We have modified the late gadolinium enhancement (LGE) MR imaging technique for patients with implanted cardiac devices by using a wideband inversion pulse; this method removes the hyperintensity artifacts that are typically seen with conventional LGE MR imaging.
Abstract
Purpose
To propose and test a modified wideband late gadolinium enhancement (LGE) magnetic resonance (MR) imaging technique to overcome hyperintensity image artifacts caused by implanted cardiac devices.
Materials and Methods
Written informed consent was obtained from all participants, and the HIPAA-compliant study protocol was approved by the institutional review board. Studies in phantoms and in a healthy volunteer were performed to test the hypothesis that the hyperintensity artifacts that are typically observed on LGE images in patients with implanted cardiac devices are caused by insufficient inversion of the affected myocardial signal. The conventional LGE MR imaging pulse sequence was modified by replacing the nonselective inversion pulse with a wideband inversion pulse. The modified LGE sequence, along with the conventional LGE sequence, was evaluated in 12 patients with implantable cardioverter defibrillators (ICDs) who were referred for cardiac MR imaging.
Results
The ICD causes 2–6 kHz in frequency shift at locations 5–10 cm away from the device. This off-resonance falls outside the typical spectral bandwidth of the nonselective inversion pulse used in conventional LGE, which results in the hyperintensity artifact. In 10 of the 12 patients, the conventional LGE technique produced severe, uninterpretable hyperintensity artifacts in the anterior and lateral portions of the left ventricular wall. These artifacts were eliminated with use of the wideband LGE sequence, thereby enabling confident evaluation of myocardial viability.
Conclusion
The modified wideband LGE MR imaging technique eliminates the hyperintensity artifacts seen in patients with cardiac devices. The technique may enable LGE MR imaging in patients with cardiac devices, in whom LGE MR imaging otherwise could not be used for diagnosis.
© RSNA, 2013
Introduction
Late gadolinium enhancement (LGE) cardiac magnetic resonance (MR) imaging plays an important role in the diagnosis and treatment of myocardial diseases. It is the clinical reference standard for noninvasive myocardial tissue characterization (1). Assessment of the presence or absence, location, size, and pattern of myocardial scars with LGE MR imaging is often the only noninvasive means of accurately diagnosing various forms of ischemic and nonischemic cardiomyopathy. LGE MR imaging also increasingly plays a role in guiding catheter ablation, a widely used treatment procedure for ventricular tachycardia. For this procedure, the assessment of myocardial scar location, size, and transmurality enabled by LGE MR imaging allows more accurate identification of arrhythmogenic substrate and ablation sites (2,3).
Despite the important role of LGE MR imaging, many patients do not undergo cardiac MR imaging because of safety and image artifact concerns associated with prior implantation of cardiac devices. It has been estimated that up to 75% of patients who could benefit from LGE MR imaging are recipients of cardiac devices, such as implantable cardioverter defibrillators (ICDs) and cardiac pacemakers (4). In the United States, approximately 116000 ICDs and 397000 cardiac pacemakers were implanted in 2009 (5). The presence of cardiac devices is traditionally considered a contraindication for cardiac MR imaging (6,7). Potential risks of MR imaging for patients with ICDs or pacemakers include heating of the tissue adjacent to lead electrodes, mechanical forces and vibration, induction of arrhythmias, and alteration of device function (6–8). Results of numerous studies (6,8–11) have demonstrated that it is safe to image patients with cardiac devices who are not device dependent and who have no abandoned leads if certain precautions are taken, including pre- and post-MR imaging interrogation of the device. While these recent advances in MR imaging safety are encouraging, the diagnostic value of LGE images for these patients, however, is often severely limited by marked imaging artifacts that arise from the device generator (2,12), a metal box that is predominantly implanted on the upper left side of the patient’s chest. The device generator results in multikilohertz off-resonance within myocardial tissue and typically causes hyperintensity artifacts on LGE images—artifacts that can appear similar to the hyperenhancement of scar tissue (Fig E1 [online]). Results of a recent study (12) have shown that these artifacts typically occur at regions of the heart that are close to the device generator, such as the left ventricular (LV) apex, lateral wall, and outflow tract. Artifacts due to the device also appear on cine and perfusion cardiac MR images, but these are not as severe as those on LGE images (2, 12).
We hypothesize that the hyperintensity LGE imaging artifacts are caused by the limited spectral bandwidth of the radiofrequency inversion pulse that is typically used in LGE MR imaging. The purpose of our study was to propose and test a modified wideband LGE MR imaging technique to overcome hyperintensity image artifacts caused by implanted cardiac devices.
Materials and Methods
Written informed consent was obtained from all participants, and the Health Insurance Portability and Accountability Act–compliant protocol was approved by the institutional review board of the University of California–Los Angeles.
Pulse Sequence Design
The conventional LGE study consists of an inversion prepared two- or three-dimensional segmented gradient-echo sequence (1,13). The subject receives gadolinium contrast material injection about 15 minutes before LGE. An inversion time of 250–300 msec is then used to nullify the signal from the healthy myocardium, which has a gadolinium-enhanced T1 of 381 msec ± 58 (14). The radiofrequency inversion pulse in the conventional LGE sequence on our 1.5-T imaging units (Avanto; Siemens Medical Systems, Malvern, Pa) is a hyperbolic secant adiabatic inversion pulse. Although the inversion pulse is spatially nonselective, it has a spectral bandwidth of 1.1 kHz. For a cardiac device generator that is typically 5–10 cm away from the patient’s heart, the expected resonance offset of the myocardium is in the 2–6 kHz range (Fig E3 [online]), well outside the bandwidth of the inversion pulse currently used. Consequently, the signal from the affected myocardium is not properly inverted (nulled) and is typically hyperintense, undermining appropriate diagnostic interpretation.
