The study findings—that younger women undergoing breast biopsy have lower short-term quality-of-life scores—suggest that tailored prebiopsy counseling may lead to an improved breast biopsy experience.
Abstract
Purpose
To examine the effects of percutaneous breast biopsy on short-term quality of life.
Materials and Methods
The institutional review board approved this HIPAA-compliant prospective study. From December 1, 2007, through February 28, 2010, women undergoing percutaneous breast biopsy in an academic medical center were recruited to participate in a mixed-mode survey 2–4 days after biopsy. Patients described their biopsy experience by using the Testing Morbidities Index (TMI), a validated instrument for assessing short-term quality of life related to diagnostic testing. The scale ranged from 0 (worst possible experience) to 100 (no adverse effects). Seven attributes were assessed: pain or discomfort before and during testing, fear or anxiety before and during testing, embarrassment during testing, and physical and mental function after testing. Demographic and clinical information were also collected. Univariate and multivariate linear regression analyses were performed to identify significant predictors of TMI score.
Results
In 188 women (mean age, 51.4 years; range, 22–80 years), the mean TMI score (±standard deviation) was 82 ± 12. Univariate analysis revealed age and race as significant predictors of the TMI score (P < .05). In the multivariate model, only patient age remained a significant independent predictor (P = .001). TMI scores decreased by approximately three points for every decade decrease in patient age, which suggests that younger women were more adversely affected by the biopsy experience.
Conclusion
Younger patient age is a significant predictor of decreased short-term quality of life related to percutaneous breast biopsy procedures. Tailored prebiopsy counseling may better prepare women for percutaneous biopsy procedures and improve their experience.
© RSNA, 2013
Introduction
In the United States, approximately 229 060 new cases of breast cancer were diagnosed in 2012 (1). Given the magnitude of this disease, efforts are continually focused on improving diagnostic techniques and treatment methods. Although the mortality benefit from screening mammography is well established, false-positive test results are a potential harm (2). Approximately 10% of women who undergo mammographic screening are recalled for additional diagnostic evaluation, and more than 500 000 women in the United States undergo a breast biopsy each year (1,3,4).
Compared with open surgical breast biopsies, percutaneous core needle biopsies are associated with decreased postprocedural complications, lead to fewer overall surgeries, and are more cost-effective (5–8). Negative effects on a patient’s short-term quality of life are smaller with percutaneous biopsy than with surgical breast biopsy, as exemplified by improved functional health domains (eg, postprocedural physical performance, physical functioning, level of pain, and social performance) (9,10). Nevertheless, there is still room for improvement. Percutaneous breast biopsies can be associated with pain and emotional distress (11,12). Understanding the factors that contribute to a woman’s diagnostic experience may help direct changes targeted at improving the percutaneous breast biopsy process. The purpose of this study was to examine the effects of percutaneous breast biopsy on short-term quality of life.
Materials and Methods
Participant Recruitment
This study was approved by the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Women undergoing breast biopsy in an academic medical center were recruited for participation in a larger study with 1285 male and female participants aimed at developing the Testing Morbidities Index (TMI) measure of short-term quality of life (13). English- and Spanish-speaking women who underwent a percutaneous breast biopsy from December 1, 2007, through February 28, 2010, with ultrasonographic (US), stereotactic, or magnetic resonance (MR) imaging guidance were eligible to participate. Participants who provided informed consent were randomly assigned to one of three groups and asked to complete surveys for the purpose of valuation of diagnostic testing attributes, index scaling, and validation. For this study, the subgroup of women (n = 188) who completed the validation surveys were included, with the sample size based on the power calculations for the primary study aims.
Data Collection
On the day of biopsy and preceding the procedure, women who agreed to participate in the study were given the survey materials. Telephone interviews occurred within 4 days after biopsy and before results from pathologic examination were known, allowing patients to review the survey and focus on the testing experience without the influence of knowing the biopsy results. Mixed-mode survey approaches, including the telephone survey, have been used successfully in previous studies (10,14–17). The participants were asked to describe their biopsy encounter in terms of the seven attributes of diagnostic testing that make up the TMI: (a) pain or discomfort before the test, (b) pain or discomfort during the test, (c) fear or anxiety before the test, (d) fear or anxiety during the test, (e) embarrassment during the test, (f) physical function after testing, and (g) mental function after testing. For the first five attributes, the patients defined their experience with use of a five-point scale, where 1 = none, 2 = mild, 3 = moderate, 4 = severe, and 5 = extreme. The following scale was used for the final two attributes: 1 = no problems, 2 = mild problems, 3 = moderate problems, 4 = severe problems, and 5 = extreme problems.
