We have shown that the widely accepted and validated CT features for a radiologic usual interstitial pneumonia (UIP) pattern used to make a diagnosis of idiopathic pulmonary fibrosis can be used to identify UIP pattern in patients with rheumatoid arthritis–associated interstitial lung disease with a high degree of confidence.
Abstract
Purpose
To determine the accuracy of computed tomography (CT) in identifying the histopathologic usual interstitial pneumonia (UIP) pattern in rheumatoid arthritis–associated interstitial lung disease (RA-ILD).
Materials and Methods
All patients were enrolled into institutional review board-approved longitudinal cohorts at their respective institution, and informed consent was obtained at the time of enrollment. Images of patients with surgical lung biopsy-proved RA-ILD (n = 69) were collected from three tertiary care centers. Two experienced thoracic radiologists independently reviewed the CT scans. The CT pattern was categorized as definite UIP, possible UIP, or inconsistent with UIP in accordance with published criteria. Findings of biopsies were reviewed by an experienced lung pathologist. The sensitivity and specificity of definite CT UIP pattern to histopathologic UIP pattern were determined. The agreement between radiologists was assessed by calculating a κ score.
Results
The histopathologic UIP pattern was present in 42 of 69 (61%) patients. Men were more likely than women to have a histopathologic UIP pattern (P = .02). Twenty patients (29%, 20 of 69) had a definite UIP pattern on CT scans. The specificity of CT UIP pattern was 96% (26 of 27; 95% confidence interval [CI]: 81%, 100%), with a negative predictive value of 53% (26 of 49). The sensitivity of CT UIP pattern was 45% (19 of 42; 95% CI: 30%, 61%), with a positive predictive value of 95% (19 of 20). The agreement between radiologists for definite UIP pattern versus not was 87% (κ = 0.67, P < .0001).
Conclusion
Definite UIP pattern on a CT scan in RA-ILD is highly specific and moderately sensitive for histopathologic UIP pattern. CT can therefore help accurately identify the UIP pattern in RA-ILD.
© RSNA, 2013
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory arthritis in the United States and affects 1% of the population (1). Interstitial lung disease (ILD) is a common extra-articular manifestation of RA and is associated with substantial morbidity and mortality (2). There are two primary histopathologic patterns of ILD that are observed in patients with RA-associated ILD (hereafter, RA-ILD), the nonspecific interstitial pneumonia (NSIP) pattern and the usual interstitial pneumonia (UIP) pattern (3–5). Other less common patterns include organizing pneumonia and obliterative bronchiolitis (2,6).
There is growing evidence to suggest that distinguishing UIP pattern from non-UIP pattern in RA-ILD may have important clinical implications with regard to treatment, disease progression, and prognosis (7–12). RA-ILD patients with UIP pattern appear to have a different clinical phenotype than do RA-ILD patients without the UIP pattern. Those with UIP pattern tend to be older, are more likely to be men, and seem to be less responsive to conventional treatment compared with RA-ILD patients with non-UIP pattern (3,8,9). In addition, the UIP pattern in RA-ILD is associated with significantly shortened survival compared with that in those without the UIP pattern (9,11,12). Therefore, accurate identification of RA-ILD patients with UIP pattern is important. Surgical lung biopsies, however, are not routinely performed in patients with RA-ILD and can be associated with morbidity and mortality (13).
Chest computed tomography (CT) has been shown to help accurately predict histopathologic UIP pattern in some patients with idiopathic pulmonary fibrosis (IPF), a fibrosing lung disease of unknown cause. In IPF, a CT pattern of UIP is characterized by the presence of reticular opacities, often associated with traction bronchiectasis, in a subpleural and basal distribution (14). Honeycombing, clustered cysts with well-defined walls involving the subpleural lung, is the key finding for making a definite diagnosis of UIP pattern. These CT features, in the absence of features inconsistent with UIP pattern such as extensive ground-glass opacity and substantial mosaic perfusion, have been shown to be highly specific, but not sensitive, for histopathologic UIP pattern and have been termed radiologic UIP (15,16). If patients do not have a radiologic UIP pattern, a surgical lung biopsy is required to make the diagnosis (14).