To address this issue, we sought to design and implement a wideband adiabatic inversion pulse to ensure proper inversion of the signal from the myocardium affected by the device generator. This inversion pulse was implemented into the existing two-dimensional inversion-recovery LGE sequence, replacing the conventional inversion pulse. The design and testing of the wideband inversion pulse with computer simulations and phantom studies are described in Appendix E1 (online).
In Vivo Study
To demonstrate the benefits of the modified wideband LGE pulse sequence, a healthy volunteer was included in the study. The volunteer received an intravenous injection of 0.15 mmol of gadolinium contrast material (Magnevist; Bayer-Schering Pharma, Berlin, Germany) per kilogram of body weight approximately 15 minutes before LGE MR imaging. A Look-Locker inversion time scout sequence (15,16) was used to identify the appropriate inversion time for suppression of left myocardial signal. Although we did not expect myocardial scars in the volunteer, our purpose was to determine whether we were able to mitigate the hyperintensity artifact that would occur with the conventional LGE sequence. In the healthy volunteer, therefore, uniform suppression of the myocardial signal would indicate successful LGE imaging. Both the conventional and the modified LGE sequence were used to acquire images of the heart in the horizontal long-axis (HLA) plane. Images were acquired with the ICD attached to the body coil, close to the infraclavicular prepectoral area of the left shoulder (which is the most common site of ICD implantation in patients). The images were immediately reacquired after removal of the ICD. Sequence parameters were as follows: repetition time msec/echo time msec, 4.1/1.5; field of view, 360 mm; flip angle, 25°; readout bandwidth, 500 Hz/pixel; section thickness, 8 mm; spatial resolution, 1.4 × 1.9 mm; and inversion time, 250–400 msec.
Subsequent to our healthy volunteer study, 12 patients with previously implanted ICDs whose heart rhythm was not device dependent were included in this study. All patients had been referred for a cardiac MR imaging examination for the purpose of evaluation of LV scarring before ventricular tachycardia ablation. The ICDs implanted in the patients included the following models: Protecta VR (Medtronic, Minneapolis, Minn), Lumax 540 DR-T (Biotronik, Berlin, Germany), Fortify DR (St Jude Medical, St Paul, Minn), and TELIGEN E110 DR and INCEPTA E163 (Boston Scientific, Natick, Mass).
The cardiac MR imaging examination included acquisitions in the heart performed by using the conventional LGE sequence. Images in which hyperintensity artifacts appeared were immediately reacquired by using the modified wideband LGE sequence. Because the wideband LGE sequence immediately followed the conventional LGE sequence, the same inversion time was used for both sequences for a given imaging section. The inversion time was increased about 10 msec for every 1–2 minutes to maintain adequate suppression of the myocardial signal. A Look-Locker inversion scout sequence (15,16) was used as needed to ensure accurate inversion time. To determine the optimal frequency offset of the inversion pulse, the wideband LGE acquisition of the first two-dimensional section was performed three times, with zero, positive, and negative frequency offsets. Positive and negative offsets were in the range of 800–1500 Hz. The frequency offset that resulted in an artifact-free image was chosen and used for any subsequent sections. Therefore, a total of three breath-hold wideband LGE acquisitions were required to determine the frequency offset in each patient.
All patients were monitored with electrocardiographic telemetry and peripheral pulse oximetry. The ICDs were interrogated and reprogrammed as appropriate before and after MR imaging according to the guidelines of the European Society of Cardiology (8) and the literature (6,10,11). An electrophysiologist, a radiologist, an advanced cardiac life support–certified attendant, and a cardiac device programmer were present during the examinations. The specific absorption rate (SAR) of the LGE sequences was limited to less than 2 W per kilogram of tissue to ensure imaging safety. All clinical images were reviewed by a board-certified radiologist (J.P.F.) with more than 20 years of experience in cardiac MR imaging.
Results
Computer simulation and phantom test results are described in Appendix E1 (online). Phantom studies demonstrated that the wideband inversion pulse can successfully invert a wider range of off-resonance spins than the conventional inversion pulse.
Figure 1 shows images in the healthy volunteer. Hyperintensity artifacts were formed at the apical region of the LV in the presence of the ICD with the conventional LGE sequence (Fig 1b), and the artifacts were completely removed on the wideband LGE images (Fig 1c). The wideband LGE sequence did not introduce additional image artifacts when compared with the conventional LGE sequence in the absence of an ICD, as shown in Figure 1a.