The surveys also included questions about the patient’s satisfaction with their clinic experience (17). Three statements were included: “the waiting room area was comfortable,” “the staff showed concern for my concerns and worries,” and “the doctor explained what to expect during my examination and biopsy.” Patients were asked to indicate their level of agreement on a scale of 1–4, where 1 = strongly agree, 2 = somewhat agree, 3 = somewhat disagree, and 4 = strongly disagree. The survey also incorporated two questions aimed at assessing the patient’s numeracy or ability to comprehend and apply numeric concepts (18): “Which of the following numbers represents the biggest risk of getting a disease?” (with answer choices of “1 in 100,” “1 in 1000,” “1 in 10,” and “don’t know”) and “Which of the following represents the biggest risk of getting a disease?” (with answer choices of “1%,” “10%,” “5%,” and “don’t know”).
Demographic and clinical history information were also collected, including age, race, personal history of breast cancer, family history of breast cancer, history of previous biopsy, and years of education. The type of imaging guidance used for the biopsy was recorded, as were the results of pathologic examination after percutaneous biopsy and any subsequent surgical excision.
Data Analysis
With use of the elicited values for each of the seven testing attributes, a TMI score was calculated for each patient’s biopsy experience with the model described separately by Swan et al (13). The TMI instrument can be used to calculate three types of scores. The first two types of scores are preference based (19,20), in which testing attributes are weighted in a function that provides values on a scale of 0 (equivalent to death) to 1 (equivalent to perfect health), enabling comparison of different health states. Preferences can incorporate the patient or the societal perspective and are most useful in economic analyses of medical interventions. The third type of score is psychometric in orientation, which gives equal weight to each attribute and ranges from 7 (all best scores) to 35 (all worst scores). We converted these scores to a scale of 0–100, where 0 represents the worst possible overall testing experience and 100 the best. For this analysis, the psychometric score was chosen as the primary outcome variable because the equal weighting of test attributes produced a broader distribution of scores than the weighted patient or societal preference-based scores.
Univariate and multivariate linear regression analyses were conducted by using software (version 9.2; SAS, Cary, NC) to identify significant predictors of the outcome variable (TMI score). P < .05 was considered indicative of a significant difference. Predictor variables included demographic information, clinical history, pathologic results, and patient numeracy. A Wilcoxon rank sum test was used for the dichotomous predictors (ie, race [white vs other], personal history of breast cancer, family history of breast cancer, final pathology results [benign vs malignant], and numeracy). The Kruskal-Wallis test was used for ordinal or categoric variables with three or more levels (eg, patient age, biopsy type, previous biopsy, and years of education). Variables with P < .1 at univariate analysis of the TMI score were then included in a multivariate regression model to identify significant independent predictors. In addition, the association between the clinic satisfaction question responses and the TMI score was tested with Spearman rank correlations. Clinic satisfaction questions that were significantly associated with TMI score were subsequently included in the multivariate model.
Results
Study Population
Of 203 women approached on the day of biopsy, four declined to participate after reviewing the survey materials and 11 could not be contacted within the period of eligibility. A total of 188 women completed the telephone survey after undergoing percutaneous breast biopsy (Table 1), for a response rate of 93% (188 of 203 patients). The mean patient age was 51.4 years (range, 22–80 years). Most patients were white, had at least 16 years of formal education, and had no personal or family history of breast cancer. Stereotactic mammography was the most frequent mode of biopsy guidance, followed by US; MR imaging guidance accounted for 17.6% of the biopsies. The final pathologic diagnosis was malignant disease in 64 of the 188 patients (34%). Of these 64 patients, 14 (22%) had ductal carcinoma in situ and 50 (78%) had invasive carcinoma. More than half of the women had previously undergone biopsy of their breast or another body part.
