Endothelialization of the aneurysm neck proceeds from the parent artery and depends on an underlying smooth muscle cell substrate.
Abstract
Purpose
To characterize the progression of healing across aneurysm necks following treatment with a flow diverter in a rabbit aneurysm model.
Materials and Methods
With institutional animal care and use committee approval, saccular aneurysms were created in 20 rabbits and treated with flow diverters. On days 1, 3, and 7 and weeks 4 and 8 after implantation, the aneurysm and the device-implanted vessel were harvested. En face staining of the gross specimen was performed for endothelial cells, endothelial progenitor cells, smooth muscle cells, and inflammatory cells.
Results
The parent artery segments covered by the flow diverters were completely devoid of endothelial cells at 1 and 3 days but had completely reendothelialized by 7 days. At all time points, the struts along the patent portions of the aneurysm necks harbored scattered tissue islands composed exclusively of inflammatory cells. At 4 and 8 weeks, all samples contiguous with the tissue along the parent arteries had translucent tissue present along the occluded segments of the aneurysm neck. The vast majority of endothelial cells were contiguous with the parent artery and had smooth muscle cells underlying them. Endothelial progenitor cells were not observed along the neck of any aneurysm. Aneurysm closure was noted only when complete or nearly complete endothelialization over the device struts was present.
Conclusion
The initial event following flow diversion treatment is adherence of clusters of inflammatory cells across the aneurysm neck. Endothelialization is relatively delayed and derived exclusively from cells in the adjacent parent artery.
© RSNA, 2013
Introduction
Flow diverters provide a noninvasive way of treating aneurysms that are otherwise difficult to treat with other endovascular techniques. Even though flow diverters are widely applied, their exact mechanism of action remains unknown. Notwithstanding the term flow diverter, which implies that the primary mode of action is diversion of flow from the aneurysm (1–4), it remains unclear whether change in intraaneurysmal flow, tissue growth across the neck, or both processes are dominant predictors of long-term occlusion. Ongoing uncertainty regarding the exact healing mechanism associated with flow diverters has myriad consequences, including but not limited to impeding our ability to develop next-generation devices. The objective of our study was to characterize the progression of healing across aneurysm necks following treatment with a flow diverter in a rabbit model.
Materials and Methods
In Vivo Experiments
The institutional animal care and use committee approved all procedures before the initiation of our study. Between January and November 2012, aneurysms were created in 20 female New Zealand white rabbits and treated by using a flow diverter (Pipeline Embolization Device; Covidien, Irvine, Calif), as previously described (1,5). Digital subtraction angiography (DSA) of the aortic arch was performed immediately after treatment. Rabbits were observed for 1 day (n = 3), 3 days (n = 3), 7 days (n = 3), 4 weeks (n = 2), or 8 weeks (n = 9). Two days before embolization, rabbits in the 8-week group were orally premedicated with aspirin (10 mg per kilogram of body weight) and clopidogrel (10 mg/kg) as per previous studies (6,7); this medication regimen was continued for 1 month after embolization. At the time of sacrifice, animals were deeply anesthetized. Follow-up DSA of the aortic arch was performed (Y.H.D., with more than 10 years experience in neurovascular imaging). The animals were then euthanized by using a lethal injection of pentobarbital. The aneurysm and flow diverter–implanted parent vessel were harvested, and samples were immediately fixed in 10% neutral buffered formalin (D.D., with more than 10 years experience in histopathology).
Angiographic Evaluation
Images from DSA obtained immediately after device implantation and just before sacrifice were evaluated. Follow-up DSA images were assessed by using a trichotomous scale (incomplete occlusion, near-complete occlusion, or complete occlusion) (Y.H.D.).
Whole-Mount En Face Immunostaining
The flow diverter–implanted arterial segment was bisected longitudinally to expose the luminal surface, photographed, and then used for whole-mount staining (D.D.) (8). Immunostaining of whole-mount specimens was then achieved by using overnight incubation with primary antibodies against CD31 (endothelial cell lineage; Dako, Carpentaria, Calif), CD34 (progenitor cell lineage; Biorbyt, San Francisco, Calif), smooth muscle actin (SMA) (smooth muscle cells; Dako), and monocytes and/or macrophages (inflammatory cells; Millipore, Billerica, Mass) at 4°C. Specific binding was visualized by using a secondary antibody: Cy3- or fluorescein isothiocyanate–conjugated immunoglobin G (Jackson Immuno Research, West Groove, Pa). Sytox green (Life Technologies, Grand Island, NY) served as a nuclear counterstain.
Histologic Analysis
After en face immunostaining, the flow diverter was removed from the implanted vessel. Then, the samples were embedded in formalin, sliced, and stained with hematoxylin-eosin (D.D.).