Figure 1a:
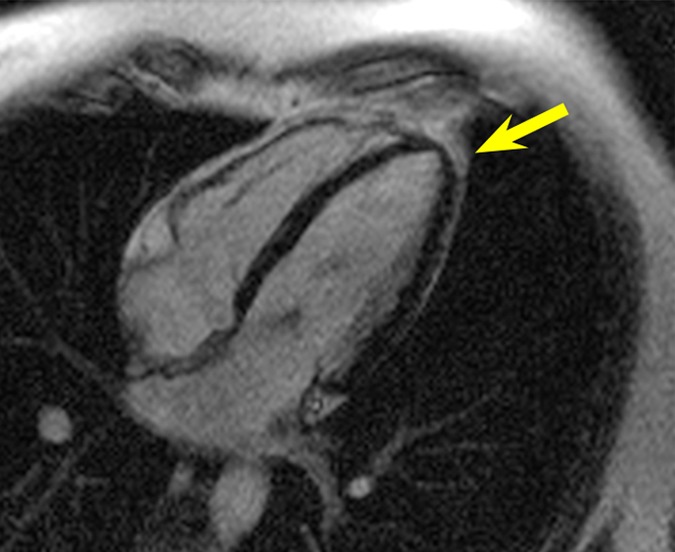
HLA LGE MR images in a healthy volunteer, acquired 15 minutes after gadolinium chelate injection. Dotted red ellipse = approximate location of ICD. (a) Image obtained by using the conventional LGE sequence, without the ICD present. (b) Image obtained by using the conventional LGE sequence, with the ICD attached near the shoulder. Hyperintensity artifacts were formed at the apex (arrow). (c) Image obtained by using the proposed wideband LGE sequence, with the ICD attached near the shoulder. Hyperintensity artifact was no longer present.
Figure 1b:
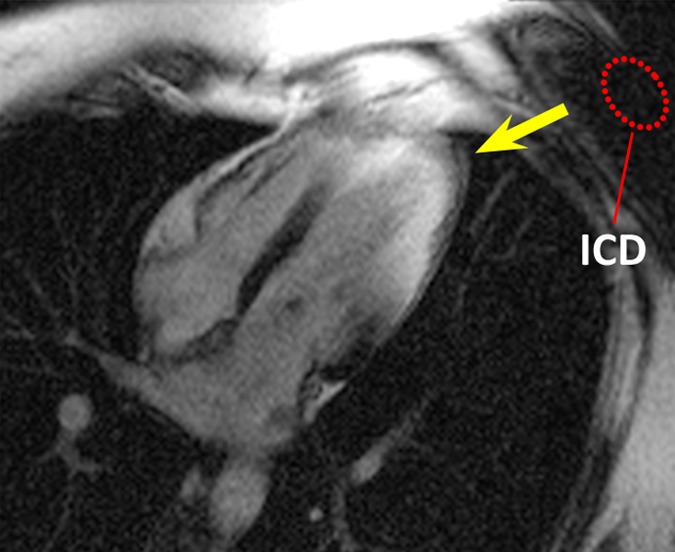
HLA LGE MR images in a healthy volunteer, acquired 15 minutes after gadolinium chelate injection. Dotted red ellipse = approximate location of ICD. (a) Image obtained by using the conventional LGE sequence, without the ICD present. (b) Image obtained by using the conventional LGE sequence, with the ICD attached near the shoulder. Hyperintensity artifacts were formed at the apex (arrow). (c) Image obtained by using the proposed wideband LGE sequence, with the ICD attached near the shoulder. Hyperintensity artifact was no longer present.
Figure 1c:
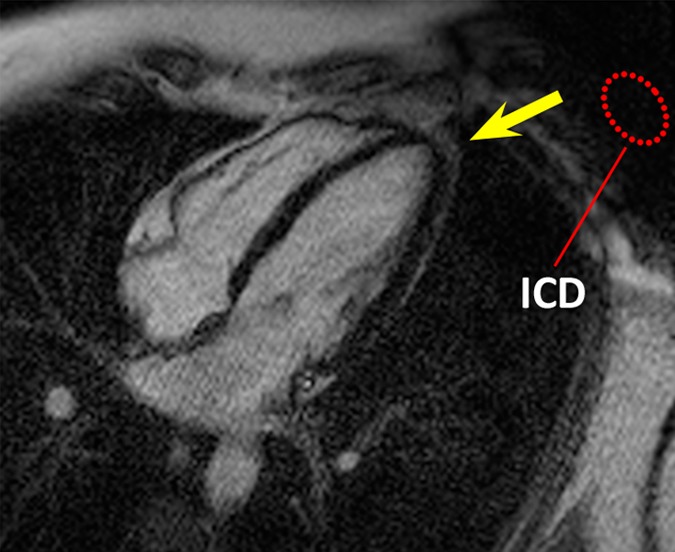
HLA LGE MR images in a healthy volunteer, acquired 15 minutes after gadolinium chelate injection. Dotted red ellipse = approximate location of ICD. (a) Image obtained by using the conventional LGE sequence, without the ICD present. (b) Image obtained by using the conventional LGE sequence, with the ICD attached near the shoulder. Hyperintensity artifacts were formed at the apex (arrow). (c) Image obtained by using the proposed wideband LGE sequence, with the ICD attached near the shoulder. Hyperintensity artifact was no longer present.
All patients were at sinus rhythm during MR imaging, except for three patients who had sporadic premature ventricular contractions. When arrhythmia occurred during image acquisition, the image was reacquired. Of the 12 patients in this study, 10 showed various degrees of hyperintensity artifact with the conventional LGE sequence, while two did not show any artifact because the ICD was at a greater distance away from the heart. One of these two patients had an ICD that had been implanted on the right side. All the artifacts were completely resolved with the modified wideband LGE sequence. The optimal frequency offset of the wideband inversion pulse was successfully determined in all of the 10 patients.