It is unknown if CT performs as well in identifying the UIP pattern in diseases other than IPF that manifest with a histopathologic UIP pattern, most commonly RA-ILD. The purpose of this study was to determine the accuracy of CT in identifying the histopathologic UIP pattern in RA-ILD.
Materials and Methods
Study Design and Patient Population
From 1997 to 2011, patients with RA-ILD were identified from three tertiary care centers. All patients were enrolled into institutional review board–approved longitudinal cohorts at their respective institution, and informed consent was obtained at the time of enrollment. This specific study was exempt from institutional review board approval due to the use of de-identified data.
Patients were included in this study if they had a diagnosis of RA and a surgical lung biopsy confirming the diagnosis of ILD. The diagnosis of RA was made according to the 1987 American College of Rheumatology classification criteria for RA (17). Patients were excluded from the study if they did not have a CT scan of the chest obtained within 12 months of their surgical lung biopsy. There were no other exclusion criteria. A total of 83 patients with RA and biopsy-proved ILD were identified from the three cohorts. Of those, 69 had a CT within 1 year of the surgical lung biopsy available for review and were included in the analysis (ie, 14 patients were excluded).
Demographics, smoking history (ever vs never), serologic profile (rheumatoid factor, anticyclic citrullinated peptide, and antinuclear antibody), medications (including prednisone and methotrexate), and pulmonary function (percentage of predicted forced vital capacity and of diffusing capacity of lung for carbon monoxide) were recorded for all patients. Patient characteristics were obtained from the medical record.
Radiologic Evaluation
CT was performed by using four, 16, or 64 multidetector scanners (Light Speed Ultra or Light Speed VCT, Bone kernel, GE Healthcare, Milwaukee, Wis; and Sensation 64, B46 kernel, Siemens, Erlangen, Germany). Axial images were acquired in the supine position at full inspiration at 1-cm intervals by using 1.25-mm section thickness. Helical images were obtained in selected patients depending on the clinical indication. These images were reconstructed at 1–1.5-mm intervals. All images were reconstructed by using an edge-enhancing algorithm. Images were reviewed on picture archiving and communication systems by using standard window levels and widths. All images were viewed at window setting optimized for assessment of lung parenchyma (width, 1500 HU; level, −700 HU). Each CT scan was independently read by two expert thoracic radiologists (B.M.E., T.H.U.; 8 and 4 years of experience, respectively). The radiologists were blinded to the patient’s diagnosis, clinical information, and histopathologic pattern of disease. The radiologic pattern of ILD was interpreted according to the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association statement on the diagnosis of IPF (14). The pattern of disease was recorded as definite UIP pattern, possible UIP pattern, and inconsistent with UIP pattern (Figure). Briefly, a definite UIP pattern is defined as subpleural, basal predominance of reticular abnormalities, honeycombing with or without traction bronchiectasis, and absence of extensive ground-glass opacities, nodules, discrete cysts, mosaic attenuation, air trapping, or segmental/lobar consolidation. Possible UIP pattern requires all features of definite UIP pattern listed above except for honeycombing. A pattern inconsistent with UIP requires the presence of any of the following abnormalities: upper or midlung zone predominance, peribronchovascular predominance, extensive ground-glass opacities, profuse micronodules, discrete cysts away from areas of honeycombing, diffuse mosaic attenuation or air trapping (bilateral, in three or more lobes), or segmental/lobar consolidation. Disagreements between the two radiologists were subsequently resolved by consensus.