Table 1.
Patient Characteristics
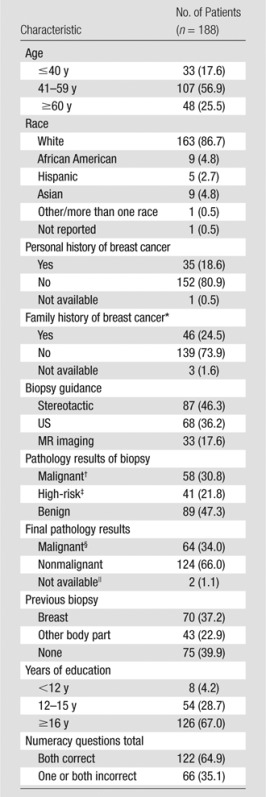
Note.—Data are numbers of patients, with percentages in parentheses. The mean patient age at enrollment was 51.4 years ± 12.1 (range, 22–80 years).
Includes any first-degree relative (mother, father, or sister).
Ductal carcinoma in situ, invasive ductal carcinoma, or invasive lobular carcinoma.
Atypical ductal hyperplasia, lobular carcinoma in situ, atypical lobular hyperplasia, flat epithelia atypia, radial scar, papilloma, phyllodes tumor, fibroepithelial lesion (cannot rule out phyllodes), or discordant pathologic findings.
Six high-risk lesions were upgraded to malignant. Fourteen of the 64 patients (22%) had ductal carcinoma in situ and 50 (78%) had invasive ductal or lobular carcinoma.
These are two cases of high-risk pathology at biopsy with no record of subsequent pathology at our institution.
Biopsy Experience
The three testing attributes with the highest mean values were fear and anxiety before the test, fear and anxiety during the test, and pain and discomfort during the test, with mean scores of 2.3, 2.1, and 2.2, respectively (Figure). Fifty-two of the 188 patients (28%) reported having moderate fear or anxiety before the test; 21 patients (11%) reporting having severe or extreme fear before the procedure. Similarly, 51 of the 188 patients (27%) reported having moderate fear or anxiety during the test; nine patients (5%) reported having severe or extreme fear during the procedure. Forty of the 188 patients (21%) reported having moderate pain or discomfort during the procedure; 15 patients (8%) reported having severe to extreme discomfort.
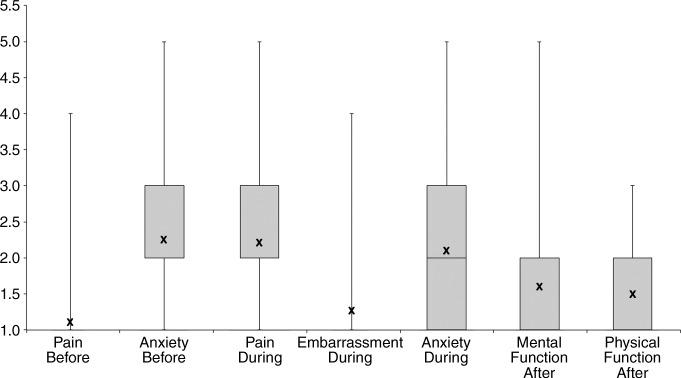
TMI attributes. Box plot shows distribution of responses to the seven components of diagnostic testing that make up the TMI. For each component, the patient rated her experience on a five-point scale. X = mean score for each component.
The mean TMI psychometric score (±standard deviation) was 82 ± 11.7 (range, 21–100). Mean TMI scores stratified according to patient characteristics are presented in Table 2.
Table 2.