In patient 1, with the conventional LGE sequence, hyperintensity artifacts were formed in the anterior LV wall, which was in close proximity to the ICD (Fig 2). The artifact could be confused with scar tissue, making an accurate diagnosis difficult. With the wideband LGE sequence, the hyperintensity artifacts were completely removed. Scarring and ventricular wall thinning were identified near the apex.
Figure 2a:
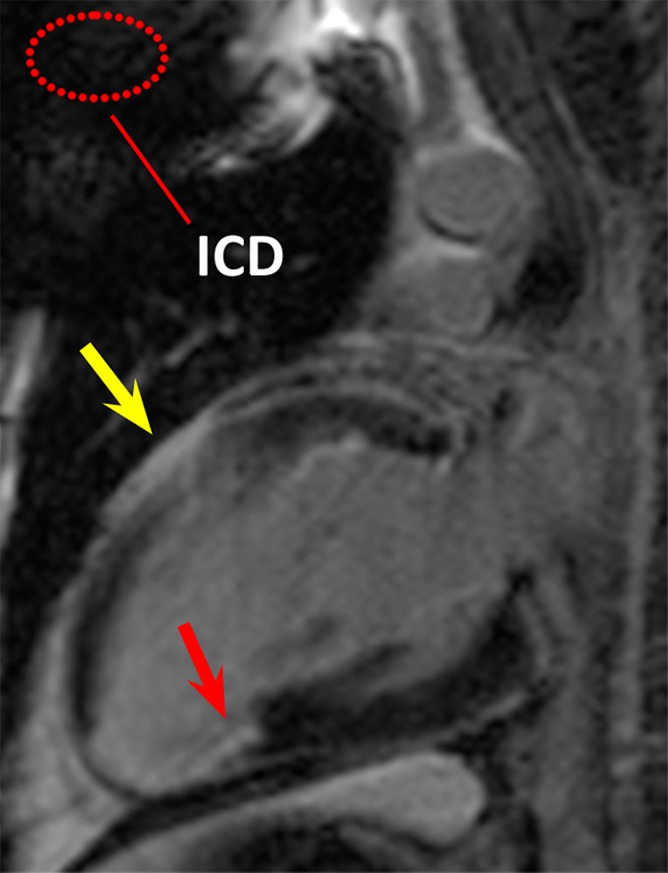
LGE images in the two-chamber vertical long-axis plane in patient 1. Dotted red ellipse = approximate location of ICD. (a) Image obtained by using the conventional LGE sequence. Hyperintensity artifact was produced in the ventricular wall (yellow arrow). (b) Image obtained by using the modified wideband LGE sequence. Hyperintensity artifact has been completely resolved (yellow arrow). In this patient, scarring and ventricular wall thinning was present near the posterior LV wall (red arrows), which is remote from the ICD.
Figure 2b:
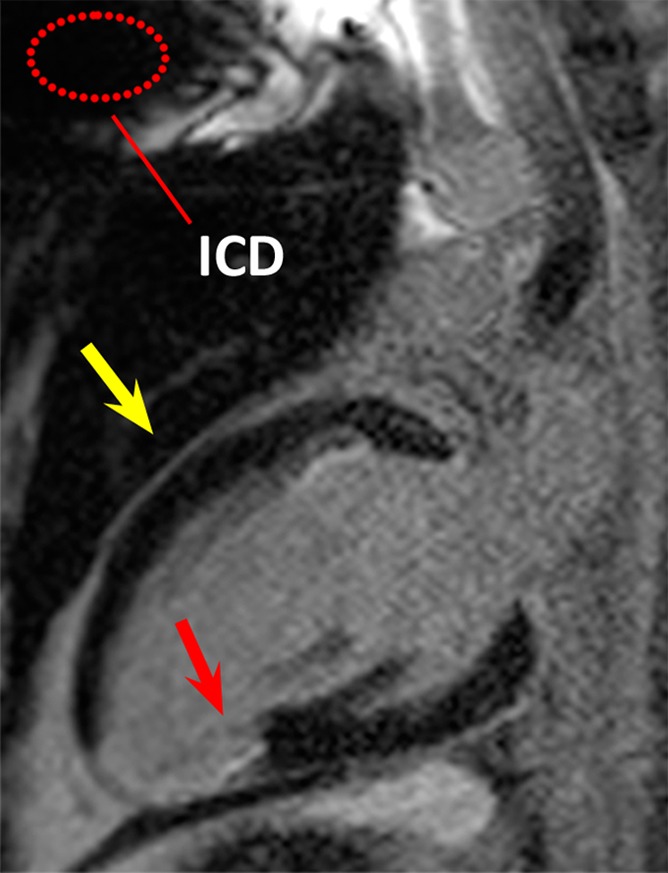
LGE images in the two-chamber vertical long-axis plane in patient 1. Dotted red ellipse = approximate location of ICD. (a) Image obtained by using the conventional LGE sequence. Hyperintensity artifact was produced in the ventricular wall (yellow arrow). (b) Image obtained by using the modified wideband LGE sequence. Hyperintensity artifact has been completely resolved (yellow arrow). In this patient, scarring and ventricular wall thinning was present near the posterior LV wall (red arrows), which is remote from the ICD.