Figure a:
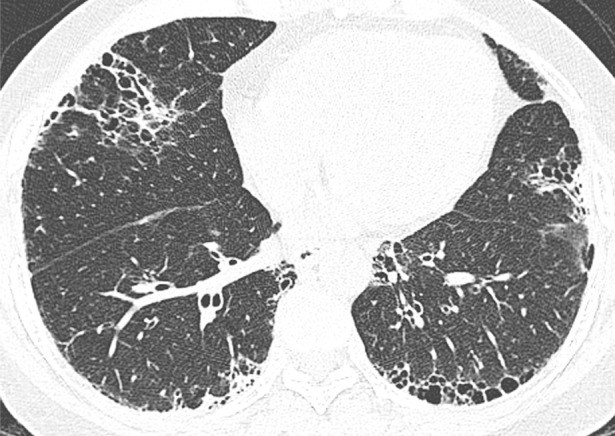
Axial thin-section CT scans (1.25-mm-thick sections) of the chest in patients with RA-ILD at full inspiration. (a) Definite UIP pattern. (b) Possible UIP pattern. (c) Pattern inconsistent with the UIP pattern with diffuse micronodules. (d) Pattern inconsistent with the UIP pattern, with ground-glass opacities in a peribronchovascular distribution.
Figure b:
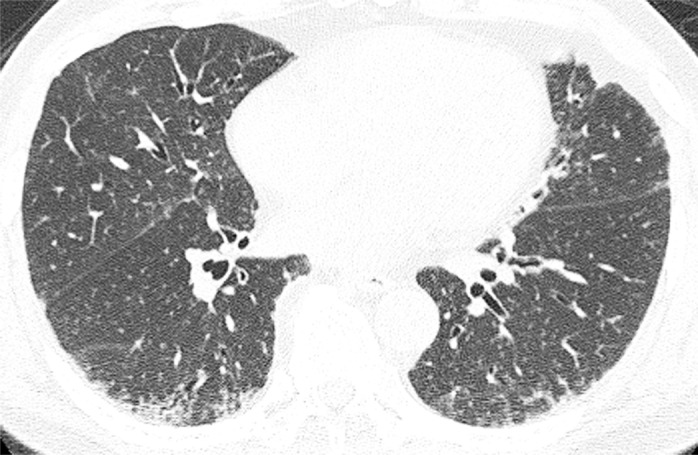
Axial thin-section CT scans (1.25-mm-thick sections) of the chest in patients with RA-ILD at full inspiration. (a) Definite UIP pattern. (b) Possible UIP pattern. (c) Pattern inconsistent with the UIP pattern with diffuse micronodules. (d) Pattern inconsistent with the UIP pattern, with ground-glass opacities in a peribronchovascular distribution.
Figure c:
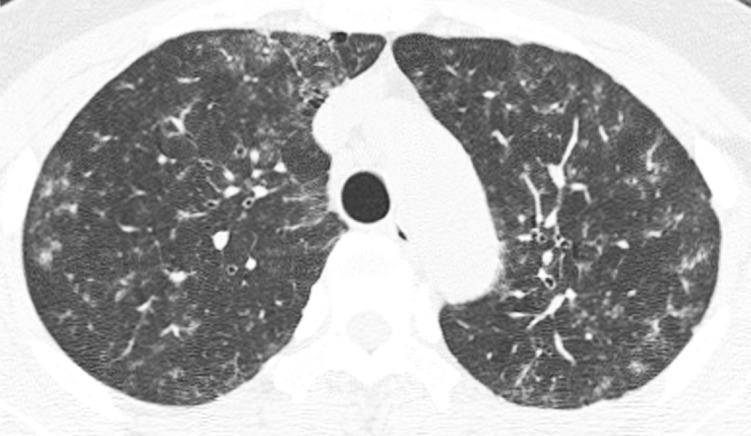
Axial thin-section CT scans (1.25-mm-thick sections) of the chest in patients with RA-ILD at full inspiration. (a) Definite UIP pattern. (b) Possible UIP pattern. (c) Pattern inconsistent with the UIP pattern with diffuse micronodules. (d) Pattern inconsistent with the UIP pattern, with ground-glass opacities in a peribronchovascular distribution.