Predictors of TMI Score at Univariate and Multivariate Analyses
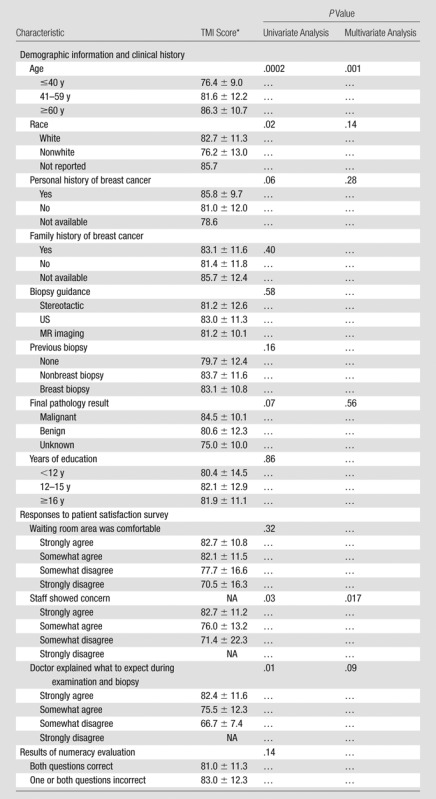
Data are means or means ± standard deviations. NA = not applicable.
Biopsy Experience and Demographic Variables
At univariate analysis (Table 2), patient age and race (white vs nonwhite) were identified as significant predictors of TMI score (P < .0002 and P < .02, respectively). Because the P values for personal history of breast cancer and final pathology results (benign vs malignant) were less than .1, they were also included in multivariate modeling. In the multivariate model, after adjustment for personal history of breast cancer, race, and cancer status, only patient age (examined as categoric and continuous predictors) remained a significant independent predictor of TMI score (P = .001). When age was examined in groups (≤40, 41–59, and ≥60 years), TMI scores were lowest in the youngest age group. When age was examined as a continuous predictor, it remained a significant predictor (P = .001), with short-term quality of life decreasing by approximately three points for every decade decrease in patient age.
Biopsy Experience and Clinic Satisfaction
Spearman correlations for the three statements assessing clinic satisfaction and TMI score showed that responses to “the staff showed concern for my concerns and worries,” and “the doctor explained what to expect” showed a negative correlation with the TMI scores (P = .03 and P = .01, respectively). Patients who disagreed with the statements had lower TMI scores. In the multivariate model, patient response to the “staff showed concern” statement was a significant predictor of TMI score (P = .017).
Discussion
In our study, the TMI was used to assess the effect of percutaneous breast biopsies on women’s short-term quality of life. The TMI was developed with both psychometric and preference-based scaling. The psychometric score, with equal weighting of each attribute of diagnostic testing, provided the broadest distribution of scores and was chosen as the primary outcome measure for this study. When participants were surveyed about their biopsy experiences, their mean TMI score was 82 out of 100. Preference-based or “utility” values for short-term health states, which are anchored on death (utility score = 0) and perfect health (utility score = 1.0) are useful for comparing different health states and are frequently used in cost-effectiveness analyses. With use of the models developed by Swan et al (13), these same 188 women had a mean preference-based score for breast biopsy of 0.84 ± 0.06.
The psychometric and utility scores were strongly correlated (r = 0.94). Because the TMI has yet to be widely applied and its psychometric and utility scores are highly correlated, comparison of published utilities for diagnostic testing and short-term interventions can provide some context for these breast biopsy experience scores. Normal prenatal testing, composed of amniocentesis and chorionic villous sampling, had a utility value of 0.92 (21), and colposcopy or cervix biopsy was given a value of 0.89 (22). In addition, an instrumental vaginal delivery had a utility value of 0.76, whereas an emergency cesarean delivery had a utility value of 0.59 (23).
Survey respondents reported that the three attributes of diagnostic testing that most influenced their short-term quality of life were anxiety before and during the biopsy and pain during the procedure. Our results showing that pre- and postprocedural anxiety contribute substantially to the adverse effects of breast biopsy on short-term quality of life are similar to those noted in previously published reports using different instruments. By using the State Trait Anxiety Inventory (24), Maxwell et al (12) demonstrated that the prebiopsy mean State Anxiety scores were significantly higher in women awaiting a core needle breast biopsy than in control subjects. A subset of these women also indicated symptoms of acute stress disorder at 5 and 30 days after the procedure. Similarly, Flory and Lang (25), also using the State Trait Anxiety Inventory, found that the preprocedural anxiety associated with a breast biopsy was greater than that associated with more invasive procedures (eg, hepatic chemoembolization and uterine fibroid embolization) and was attributable to the diagnostic uncertainty associated with the breast biopsy.
