).
There were a total of 288 data points for 16 patients and 18 time-point combinations. The CT perfusion algorithm did not compute parameter values for 17 data points, all of which occurred with T2 time points less than 14 seconds (ie, very short acquisition durations). An average of 17 repeated time-point measurements per patient were analyzed.
Statistical Analysis
We used nonparameteric regression to evaluate CT perfusion parameters for evidence of time-invariance as functions of acquisition duration during a period of 12–590 seconds by inferring the fitted derivatives to assess their proximity to zero as a function of acquisition time by using an equivalence testing framework. Stability criteria were evaluated at three levels of confidence (low, moderate, and high, corresponding to 20%, 70%, and 90% confidence intervals, respectively). Larger confidence levels corresponded to more stringent criteria for stabilization, requiring more evidence of time invariance. A linear mixed model was used to compare CT perfusion parameter values acquired at 360 seconds between tumor and normal tissue, and a P value less than .05 indicated a significant difference. Further details appear in Appendix E2 (online).
Results
Acquisition Duration and Stabilization
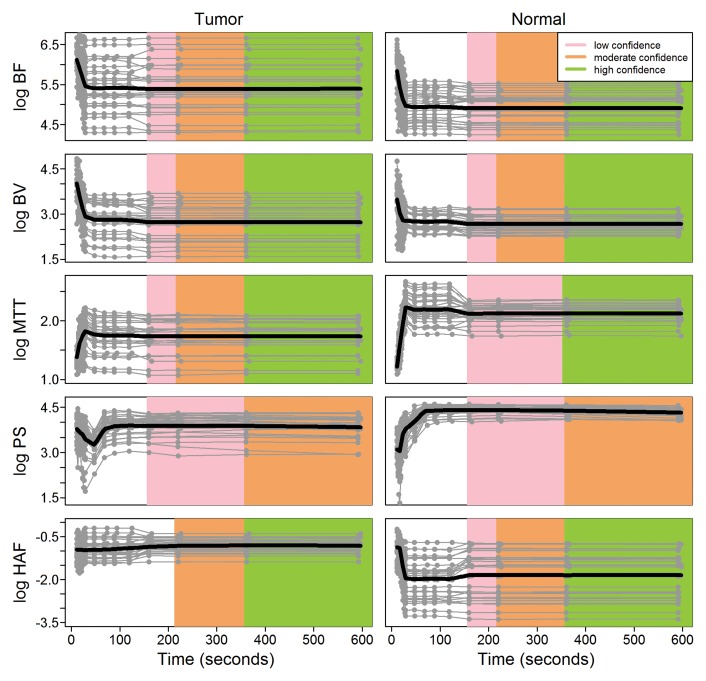
Discrete acquisition durations are summarized in Table 1. The observed evidence did not satisfy our minimum stability criteria (ie, 20% confidence) for acquisition durations up to 160 seconds for any of the CT perfusion parameters. For many of the parameters, there were substantial changes in CT perfusion parameter values in the initial 90 seconds of acquisition (Fig 3). At 160 seconds of acquisition, all five CT perfusion parameters stabilized with low confidence in tumor and normal tissue, with the exception of HAF in tumors (Table 2). After 220 seconds of acquisition, there was stabilization with moderate confidence for blood flow, blood volume, and HAF in tumors and in normal tissue,and for MTT in tumors; however, PS did not satisfy the moderate stabilization criteria in both tumor and normaltissue until 360 seconds. After 360 seconds of acquisition, four of the five CT perfusion parameters, blood flow, blood volume, MTT, and HAF, stabilized with high confidence, for both tumors and normal tissue. In comparison, PS stabilized with moderate confidence, and did not attain high confidence at our full acquisition duration of 590 seconds. Detailed scatterplots for PS are presented in Figure 4. The estimated derivatives for each of the CT perfusion parameters as functions of acquisition duration are presented in Figure 5.
Table 1.