In patient 2, hyperintensity artifacts were formed over a large portion of the ventricle near the apex when the conventional LGE sequence was used (Fig 3a). With the wideband LGE sequence, the artifacts were eliminated and the myocardium near the apex was clearly visible. On the basis of the wideband LGE images, scarring was identified near the apex, which was obscured on conventional LGE images. In the volunteer and in patients 1 and 2, parallel imaging (with a generalized autocalibrating partially parallel acquisitions, or GRAPPA, rate of two) was used for the wideband LGE but not the conventional LGE images, resulting in an apparent increase in noise on the wideband images in Figures 1–3. The imaging parameters were subsequently matched between the two LGE sequences in the later stage of our study, and, hence, examples in Figures 4 and 5 do not show increased noise with the wideband LGE sequence.
Figure 3a:
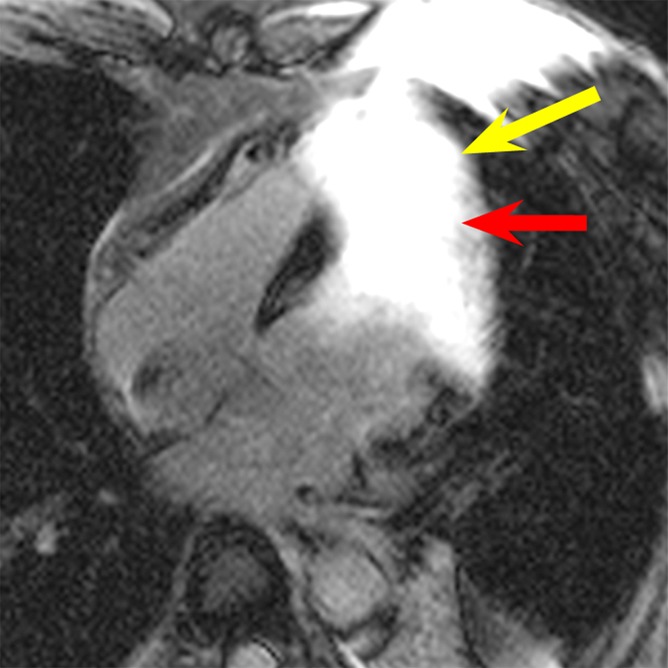
LGE images in the four-chamber HLA plane in patient 2. (a) Image obtained by using the conventional LGE sequence. Severe hyperintensity artifact was produced over a large portion of the ventricular wall (yellow arrow). (b) Image obtained by using the modified wideband LGE sequence. The hyperintensity artifact was completely eliminated (yellow arrow). Scar tissue near the apex is clearly visible in the wideband image (red arrow in b) but is completely obscured by the artifact in the conventional LGE image (red arrow in a).
Figure 4a:
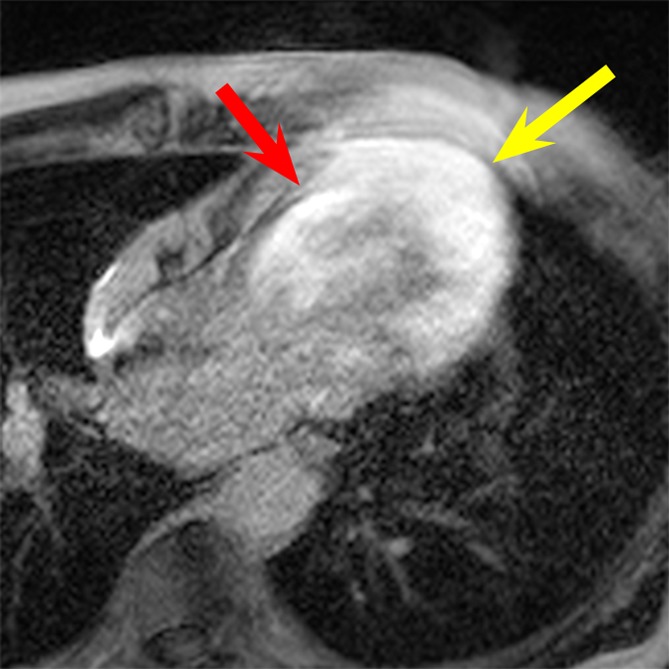
LGE images in the four-chamber HLA plane in patient 3. (a) Image obtained by using the conventional LGE sequence. Severe hyperintensity artifact was formed throughout the LV wall (yellow arrow). (b) Image obtained by using the wideband LGE sequence. The hyperintensity artifact was completely eliminated (yellow arrow). Scar tissue at the septum (red arrow in b) is clear in the wideband image but is completely obscured by the hyperintensity artifact in the conventional LGE image (red arrow in a).