Results
Angiographic Findings
Findings from follow-up angiography are summarized in the Table. Angiograms showed incomplete occlusion at both 1 and 3 days; near-complete occlusion of one aneurysm and incomplete occlusion of two aneurysms at 7 days; complete occlusion of one aneurysm and incomplete occlusion of the other aneurysm at 4 weeks; and complete occlusion of four aneurysms, near-complete occlusion of two aneurysms, and incomplete occlusion of the remaining three aneurysms at 8 weeks.
Angiographic Findings in Aneurysms Treated with Flow Diverters

Histologic Findings
At 1 and 3 days, all samples showed the parent artery to be mostly devoid of endothelial cells along the arterial segments harboring the flow diverters. Small islands of tissue, primarily at the intersections of metallic struts, were scattered across the aneurysm neck. These tissue islands contained cells negative for CD31, CD34, and SMA staining. At 3 days, SMA-positive cells started to grow over the flow diverter struts in direct contiguity with the underlying arterial wall.
At 7 days, all three samples showed complete endothelialization on the surface of the flow diverter within the parent artery wall. The scattered tissue islands in the neck area consisted of inflammatory cells. A few cells within the tissue islands showed positive staining for CD31 in one sample. The cells within the tissue islands at the neck were negative for SMA and CD34 and positive for inflammatory cells in all three samples. There were some SMA-positive cells on struts in the periphery of the neck, which were contiguous with the parent artery, and it appeared that these cells had migrated or originated from the parent artery. The CD31-positive cells were observed only over SMA-positive cells.
At 4 weeks, one sample showed that the device struts at the neck orifice were covered by a thin translucent tissue. Scattered, patchy CD31-positive cells were present in the neck area covering the struts contiguous with the parent artery. Areas devoid of endothelial cells were covered with SMA-positive cells. The other 4-week sample showed bare metal struts covering the neck orifice. Both samples had complete endothelialization where the device had contact with the parent artery.
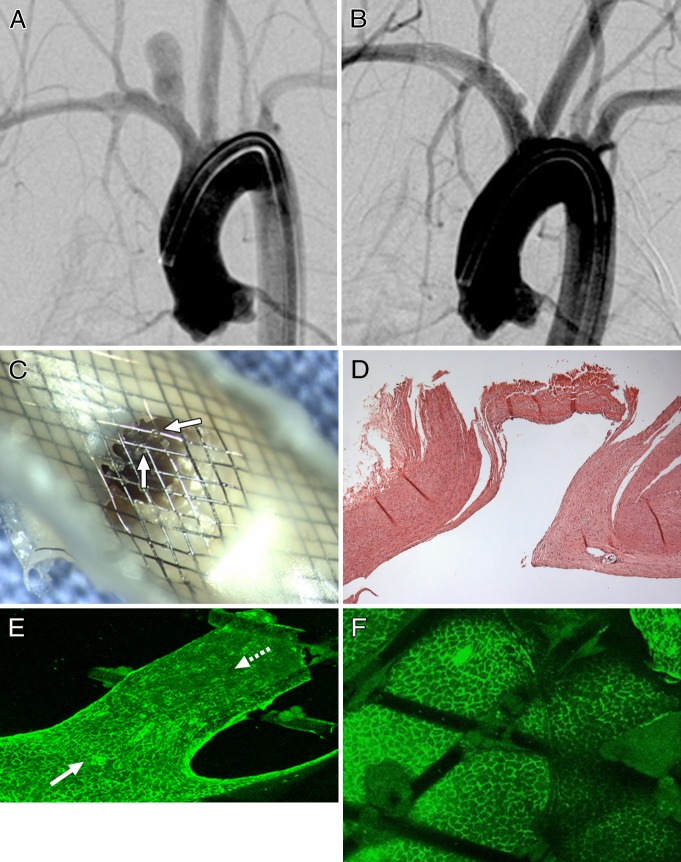
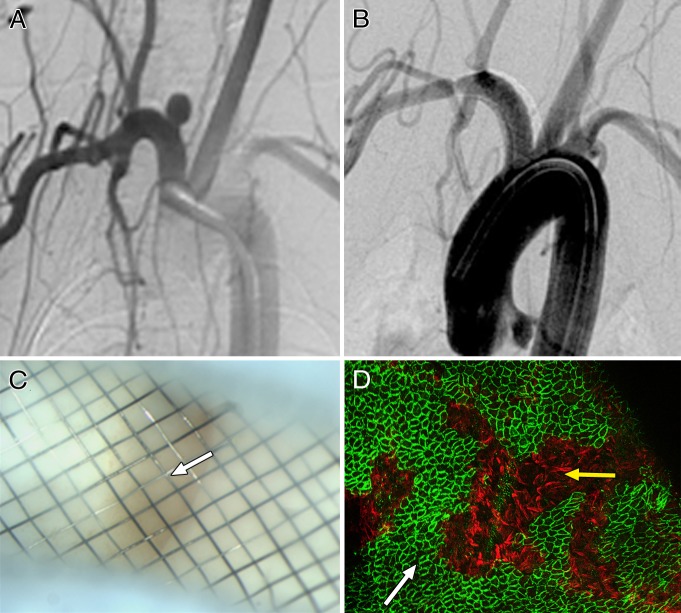
At 8 weeks, the necks of the completely occluded aneurysms were covered with translucent tissue. The flow-diverter struts adjacent to the parent artery wall were completely covered with a white neointima, which consisted of CD31- and SMA-positive cells. Discontinuous tissue islands were found attached to the devices covering the neck areas of all incompletely occluded specimens. The tissue islands present on the flow diverter surface located in the center of the neck consisted primarily of monocytes and macrophages. These were CD31-, CD34-, and SMA-negative cells. However, CD31-positive cells were noted within the tissue islands along the peripheral area of the neck, contiguous with the parent artery wall (Fig 1). Histologic analysis revealed that the domes of these aneurysms were filled with poorly organized thrombus. Two incompletely occluded aneurysms showed contiguity of endothelial cells from the parent vessel to the neck of the aneurysm (Fig 2).