Figure d:
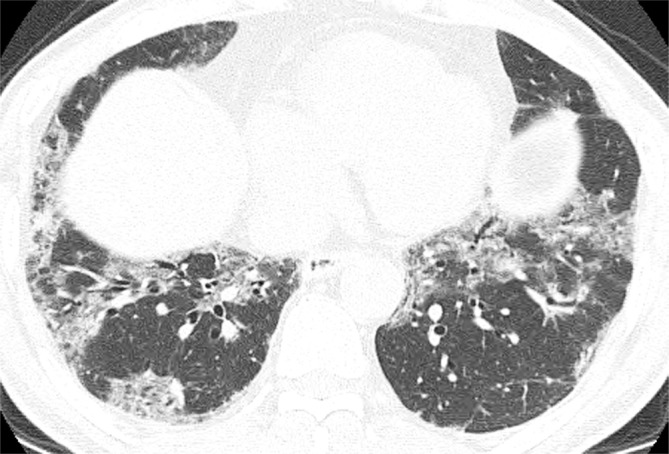
Axial thin-section CT scans (1.25-mm-thick sections) of the chest in patients with RA-ILD at full inspiration. (a) Definite UIP pattern. (b) Possible UIP pattern. (c) Pattern inconsistent with the UIP pattern with diffuse micronodules. (d) Pattern inconsistent with the UIP pattern, with ground-glass opacities in a peribronchovascular distribution.
Histopathologic Evaluation
All surgical lung biopsy specimens were read prospectively at the time of initial enrollment into the participating center’s cohort by lung pathologists who are experts in the interpretation of ILD. All lung biopsy specimens that were available for re-review for this study were read by a second lung pathologist (T.V.C.). Histopathologic UIP pattern was determined by using the criteria described in the 2001 American Thoracic Society and European Respiratory Society International Consensus Classification of idiopathic interstitial pneumonias (18).
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation. Intergroup comparisons between subjects with and those without UIP pattern at histopathologic analysis were performed by using χ2 test for categorical variables and t test for numeric variables. The test characteristics of radiologic UIP pattern were obtained by comparing the UIP pattern on a CT scan, using the consensus readings from both radiologists, to the histopathologic UIP pattern as the reference standard. These included measures of sensitivity, specificity, and positive and negative predictive values. We performed two separate analyses, one using strict criteria for radiologic UIP pattern (definite vs nondefinite [possible plus inconsistent]) and one using broad criteria for radiologic UIP pattern (definite plus possible vs inconsistent). Test characteristics were obtained by using the consensus CT interpretations. Interrater agreement of the radiologic UIP pattern was determined by calculating the κ value, using the CT readings that were performed initially, independently. A κ value was also calculated for the histopathologic UIP pattern on the subset of lung biopsies that were read by a second pathologist.
Results
The patients were predominantly female (65%, 45 of 69), with a mean age of 58 years (mean age, 55 years for women and 63 years for men; P = .003) (Table 1). Forty-five percent (31 of 69) of patients had a history of cigarette smoking. The majority of patients reported prednisone use at baseline (77%, 53 of 69), while only 25% (17 of 69) reported use of methotrexate. The patients had moderately reduced pulmonary function, with a mean forced vital capacity of 69% predicted and mean diffusing capacity of lung for carbon monoxide of 61% predicted.
Table 1.
Baseline Characteristics
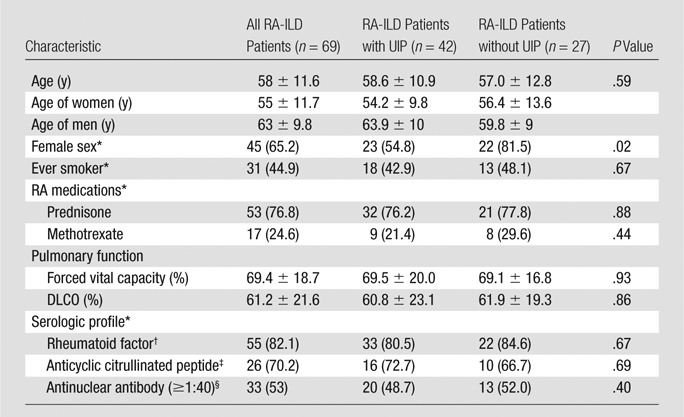
Note.— DLCO = diffusing capacity of lung for carbon monoxide. Unless otherwise indicated, data are means ± standard deviation.
Data are the number of patients and data in parentheses are percentages.
Data available in 67 patients (41 with UIP and 26 without UIP).
Data available in 37 patients (22 with UIP and 15 without UIP).