Univariate analyses demonstrated that age and race were significant predictors of short-term quality of life related to breast biopsy. However, the multivariate model showed only patient age to be an independent predictor of the overall TMI score, suggesting that younger women are more adversely affected by percutaneous breast biopsies. The perceived concern of the staff in the radiology clinic was identified as an additional significant predictor of the TMI score, independent of patient age, serving as a reminder that all breast clinic staff who encounter patients can influence their care experiences.
We included guidance for percutaneous biopsy as a predictor variable, hypothesizing that women who underwent a stereotactic core biopsy or MR imaging–guided core biopsy might have lower TMI scores than women who underwent US-guided procedures. Although stereotactic and MR imaging–guided biopsies have less comfortable positioning, require breast compression throughout the procedure, and have longer average biopsy procedure times than US-guided procedures, there was no significant association between biopsy guidance and TMI score. We attribute this finding to the larger relative contribution of pre- and periprocedural anxiety to the observed effects on short-term quality of life.
Previous investigations examining the relationship between age and the functional impact of percutaneous breast biopsy have demonstrated that younger women have greater stress in the diagnostic period of breast-related disease, similar to our findings. In women who underwent breast biopsy and had benign results, Steffens et al (26) found that younger women were more distressed not only by the biopsy experience but also by some of the steps leading up to the biopsy, such as awaiting diagnostic mammography after a screening examination with abnormal findings. Using the State Trait Anxiety Inventory, Seckel and Birney (27) found that as women approach the age of 40 years, biopsy-associated stress increased; however, beyond age 40 years the degree of stress decreased with patient age.
Different theories exist as to why younger women are more negatively affected by percutaneous breast biopsies, regardless of final pathology results. Younger women, who are generally healthier than older women, probably have had less experience with the health care system (28), leading to greater uncertainty and anxiety during the diagnostic process. In our cohort, the mean age of the participants who had never undergone biopsy was 48.4 years, whereas the mean age of women with previous biopsy experience was 53.4 years. Second, younger women are more negatively influenced by a diagnosis of breast cancer. Younger women are more likely to be engaged in child-rearing and professional activities and thus may face the possibility of a life-threatening illness with greater fear and anxiety than older women who have completed that phase of life (29). Kroenke et al (30) reported that women younger than 40 years in whom invasive breast cancer was diagnosed had the greatest absolute and relative decline in their physical and social function, as well as mental health, compared with middle-aged or elderly women given a recent diagnosis. Finally, some authors have proposed that younger women may have coping mechanisms that are less developed than those of older women (31).
A review of the effect of cancer prevention and screening on short-term quality of life (32) noted that descriptive measurement of psychologic states and symptoms informs and improves shared decision-making between individual patients and clinicians; however, if study goals included assessment and comparison of programmatic benefits, harms, or costs, then preference-based instruments would be needed. Another methodologic challenge noted by Cullen et al (32) is the possibility of decreased sensitivity of chronic health state instruments, such as the EuroQol (33) or Time-Trade-Off (34), when used for measuring transient effects. The TMI addresses this challenge of measuring short-term functional health on a preference-based or psychometric scale, and previous studies have shown early evidence of content, construct, and discriminative validity of the TMI (13,35).
Limitations of this study include those intrinsic to mixed-mode surveys, particularly nonresponse bias. However, our response rate of 93% mitigates the potential effect of this bias. In addition, the demographic characteristics of the study population—which primarily consisted of well-educated white women—reflect those of the institution and geographic region (36). A larger sample size with a greater representation across races and education levels would have allowed for more generalizable results.
Percutaneous breast biopsies are the current standard for breast cancer diagnosis, and, given the number of women who will ultimately undergo such a biopsy, continued efforts to improve the experience are warranted. Our study findings—that younger women undergoing breast biopsy have lower short-term quality-of-life scores—suggest that tailored prebiopsy counseling may lead to an improved breast biopsy experience.
Advances in Knowledge
■ The Testing Morbidities Index indicates that percutaneous breast biopsy is associated with a mild-to-moderate short-term decline in a patient’s quality of life.