Figure 5a:
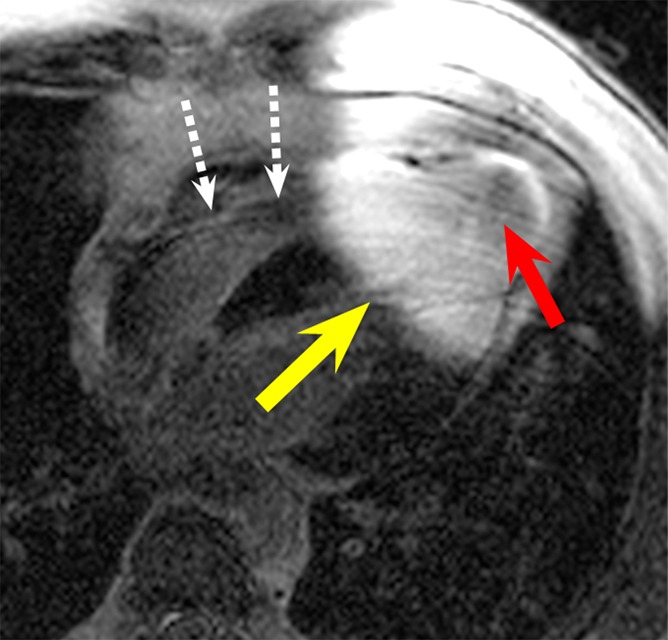
LGE images in the four-chamber HLA plane in patient 4. (a) Image obtained by using the conventional LGE sequence. Severe hyperintensity artifact was formed in the ventricular wall around the apex (yellow arrow). (b) Image obtained by using the wideband LGE sequence. The hyperintensity artifact was completely eliminated. This patient has an apical aneurysm, which is obscured in the conventional LGE image (red arrow in a) but is much clearer in the wideband LGE image (red arrow in b). A device lead is visible in the right ventricle (white dotted arrows), but it did not produce any noticeable artifact in the wideband image.
Figure 3b:
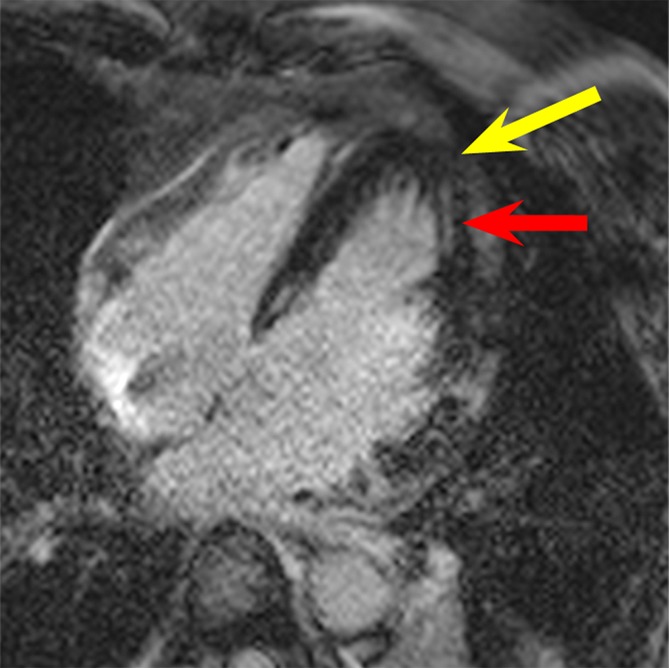
LGE images in the four-chamber HLA plane in patient 2. (a) Image obtained by using the conventional LGE sequence. Severe hyperintensity artifact was produced over a large portion of the ventricular wall (yellow arrow). (b) Image obtained by using the modified wideband LGE sequence. The hyperintensity artifact was completely eliminated (yellow arrow). Scar tissue near the apex is clearly visible in the wideband image (red arrow in b) but is completely obscured by the artifact in the conventional LGE image (red arrow in a).
Hyperintensity artifacts were produced throughout the LV in patient 3 (Fig 4a) and in the apical regions of the LV in patient 4 (Fig 5a) with the conventional LGE sequence. The artifacts were completely resolved when the wideband LGE sequence was used (Figs 4b, 5b). Patient 3 had scarring at the septum, which was completely obscured on the conventional LGE image (Fig 4a) but was visible on the wideband LGE image (Fig 4b). Patient 4 had an apical aneurysm, which was obscured on the conventional LGE image (Fig 5a) but was much clearer on the wideband LGE image (Fig 5b).
Figure 4b:
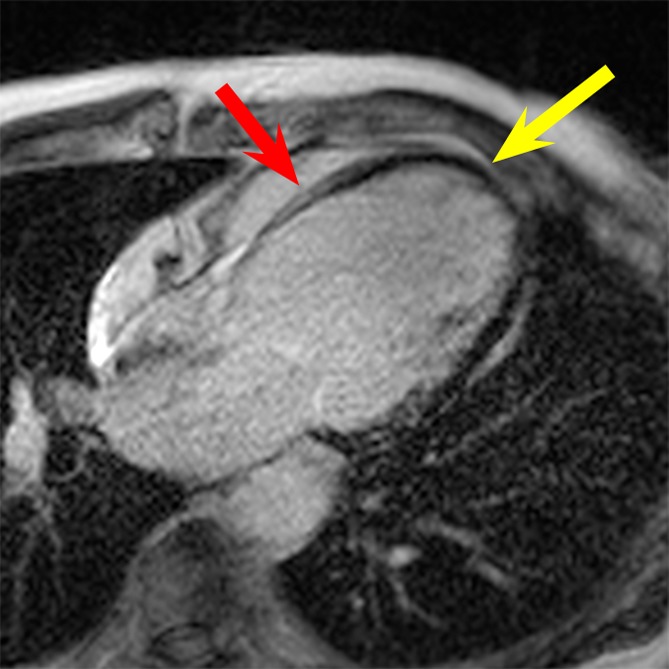
LGE images in the four-chamber HLA plane in patient 3. (a) Image obtained by using the conventional LGE sequence. Severe hyperintensity artifact was formed throughout the LV wall (yellow arrow). (b) Image obtained by using the wideband LGE sequence. The hyperintensity artifact was completely eliminated (yellow arrow). Scar tissue at the septum (red arrow in b) is clear in the wideband image but is completely obscured by the hyperintensity artifact in the conventional LGE image (red arrow in a).