Summary of CT Perfusion Values
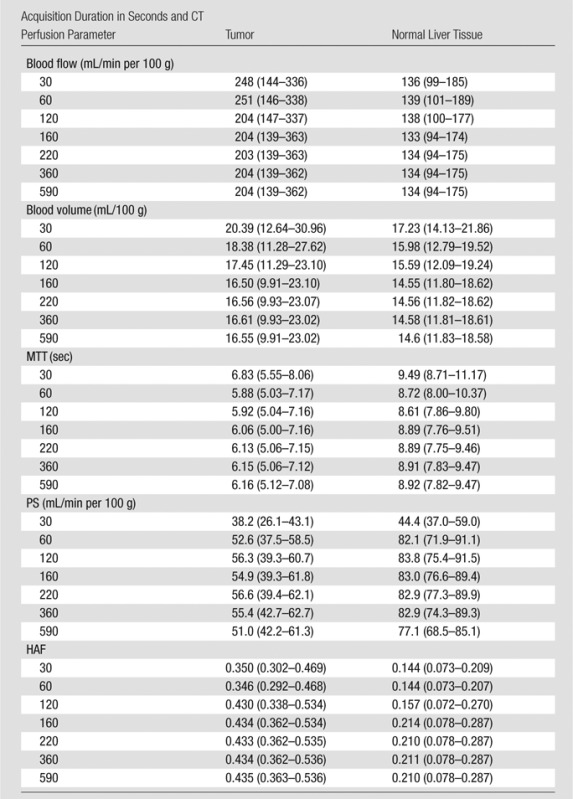
Note.—Unless otherwise indicated, data are medians, with interquartile range in parentheses.
Figure 3:
Scatterplots of CT perfusion parameter values on the natural logarithm (log) scale as functions of acquisition time for liver tumor (left column) and normal liver (right column) tissues. Gray lines are used to connect the observed repeated measurements from the same ROI in a patient. Nonparameteric regression fits are represented with black lines. Shaded regions show inferred levels of confidence for stabilization (a steady state) as functions of acquisition duration for three levels of confidence, low (20%), moderate (70%), and high (90%), as in Table 2.
Table 2.
Confidence Level and Acquisition Duration for CT Perfusion Parameters

Note.—PS did not stabilize with high confidence during the available 590 seconds of acquisition.
Figure 4:
Detailed scatterplots of PS on natural logarithm (log) scale as functions of acquisition duration for both tumor (left plot) and normal tissue (right plot). Plots illustrate relative absence of steady states or stabilization in duration of acquired data, as shown by drift in lines observed at later acquisition times. Gray lines are used to connect observed repeated measurements from same ROI in a patient. The nonparameteric regression fits are represented with black lines. Shaded regions are inferred levels of confidence for stabilization as functions of acquisition duration, as in Table 2.
Figure 5:
Graphs show estimated derivatives for five CT perfusion parameters as functions of acquisition duration scaled by their respective estimated residual error standard deviations. Shaded regions are intervals of likely values for three levels of confidence: low (20%), moderate (70%), and high (90%). Stabilization requires that these confidence intervals be bounded in equivalence region for all subsequent acquisition times. The gray horizontal lines characterize equivalence region used in stability inference. Drift for PS is illustrated by the negative shift after 360 seconds. Variability bounds observed for PS at high confidence level extend beyond lower bound of equivalence region, precluding stabilization in both tumor and normal liver tissue.
Tumor versus Normal Liver Tissue
CT perfusion values from tumor and normal liver sites were compared after 360 seconds of acquisition (Table 3); for four parameters (blood flow, MTT, PS, and HAF), the mean CT perfusion values obtained in tumor and normal liver tissue were significantly different (P = .002).
Table 3.
Tumor CT Perfusion Parameters for Tumor and Normal Liver Tissue
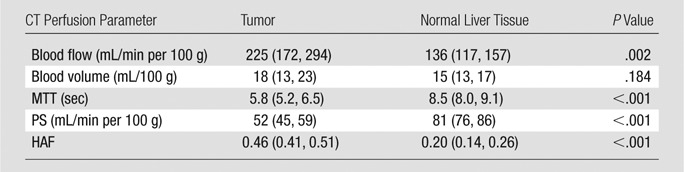
Note.—Data are means, with 95% confidence intervals in parentheses. Values were acquired after 360 seconds.
Discussion
Our results indicated that CT perfusion parameter values were affected by the acquisition duration of data available for analysis. With increasing acquisition durations, blood flow, blood volume, MTT, and HAF for both tumor and normal liver tissue approached steady-state or stable values. This was demonstrated by means of the CT perfusion parameter plots as functions of acquisition duration, which approached horizontal plateaus; and by the approach toward zero of the corresponding derivative plots with increasing acquisition times, which are further noted to do so with increasingly tighter confidence bounds.
Determining a suitable acquisition duration that might be suggested or used is a matter of defining a required level of confidence for the resultant values. Our results indicated that, with acquisition durations of 220 seconds, parameter values for blood flow, blood volume, and HAF of tumors and of normal liver tissue, and MTT of tumors, can be estimated with moderate (70%) confidence. This acquisition duration, however, is not sufficient for estimation of PS in either tumor or normal tissue, or of MTT in normal liver tissue, with the same moderate confidence. After 360 seconds, moderate or high (90%) confidence was achieved for the estimation of all five CT perfusion parameter values. This longer acquisition duration of 360 seconds allowed high-confidence estimation of all CT perfusion parameters except for PS.