■ Women aged 40 years or younger had lower quality-of-life scores when undergoing percutaneous breast biopsy than women older than 40 years, suggesting that younger women are more adversely affected by the biopsy experience.
Implication for Patient Care
■ Tailored prebiopsy counseling may better prepare women for percutaneous breast biopsy and improve their overall experience.
Current address: Department of Radiology, University of Washington, Seattle, Wash.
Current address: Department of Medicine, Renal Division, Emory University, Atlanta, Ga.
Current address: Department of Obstetrics and Gynecology, Wayne State University, Detroit, Mich.
Received April 11, 2013; revision requested May 20; revision received July 1; accepted July 15; final version accepted August 15.
J.M.L., K.D., C.Y.K., O.W., O.I., E.F.H., J.S.S. are supported by the American Cancer Society (114130-RSGHP-07-266-01-CPHPS). J.S.S. is supported by the Massachusetts General Hospital Executive Committee on Research (Bridge Fund 1200-218421).
Funding: This research was supported by the National Institutes of Health (grants K07-CA128816 and K25-CA133141).
Disclosures of Conflicts of Interest: K.L.H. No relevant conflicts of interest to disclose. J.M.L. No relevant conflicts of interest to disclose. K.D. No relevant conflicts of interest to disclose. C.Y.K. No relevant conflicts of interest to disclose. O.W. No relevant conflicts of interest to disclose. O.I. No relevant conflicts of interest to disclose. E.F.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for Hologic; received payment for expert testimony from Ameritox; received payment for lectures including service on speakers bureaus from Barnett International. Other relationships: none to disclose. B.J.G. No relevant conflicts of interest to disclose. E.A.R. No relevant conflicts of interest to disclose. J.S.S. No relevant conflicts of interest to disclose.
Abbreviation:
- TMI
- Testing Morbidities Index
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151(10):727–737, W237–W242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006;241(1):55–66. [DOI] [PubMed] [Google Scholar]
- 4.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology 2005;235(3):775–790. [DOI] [PubMed] [Google Scholar]
- 5.Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med 2010;152(4):238–246. [DOI] [PubMed] [Google Scholar]
- 6.Fajardo LL, Pisano ED, Caudry DJ, et al. Stereotactic and sonographic large-core biopsy of nonpalpable breast lesions: results of the Radiologic Diagnostic Oncology Group V study. Acad Radiol 2004;11(3):293–308. [DOI] [PubMed] [Google Scholar]
- 7.White RR, Halperin TJ, Olson JA, Jr, Soo MS, Bentley RC, Seigler HF. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg 2001;233(6):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt JH, Sunshine JH. Core-needle and surgical breast biopsy: comparison of three methods of assessing cost. Radiology 1999;212(1):181–188. [DOI] [PubMed] [Google Scholar]
- 9.Verkooijen HM, Buskens E, Peeters PH, et al. Diagnosing non-palpable breast disease: short-term impact on quality of life of large-core needle biopsy versus open breast biopsy. Surg Oncol 2002;10(4):177–181. [DOI] [PubMed] [Google Scholar]
- 10.Swan JS, Lawrence WF, Roy J. Process utility in breast biopsy. Med Decis Making 2006;26(4):347–359. [DOI] [PubMed] [Google Scholar]
- 11.Hemmer JM, Kelder JC, van Heesewijk HP. Stereotactic large-core needle breast biopsy: analysis of pain and discomfort related to the biopsy procedure. Eur Radiol 2008;18(2):351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell JR, Bugbee ME, Wellisch D, Shalmon A, Sayre J, Bassett LW. Imaging-guided core needle biopsy of the breast: study of psychological outcomes. Breast J 2000;6(1):53–61. [DOI] [PubMed] [Google Scholar]
- 13.Swan JS, Kong CY, Lee JM, et al. Patient and societal value functions for the testing morbidities index. Med Decis Making 2013;33(6):819–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Wijck EE, Bosch JL, Hunink MG. Time-tradeoff values and standard-gamble utilities assessed during telephone interviews versus face-to-face interviews. Med Decis Making 1998;18(4):400–405. [DOI] [PubMed] [Google Scholar]