Figure 5b:
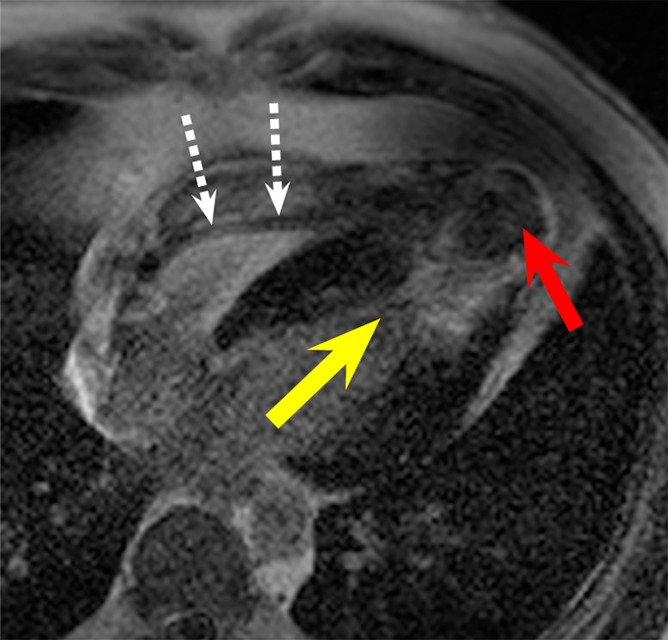
LGE images in the four-chamber HLA plane in patient 4. (a) Image obtained by using the conventional LGE sequence. Severe hyperintensity artifact was formed in the ventricular wall around the apex (yellow arrow). (b) Image obtained by using the wideband LGE sequence. The hyperintensity artifact was completely eliminated. This patient has an apical aneurysm, which is obscured in the conventional LGE image (red arrow in a) but is much clearer in the wideband LGE image (red arrow in b). A device lead is visible in the right ventricle (white dotted arrows), but it did not produce any noticeable artifact in the wideband image.
ICD leads were noted in the right ventricle, adjacent to the septum, on LGE images in a few patients (Fig 5). No artifacts were seen in the myocardium that could be directly caused by leads.
Discussion
The results of our study indicate that diagnostic-quality LGE MR imaging in patients with ICDs is feasible using our wideband technique. Data collected in 10 patients with ICDs demonstrate that the commonly seen hyperintensity artifacts can be removed by using the wideband LGE technique. We also measured the off-resonance induced by the generator of an ICD and have shown that this off-resonance is in the 2–6 kHz range at distances of 5–10 cm, which is often the typical distance of an ICD from the myocardium in patients (Appendix E1 [online]). Our results show that the off-resonance induced by an ICD decreases with distance and that the standard LGE sequence produces hyperintensity artifacts if an ICD is implanted less than about 16 cm from the heart.
The main advantage of using a wideband inversion pulse is that it allows us to eliminate the hyperintensity artifacts that appear on LGE images in patients with ICDs without requiring additional hardware, time, or reconstruction. One disadvantage of the proposed sequence is that a wider bandwidth adiabatic radiofrequency pulse requires a higher B1 amplitude. This results in an increased SAR. In our in vivo studies, SAR for the conventional LGE sequence varied from 0.05 to 0.08 W/kg, while SAR for the proposed wideband LGE sequence varied from 0.07 to 0.1 W/kg. The increase in SAR from the conventional sequence to the proposed sequence varied from 19% to 56%. Although we did not approach the SAR limit of 2 W/kg in this study, the B1 requirement has to be accounted for when designing radiofrequency pulses of higher bandwidth, to ensure that the imaging unit’s B1 limit is not exceeded.
The wideband LGE sequence does not correct any geometric distortions caused by the off-resonance. An off-resonance in the kilohertz range could potentially translate to prominent image distortion in both the frequency encoding direction (in plane) and the section-select direction (through plane). Image distortions due to the presence of metallic implants have been addressed by numerous techniques (17–21). The most widely used are multiacquisition variable-resonance image combination, or MAVRIC (22), and slice encoding for metal artifact correction, or SEMAC (23). These methods can potentially be applied to our wideband LGE imaging technique to reduce image distortions. A disadvantage of these sequences is that multiple acquisitions are required, which, together with cardiac gating, could make the imaging duration unfeasible for cardiac imaging.
We have modified the LGE MR imaging technique for patients with implanted cardiac devices by using a wideband inversion pulse. The wideband LGE technique removes the hyperintensity artifacts that are typically seen with conventional LGE MR imaging. Our feasibility study suggests that the technique may enable the successful use of LGE MR imaging in patients with cardiac devices who would otherwise be inaccessible to diagnosis. Further clinical studies are warranted to validate the reliability of the wideband technique in routine clinical practice and to determine whether most or all patients with ICDs who require cardiac MR imaging may now be imaged successfully.