Although four of the five CT perfusion parameters achieved stabilization of values with high confidence at 360 seconds of data acquisition, PS did not do so at the full 590 seconds of our acquired data. PS requires longer durations of data acquisition than the other CT perfusion parameters for appropriate characterization (3). Our data provide some indication of adequate acquisition times to achieve a moderate level of confidence. It is possible that PS attains stabilization with greater levels of confidence beyond 10 minutes, for which further studies would be required to determine when, or indeed if, this occurs.
Computation of the various perfusion parameter values is derived from the deconvolution of the tissue and vascular input functions, and the resultant impulse residue function. The functional relationships are mathematically complex and nonlinear, with no requirement that parameters or their associated functional forms attain stability at the same time.
There have been relatively few studies examining the effects of acquisition duration on CT perfusion parameter values by using the same underlying physiologic model as in our study (ie, the distributed parameter model). Studies in the brain have suggested that acquisition durations can substantially affect CT perfusion parameter values and that durations of 48 or 60 seconds might suffice (23,24,27). In relation to body tumors, Goh et al (8) reported that acquisition duration of 45 seconds was satisfactory for blood flow, blood volume, and MTT in colorectal tumors. Kambadakone et al (12) reported that acquisition duration of 30 seconds issatisfactory for blood flow and MTT (but not blood volume) in rectal tumors and retroperitoneal sarcomas. Neither study included comment on a suitable acquisition duration for PS.
The conclusions of these studies were derived by using statistical analyses that might be considered limited. For example, Goh et al (8) and Kambadakone et al (12) consider pairwise comparisons (t tests) among three and five discrete time points, respectively; stabilization was inferred from the failure to observe significant differences (P > .05) between values for various acquisition durations. In our study, we used the entirety of the observed data to estimate the underlying functional relationship between CT perfusion quantification and acquisition time.
Many previous CT perfusion studies, particularly those involving body tissues and tumors, have used acquisition durations of less than 65 seconds (7,9–11,13–17,19,21,22,28). Although these studies did not all include the specific organ and tissues we used, some of these studies may not have characterized the CT perfusion parameters adequately.
There have been some suggestions that an acquisition duration of 2 minutes should suffice for evaluation of tissue perfusion (1,3). Our analysis indicates that sufficient acquisition duration is largely dictated by the level of confidence required from the resultant CT perfusion parameter values, with a requirement for at least 160 seconds to provide a low level of confidence for most CT perfusion parameters (at least for liver analyses for neuroendocrine metastates). If at least moderate confidence were required in the stabilization of all CT perfusion parameters, then our analysis suggests that acquisition durations of at least 220–360 seconds are necessary.
Both tumors and normal liver tissue had broadly similar patterns of behavior with respect to CT perfusion values and acquisition durations. CT perfusion values approached stabilization with increasing acquisition duration and achieved our confidence thresholds at broadly similar times.
There was a noticeably wider range of blood flow, blood volume, MTT, and PS values among the tumor group compared with those of normal tissue, and a narrower range of HAF. The wider range of values for four of the five CT perfusion parameters is probably a reflection of the greater heterogeneity in perfusion among tumors, even in those derived from a single histologic type, than among normal liver tissue. Values for both blood flow and HAF were significantly higher for tumors than those for normal tissue; the opposite relationship was observed for MTT and PS. The higher values for blood flow and HAF confirmed the well-documented relatively increased arterial blood supply of tumors compared with that of normal liver tissue (1).
Although our study was not (and was not intended to be) a validation study, our blood flow and HAF values for normal livers (134 mL/min per 100 g and 0.21, respectively) were in good agreement with values in the literature. Validation studies have indicated normal liver blood flow to be in the range of 52.8–122.6 mL/min per 100 g (29), and normal liver has a well-documented HAF of 0.2.
We recognize several limitations in our study. We had a relatively small number of patients. Our study was limited only to the liver and metastases in the liver from a specific tumor. The extent to which our conclusions might be generalizable to other tissues and tumors requires further work. Our data acquisition period was nearly 10 minutes, but it appeared to be insufficient to attain stabilization in PS with high confidence. Further studies extending beyond 10 minutes are required to characterize and define the behavior of PS with acquisition time.