Data available in 66 patients (41 with UIP and 25 without UIP).
Histopathologic UIP pattern was present in 61% of the patients (42 of 69). Other histopathologic patterns of ILD included NSIP (n = 14), organizing pneumonia (n = 6), acute lung injury (n = 3), and others (obliterative and constrictive bronchiolitis, methotrexate toxicity, nonclassifiable interstitial fibrosis, n = 4). Male sex was associated with histopathologic UIP pattern (P = .02) (Table 1). No other clinical variables were associated with histopathologic UIP pattern. There were 52 surgical lung biopsies that were available for a second review. The interrater agreement for UIP at histopathologic examination was 87%, with a κ value of 0.72 (P < .001) (Table E1 [online]).
Radiologic UIP pattern was identified in 29% of patients (20 of 69). The remainder had possible UIP pattern (n = 18) and pattern inconsistent with UIP (n = 31) (Table 2). The reasons for an inconsistent UIP pattern included presence of extensive ground-glass opacities (n = 22), peri-bronchovascular predominance (n = 12), discrete cysts (n = 4), presence of consolidation (n = 4), diffuse mosaicism and/or air-trapping in more than three lobes (n = 2), upper-mid lung zone predominance (n = 1), and/or presence of profuse micronodules (n = 1). The interrater agreement for radiologic UIP pattern on CT scans with use of the strict criteria (definite vs possible plus inconsistent) was 87%, with a κ value of 0.67 (P < .0001) (Table E2 [online]). The interrater agreement for radiologic UIP pattern on CT scans with use of the broad criteria (definite plus possible vs inconsistent) was 75%, with a κ value of 0.52 (P < .0001).
Table 2.
UIP Pattern on CT Scan Compared with Histopathologic Examination (14)
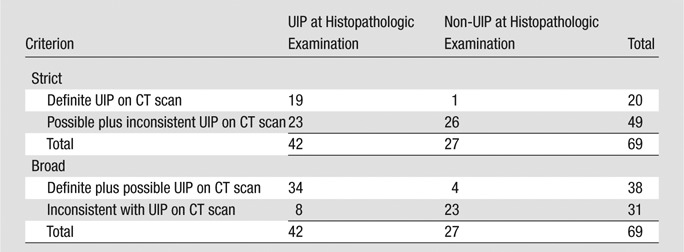
By using the strict criteria for radiologic UIP pattern, a definite UIP pattern on a CT scan was highly specific for a UIP pattern on histopathologic examination (96%, 26 of 27; 95% confidence interval [CI]: 81%, 100%) (Table 3). This was associated with a positive predictive value of 95% (19 of 20; 95% CI: 75%, 100%). The sensitivity of radiologic UIP pattern on a CT scan was 45% (19 of 42; 95% CI: 30%, 61%) when compared with that on histopathologic examination. This was associated with a negative predictive value of 53% (26 of 49; 95% CI: 38%, 68%). By using the broad criteria for radiologic UIP pattern, the sensitivity of CT improved to 81% (34 of 42; 95% CI: 66%, 91%), with a slight reduction in specificity (85%, 23 of 27; 95% CI: 66%, 96%). This increased the area under the receiver operating characteristic curve from 0.71 to 0.83.
Table 3.
Test Characteristics of CT in Determining Histopathologic UIP Pattern
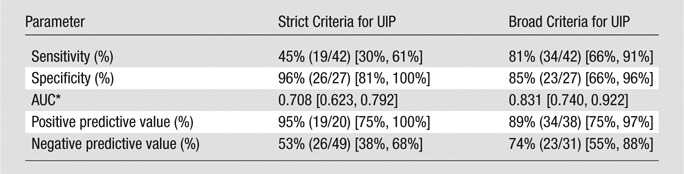
Note.—Data in parentheses are the raw data used to calculate percentages. Data in brackets are 95% confidence intervals.
AUC = Area under the receiver operating characteristic curve.