- 15.Swan JS, Fryback DG, Lawrence WF, Sainfort F, Hagenauer ME, Heisey DM. A time-tradeoff method for cost-effectiveness models applied to radiology. Med Decis Making 2000;20(1):79–88. [DOI] [PubMed] [Google Scholar]
- 16.Swan JS, Sainfort F, Lawrence WF, Kuruchittham V, Kongnakorn T, Heisey DM. Process utility for imaging in cerebrovascular disease. Acad Radiol 2003;10(3):266–274. [DOI] [PubMed] [Google Scholar]
- 17.Donelan K, Mailhot JR, Dutwin D, et al. Patient perspectives of clinical care and patient navigation in follow-up of abnormal mammography. J Gen Intern Med 2011;26(2):116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making 2001;21(1):37–44. [DOI] [PubMed] [Google Scholar]
- 19.Hunink M, Glasziou P, Siegel J, et al. Decision making in health and medicine: integrating evidence and values. Cambridge, England: Cambridge University Press, 2001; 88–127. [Google Scholar]
- 20.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. New York, NY: Oxford University Press, 2005; 137–209. [Google Scholar]
- 21.Harris RA, Washington AE, Nease RF, Jr, Kuppermann M. Cost utility of prenatal diagnosis and the risk-based threshold. Lancet 2004;363(9405):276–282. [DOI] [PubMed] [Google Scholar]
- 22.Insinga RP, Glass AG, Myers ER, Rush BB. Abnormal outcomes following cervical cancer screening: event duration and health utility loss. Med Decis Making 2007;27(4):414–422. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Ivy JS, Patel DA, et al. Pelvic floor consequences of cesarean delivery on maternal request in women with a single birth: a cost-effectiveness analysis. J Womens Health (Larchmt) 2010;19(1):147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spielberger C. Manual for state-trait anxiety inventory (STAI). Redwood, Calif: Mindgarden, 1983. [Google Scholar]
- 25.Flory N, Lang EV. Distress in the radiology waiting room. Radiology 2011;260(1):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffens RF, Wright HR, Hester MY, Andrykowski MA. Clinical, demographic, and situational factors linked to distress associated with benign breast biopsy. J Psychosoc Oncol 2011;29(1):35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seckel MM, Birney MH. Social support, stress, and age in women undergoing breast biopsies. Clin Nurse Spec 1996;10(3):137–143. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau. Health and nutrition: health care utilization. In: Statistical abstract of the United States 2012. http://www.census.gov/compendia/statab/cats/health_nutrition/health_care_utilization.html. Accessed November 20, 2012.
- 29.Blank TO, Bellizzi KM. A gerontologic perspective on cancer and aging. Cancer 2008;112(11 Suppl):2569–2576. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke CH, Roster B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol 2004;22(10):1849–1856. [DOI] [PubMed] [Google Scholar]
- 31.Mehlsen M, Jensen AB, Christensen S, Pedersen CG, Lassesen B, Zachariae R. A prospective study of age differences in consequences of emotional control in women referred to clinical mammography. Psychol Aging 2009;24(2):363–372. [DOI] [PubMed] [Google Scholar]
- 32.Cullen J, Schwartz MD, Lawrence WF, Selby JV, Mandelblatt JS. Short-term impact of cancer prevention and screening activities on quality of life. J Clin Oncol 2004;22(5):943–952. [DOI] [PubMed] [Google Scholar]
- 33.Dolan P, Roberts J. Modelling valuations for Eq-5d health states: an alternative model using differences in valuations. Med Care 2002;40(5):442–446. [DOI] [PubMed] [Google Scholar]
- 34.Torrance GW. Social preferences for health states: an empirical evaluation of three measurement techniques. Socioecon Plann Sci 1976;10(3):129–136. [Google Scholar]
- 35.Swan JS, Ying J, Stahl J, et al. Initial development of the temporary utilities index: a multiattribute system for classifying the functional health impact of diagnostic testing. Qual Life Res 2010;19(3):401–412. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Census Bureau. 2010 Census data. http://2010.census.gov/2010census/data/. Published online 2010. Accessed November 20, 2012.