Our data had a specific acquisition protocol based on relatively high temporal sampling initially (in the first 30 seconds), and more sparsely sampled data in its second phase (590 seconds). An ideal dataset might be one that was acquired at a high temporal sampling in the second phase as well. However, obtaining such data would require high overall radiation exposures and has practical difficulties because of the constraints of breathing motion and registration. Finally, different versions of CT perfusion software may generate different results (30). In our analysis, we used a more recent version of the software (CT Perfusion 4, GE Healthcare) than did authors of previous studies, who used CT Perfusion 3 or older (8,12,24,27).
In summary, CT perfusion parameter values are affected by the duration of acquisition of data. In our particular setting, an acquisition duration of 360 seconds yielded stable blood flow, blood volume, MTT, and HAF CT perfusion values with high confidence, and PS values with moderate confidence. Further work is required to determine the generalizability of our results to other tissues and acquisition protocols, including contrast injection volumes and rates, and other analysis software.
Advances in Knowledge
■ CT perfusion parameter values approach steady-state or stable values with increasing acquisition time; at 160 seconds, all five CT perfusion parameters stabilized with low confidence in tumor and normal tissue, with the exception of hepatic arterial fraction in tumors.
■ After 220 seconds of acquisition, there was stabilization with moderate confidence for blood flow, blood volume, and hepatic arterial fraction in tumors and in normal tissue, and for mean transit time in tumors; however, permeability surface area product did not satisfy the criteria for moderate stabilization in both tumor and normal tissue until 360 seconds.
■ Permeability surface area product values require longer acquisition times than do blood flow, blood volume, mean transit time, and hepatic arterial fraction in the liver to reach comparable levels of stabilization in CT perfusion values (eg, 360 seconds versus 220 seconds for moderate and 220 seconds versus 160 seconds for low confidence).
Implication for Patient Care
■ Determining appropriate acquisition duration depends on the degree of confidence required of the resultant CT perfusion values, which can vary according to the specific CT perfusion parameter of interest.
APPENDIX
Received December 8, 2012; revision requested January 16, 2013; revision received February 25; accepted March 18; final version accepted April 11.
Funding: This research was supported by the National Institutes of Health (grant P30 CA016672).
Partial funding support provided by Novartis and Genentech.
Current address: Department of CT Research, GE Healthcare, Waukesha, Wis.
Disclosures of Conflicts of Interest: C.S.N. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: grants/grants pending from GE Healthcare. Other relationships: none to disclose. B.P.H. No relevant conflicts of interest to disclose. A.G.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: employee of GE Healthcare. Other relationships: none to disclose. E.F.A. No relevant conflicts of interest to disclose. D.H.H. No relevant conflicts of interest to disclose. C.C. Financial activities related to the present article: Consulting fee or honorarium from Novartis Pharmaceuticals. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. J.Y. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Consultancy for Novartis, grants/grants pending from Genentech. Other relationships: none to disclose.
Abbreviations:
- HAF
- hepatic arterial fraction
- MTT
- mean transit time
- PS
- permeability-surface area product
- ROI
- region of interest
References
- 1.Miles KA, Charnsangavej C, Lee FT, Fishman EK, Horton K, Lee TY. Application of CT in the investigation of angiogenesis in oncology. Acad Radiol 2000;7(10):840–850. [DOI] [PubMed] [Google Scholar]
- 2.Miles KA. Functional computed tomography in oncology. Eur J Cancer 2002;38(16):2079–2084. [DOI] [PubMed] [Google Scholar]
- 3.Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 2003;76(Spec No 1):S36–S42. [DOI] [PubMed] [Google Scholar]
- 4.Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am 2009;47(1):161–178. [DOI] [PubMed] [Google Scholar]
- 5.Lee TY. Functional CT: physiological models. Trends Biotechnol 2002;20(8):S3–S10. [Google Scholar]
- 6.Henderson E, Milosevic MF, Haider MA, Yeung IW. Functional CT imaging of prostate cancer. Phys Med Biol 2003;48(18): 3085–3100. [DOI] [PubMed] [Google Scholar]