Discussion
Findings of our study suggest that histopathologic UIP in RA-ILD can be determined with a high degree of confidence when the radiologic UIP pattern is present on a CT scan. Given emerging data that identifying histopathologic UIP has important clinical implications for RA-ILD patients, a method of identifying it without the risk and cost of surgical lung biopsy is valuable. Although loosening the radiologic criteria to include those with possible UIP pattern on a CT scan resulted in a better overall performance (as judged by the area under the curve analysis), specificity and interobserver agreement between radiologists suffers. Because reliability and a high positive predictive value are critical for a surrogate diagnostic test for histopathologic UIP, we propose that only radiologic UIP pattern (and not radiologic possible UIP pattern) be considered diagnostic of histopathologic UIP in RA-ILD.
The criteria for radiologic UIP pattern on a CT scan used in this study were derived and validated by using patients suspected of having IPF (14). To our knowledge, the radiologic UIP pattern has not been validated in other conditions that manifest with UIP pattern, primarily RA-ILD. Our findings are consistent with the test performance of CT in depicting histopathologic UIP pattern in IPF. Raghu et al (15) prospectively assessed the correlation between clinical, radiologic, and pathologic diagnosis of ILD in patients referred to a tertiary care center. Among 29 patients with a histopathologic diagnosis of IPF, CT had a specificity of 90% and a sensitivity of 79%. Hunninghake et al (16) identified 91 patients suspected of having IPF. Among those, 54 patients had UIP pattern on histopathologic examination. The specificity of CT for a radiologic UIP pattern was 95%, with a sensitivity of 85%. Subsequently, Flaherty et al (19) assessed a prospective cohort of 96 patients with idiopathic interstitial pneumonia, including 73 with histopathologic UIP pattern. All 27 patients who had a radiologic UIP pattern on a CT scan had UIP pattern on histopathologic examination, with a specificity of 100% and a sensitivity of 37%. More recently, Aalokken et al (20) studied 91 patients with idiopathic interstitial lung disease and found the specificity of CT UIP pattern to be 96%, with a sensitivity of 63%.
We found good interrater agreement of radiologic UIP. Our observed agreement is similar to what has been previously published in the IPF literature. In the study by Hunninghake et al (16), the interrater agreement for radiologists in determining the radiologic UIP pattern was fair at 77%, with a κ value of 0.54. Aalokken et al (20) found very good agreement between the two radiologists reading the CT scans (with 18 and 12 years of experience), with a κ value of 0.79. The differences in agreement may be due in part to differences in the prevalence of histopathologic UIP in each of the cohorts.
Our study had some limitations. First, given that the CT images were obtained at different centers, there may have been some differences in the techniques of image acquisition, patient positioning, and processing of the scan. However, all three centers were tertiary care hospitals with expertise in ILD and protocols for obtaining CT scans in these patients. Second, the histopathologic diagnosis of UIP was determined by separate pathologists at their respective institutions. However, one pathologist (T.V.C.) provided a second reading for 52 of 69 patients in the study. We found good agreement between the readers for the histopathologic UIP pattern. This is similar to what has been previously described in the literature for the idiopathic interstitial pneumonias (21,22). Last, given that this population includes only biopsy-proved RA-ILD cases, there may be spectrum bias and these findings may be less generalizable to all patients with RA-ILD.
In summary, we have shown that the widely accepted and validated CT features for a radiologic UIP pattern used to make a diagnosis of IPF can be used to identify the UIP pattern in RA-ILD patients, with a high degree of confidence. Similar to the literature for the idiopathic interstitial pneumonias, the histopathologic subtype likely has important prognostic and potentially therapeutic implications in patients with RA-ILD (7–9). Given the physiologic impairment seen in this patient population and the risks associated with surgical lung biopsy, the accurate identification of the UIP pattern in patients with RA-ILD by using noninvasive diagnostic tests such as CT is important.
Advances in Knowledge
■ CT of the chest can be used to correctly identify the histopathologic usual interstitial pneumonia (UIP) pattern in patients with rheumatoid arthritis–associated interstitial lung disease (RA-ILD), with a positive predictive value of 95% (19 of 20).
■ In patients with RA-ILD, a definite CT UIP pattern (composed of basal and subpleural interstitial reticulations and fibrosis, traction bronchiectasis, and honeycombing) has a specificity of 96% (26 of 27) and a sensitivity of 45% (19 of 42) for the histopathologic UIP pattern.