Advance in Knowledge
■ Late gadolinium enhancement (LGE) MR imaging performed with a wide-bandwidth radiofrequency inversion pulse can eliminate hyperintensity artifacts induced by implanted cardiac devices.
Implication for Patient Care
■ The modified wideband LGE technique may enable the widespread use of LGE MR imaging in patients with implanted cardiac devices, in whom LGE MR imaging otherwise could not be used for diagnosis.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgment
The authors acknowledge the use of a Bloch equation simulator developed by Brian Hargreaves, PhD, in the design and testing of our wideband inversion pulses.
Received April 24, 2013; revision requested May 31; revision received July 23; accepted July 28; final version accepted July 31.
Disclosures of Conflicts of Interest: S. Rashid No relevant conflicts of interest to disclose. S. Rapacchi No relevant conflicts of interest to disclose. M.V. No relevant conflicts of interest to disclose. R.T. No relevant conflicts of interest to disclose. K.S. No relevant conflicts of interest to disclose. J.P.F. No relevant conflicts of interest to disclose. P.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution may receive money from pending patents describing the proposed technique. Other relationships: none to disclose.
Funding: This research was supported by the National Institutes of Health (grant 1R21HL118533).
Abbreviations:
- HLA
- horizontal long axis
- ICD
- implantable cardioverter defibrillator
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- SAR
- specific absorption rate
References
- 1.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001;218(1):215–223. [DOI] [PubMed] [Google Scholar]
- 2.Dickfeld T, Tian J, Ahmad G, et al. MRI-guided ventricular tachycardia ablation: integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol 2011;4(2):172–184. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Ahmad G, Mesubi O, Jeudy J, Dickfeld T. Three-dimensional delayed-enhanced cardiac MRI reconstructions to guide ventricular tachycardia ablations and assess ablation lesions. Circ Arrhythm Electrophysiol 2012;5(2):e31–e35. [DOI] [PubMed] [Google Scholar]
- 4.Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28(4):326–328. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics: 2012 update—a report from the American Heart Association. Circulation 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation 2006;114(12):1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shellock FG, Crues JV. MR procedures: biologic effects, safety, and patient care. Radiology 2004;232(3):635–652. [DOI] [PubMed] [Google Scholar]
- 8.Roguin A, Schwitter J, Vahlhaus C, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 2008;10(3):336–346. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JD, Costa HS, Russo RJ. Determining the risks of magnetic resonance imaging at 1.5 tesla for patients with pacemakers and implantable cardioverter defibrillators. Am J Cardiol 2012;110(11):1631–1636. [DOI] [PubMed] [Google Scholar]
- 10.Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm 2009;6(1):138–143. [DOI] [PubMed] [Google Scholar]
- 11.Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011;155(7):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki T, Hansford R, Zviman MM, et al. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter-defibrillators. Circ Cardiovasc Imaging 2011;4(6):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peukert D, Laule M, Taupitz M, Kaufels N, Hamm B, Dewey M. 3D and 2D delayed-enhancement magnetic resonance imaging for detection of myocardial infarction: preclinical and clinical results. Acad Radiol 2007;14(7):788–794. [DOI] [PubMed] [Google Scholar]
- 14.Hu P, Chan J, Ngo LH, et al. Contrast-enhanced whole-heart coronary MRI with bolus infusion of gadobenate dimeglumine at 1.5 T. Magn Reson Med 2011;65(2):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Lee VS, Chung YC, Babb JS, Simonetti OP. Myocardial infarction: optimization of inversion times at delayed contrast-enhanced MR imaging. Radiology 2004;233(3):921–926. [DOI] [PubMed] [Google Scholar]
- 16.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 1970;41(2):250–251. [Google Scholar]
- 17.Butts K, Pauly JM, Gold GE. Reduction of blurring in view angle tilting MRI. Magn Reson Med 2005;53(2):418–424. [DOI] [PubMed] [Google Scholar]
- 18.Cho ZH, Kim DJ, Kim YK. Total inhomogeneity correction including chemical shifts and susceptibility by view angle tilting. Med Phys 1988;15(1):7–11. [DOI] [PubMed] [Google Scholar]
- 19.Kolind SH, MacKay AL, Munk PL, Xiang QS. Quantitative evaluation of metal artifact reduction techniques. J Magn Reson Imaging 2004;20(3):487–495. [DOI] [PubMed] [Google Scholar]
- 20.Ramos-Cabrer P, van Duynhoven JP, Van der Toorn A, Nicolay K. MRI of hip prostheses using single-point methods: in vitro studies towards the artifact-free imaging of individuals with metal implants. Magn Reson Imaging 2004;22(8):1097–1103. [DOI] [PubMed] [Google Scholar]
- 21.Venook RD, Matter NI, Ramachandran M, et al. Prepolarized magnetic resonance imaging around metal orthopedic implants. Magn Reson Med 2006;56(1):177–186. [DOI] [PubMed] [Google Scholar]
- 22.Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med 2009;61(2):381–390. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: slice encoding for metal artifact correction in MRI. Magn Reson Med 2009;62(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