- 7.Goh V, Halligan S, Hugill JA, Bassett P, Bartram CI. Quantitative assessment of colorectal cancer perfusion using MDCT: inter- and intraobserver agreement. AJR Am J Roentgenol 2005;185(1):225–231. [DOI] [PubMed] [Google Scholar]
- 8.Goh V, Halligan S, Hugill JA, Gartner L, Bartram CI. Quantitative colorectal cancer perfusion measurement using dynamic contrast-enhanced multidetector-row computed tomography: effect of acquisition time and implications for protocols. J Comput Assist Tomogr 2005;29(1):59–63. [DOI] [PubMed] [Google Scholar]
- 9.Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT—do tumor perfusion measurements reflect angiogenesis? Radiology 2008;249(2):510–517. [DOI] [PubMed] [Google Scholar]
- 10.Sahani DV, Kalva SP, Hamberg LM, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 2005;234(3):785–792. [DOI] [PubMed] [Google Scholar]
- 11.Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology 2007;244(2):486–493. [DOI] [PubMed] [Google Scholar]
- 12.Kambadakone AR, Sharma A, Catalano OA, Hahn PF, Sahani DV. Protocol modifications for CT perfusion (CTp) examinations of abdomen-pelvic tumors: impact on radiation dose and data processing time. Eur Radiol 2011;21(6):1293–1300. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi D, Hoeffner EG, Carlos RC, Case I, Mukherji SK. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract. Initial results. J Comput Assist Tomogr 2003;27(5):687–693. [DOI] [PubMed] [Google Scholar]
- 14.Bisdas S, Baghi M, Wagenblast J, et al. Differentiation of benign and malignant parotid tumors using deconvolution-based perfusion CT imaging: feasibility of the method and initial results. Eur J Radiol 2007;64(2):258–265. [DOI] [PubMed] [Google Scholar]
- 15.Makari Y, Yasuda T, Doki Y, et al. Correlation between tumor blood flow assessed by perfusion CT and effect of neoadjuvant therapy in advanced esophageal cancers. J Surg Oncol 2007;96(3):220–229. [DOI] [PubMed] [Google Scholar]
- 16.Ma SH, Xu K, Xiao ZW, et al. Peripheral lung cancer: relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and cyclin D1 expression. Clin Imaging 2007;31(3):165–177. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol 2009;193(4):1090–1096. [DOI] [PubMed] [Google Scholar]
- 18.Ng CS, Chandler AG, Wei W, et al. Reproducibility of perfusion parameters obtained from perfusion CT in lung tumors. AJR Am J Roentgenol 2011;197(1):113–121. [DOI] [PubMed] [Google Scholar]
- 19.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue—initial experience. Radiology 2007;243(3):736–743. [DOI] [PubMed] [Google Scholar]
- 20.Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist 2008;13(2):120–125. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Yuan ZG, Wang DQ, Yan ZH, Tang J, Liu ZQ. Perfusion CT findings in liver of patients with tumor during chemotherapy. World J Gastroenterol 2010;16(25):3202–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda T, Yoshikawa T, Ohno Y, et al. CT hepatic perfusion measurement: comparison of three analytic methods. Eur J Radiol 2012;81(9):2075–2079. [DOI] [PubMed] [Google Scholar]
- 23.Yeung TP, Yartsev S, Bauman G, He W, Fainardi E, Lee TY. The effect of scan duration on the measurement of perfusion parameters in CT perfusion studies of brain tumors. Acad Radiol 2013;20(1):59–65. [DOI] [PubMed] [Google Scholar]
- 24.Sanelli PC, Lev MH, Eastwood JD, Gonzalez RG, Lee TY. The effect of varying user-selected input parameters on quantitative values in CT perfusion maps. Acad Radiol 2004;11(10):1085–1092. [DOI] [PubMed] [Google Scholar]
- 25.Stewart EE, Chen X, Hadway J, Lee TY. Correlation between hepatic tumor blood flow and glucose utilization in a rabbit liver tumor model. Radiology 2006;239(3):740–750. [DOI] [PubMed] [Google Scholar]
- 26.Chandler A, Wei W, Herron DH, Anderson EF, Johnson VE, Ng CS. Semiautomated motion correction of tumors in lung CT-perfusion studies. Acad Radiol 2011;18(3):286–293. [DOI] [PubMed] [Google Scholar]
- 27.Hirata M, Sugawara Y, Fukutomi Y, et al. Measurement of radiation dose in cerebral CT perfusion study. Radiat Med 2005;23(2):97–103. [PubMed] [Google Scholar]
- 28.Hegenscheid K, Behrendt N, Rosenberg C, et al. Assessing early vascular changes and treatment response after laser-induced thermotherapy of pulmonary metastases with perfusion CT: initial experience. AJR Am J Roentgenol 2010;194(4):1116–1123. [DOI] [PubMed] [Google Scholar]
- 29.Sherriff SB, Smart RC, Taylor I. Clinical study of liver blood flow in man measured by 133Xe clearance after portal vein injection. Gut 1977;18(12):1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goh V, Shastry M, Engledow A, et al. Commercial software upgrades may significantly alter Perfusion CT parameter values in colorectal cancer. Eur Radiol 2011;21(4):744–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.