Implication for Patient Care
■ The use of CT in RA-ILD can help confirm the presence of UIP pattern, a histopathologic phenotype associated with increased mortality in this population.
SUPPLEMENTAL TABLES
Received January 30, 2013; revision requested March 28; revision received May 20; accepted June 14; final version accepted August 7.
Funding: This research was supported by the National Institutes of Health (grant KL2 RR024130).
Disclosures of Conflicts of Interest: D.A. No relevant conflicts of interest to disclose. B.M.E. No relevant conflicts of interest to disclose. T.H.U. No relevant conflicts of interest to disclose. T.V.C. No relevant conflicts of interest to disclose. B.H.K. No relevant conflicts of interest to disclose. J.H.R. No relevant conflicts of interest to disclose. T.E.K. No relevant conflicts of interest to disclose. H.R.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received consultancy fees from Biogen, FibroGen, Genoa, Gilead, InterMune, MedImmune, Mesoblast, and Promedior; grants from Boehringer-Ingelheim, Genentech, and NIH/NHLBI; royalties from UpToDate; and payment for development of educational presentations from MedScape. Other relationships: none to disclose. D.S.K. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author received fees as an advisory board (steering committee) member for Nintedanib (BIBF 1120). Other relationships: none to disclose. J.S.L. Financial activities related to the present article: author and institution received a grant from KL2 (career investigator award). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose.
Abbreviations:
- ILD
- interstitial lung disease
- IPF
- idiopathic pulmonary fibrosis
- NSIP
- nonspecific interstitial pneumonia
- RA
- rheumatoid arthritis
- UIP
- usual interstitial pneumonia
References
- 1.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 2001;27(2):269–281. [DOI] [PubMed] [Google Scholar]
- 2.Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc 2007;4(5):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005;127(6):2019–2027. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinouchi T, Ohtsuki Y, Fujita J, et al. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol Int 2005;26(2):121–125. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Kim JS, Newell JD, et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology 2004;232(1):81–91. [DOI] [PubMed] [Google Scholar]
- 6.Kim EJ, Collard HR, King TE, Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009;136(5):1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132(1):214–220. [DOI] [PubMed] [Google Scholar]
- 8.Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Curr Opin Rheumatol 2008;20(3):340–346. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35(6):1322–1328. [DOI] [PubMed] [Google Scholar]
- 10.Akira M, Sakatani M, Hara H. Thin-section CT findings in rheumatoid arthritis-associated lung disease: CT patterns and their courses. J Comput Assist Tomogr 1999;23(6):941–948. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya Y, Takayanagi N, Sugiura H, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J 2011;37(6):1411–1417. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Suda T, Kaida Y, et al. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir Med 2012;106(8):1164–1169. [DOI] [PubMed] [Google Scholar]
- 13.Caples SM, Utz JP, Allen MS, Ryu JH. Thoracic surgical procedures in patients with rheumatoid arthritis. J Rheumatol 2004;31(11):2136–2141. [PubMed] [Google Scholar]
- 14.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis—evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183(6):788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest 1999;116(5):1168–1174. [DOI] [PubMed] [Google Scholar]
- 16.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164(2):193–196. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31(3):315–324. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society; European Respiratory Society . American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165(2):277–304. [Published correction appears in Am J Respir Crit Care Med 2002;166(3):426.] [DOI] [PubMed] [Google Scholar]
- 19.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58(2):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaløkken TM, Naalsund A, Mynarek G, et al. Diagnostic accuracy of computed tomography and histopathology in the diagnosis of usual interstitial pneumonia. Acta Radiol 2012;53(3):296–302. [DOI] [PubMed] [Google Scholar]
- 21.Thomeer M, Demedts M, Behr J, et al. Multidisciplinary interobserver agreement in the diagnosis of idiopathic pulmonary fibrosis. Eur Respir J 2008;31(3):585–591. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson AG, Addis BJ, Bharucha H, et al. Inter-observer variation between pathologists in diffuse parenchymal lung disease. Thorax 2004;59(6):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