Figure 1:
A, DSA image obtained immediately before flow diverter implantation shows patent aneurysm cavity. B, DSA image obtained 8 weeks after flow diverter implantation shows substantial aneurysm remnant. C, Gross image of aneurysm neck, viewed from parent artery, shows multiple separate tissue islands (arrows) partially covering the neck. Two open pores (red and white stars) along the aneurysm periphery are shown on C and D. D, Immunostained confocal microscopic image of distal aspect of aneurysm neck–parent artery interface (CD31 stain; original magnification, ×20). Note confluent coverage with CD31-positive endothelial cells along more peripherally located struts (solid arrow) contiguous with parent artery, with well-demarcated interface between CD31-positive and CD31-negative cells (dashed arrow) covering more centrally located struts.
Figure 2:
A, DSA image obtained immediately before flow diverter implantation shows patent aneurysm cavity. B, DSA image obtained 8 weeks after flow diverter implantation shows near-complete occlusion. C, Gross image shows scattered tissue islands over neck (arrows). D, Photomicrograph of histopathologic slice through midportion of neck (hematoxylin-eosin stain; original magnification, ×100) shows neck remnant with endothelialized organized thrombus deep to neck. Tissue seen on C covering the neck was dislodged during processing, so no tissue is present over the neck on this slice. E, Immunostained confocal microscopic image of a tissue island noted in C (CD31 stain; original magnification, ×20). Note confluent coverage with CD31-positive endothelial cells along more peripherally located struts (solid arrow) in direct contiguity with parent artery, with a well-demarcated interface between CD31-positive and CD31-negative (dashed arrow) cells covering more centrally located struts. F, Confocal microscopic image of center of neck (CD31 stain; original magnification, ×20) shows, in foreground, CD31-negative cells attached to intersections of struts and corresponding to tissue islands on C and, in background, deep to struts, confluent endothelial cells as on D.
At 8 weeks, the necks of the completely occluded aneurysms were covered with a thin tissue layer in which the luminal surface layer was CD31-positive cells over SMA-positive cells. Small, localized, nonendothelialized areas (CD31-negative) at the neck in these completely occluded aneurysms were covered with smooth muscle cells (SMA-positive) (Fig 3). We did not observe any CD34-positive cells in any aneurysm, suggesting that circulating progenitor cells were not the cell of origin in and around the aneurysm neck. Histologic analysis revealed that the aneurysm cavities of these completely occluded aneurysms were filled with collagenized, dense connective tissue.
Figure 3:
A, DSA image obtained immediately before flow diverter implantation shows aneurysm cavity. B, DSA image obtained 8 weeks after flow diverter implantation shows occlusion of aneurysm. C, Gross image of aneurysm neck, viewed from parent artery, shows confluent tissue covering the neck (arrow). D, Dual immunofluorescence–stained confocal microscopic image of aneurysm neck (SMA and CD31 stains; original magnification, ×20) shows a confluent smooth muscle cell layer (red area, yellow arrow) deep to an incomplete layer of endothelial cells (green area, white arrow).
In no samples did we identify thrombus alone without overlying smooth muscle and endothelial cell coverage along occluded segments of the aneurysm. Indeed, in many samples, histologic evaluation showed moderate amounts of tissue over the struts with relatively few remaining segments that were patent; however, there was substantial, ongoing aneurysm filling. This constellation of findings suggests that tissue growth, rather than diversion of flow, is required for final aneurysm occlusion at the neck.
Discussion
Clinical case series have demonstrated remarkably high rates of complete aneurysm occlusion with use of these flow diversion devices (9–12). However, our basic understanding of the mechanism of the flow diverters remains poor. This study offers clarity regarding the cellular events associated with healing of flow diversion devices placed across the necks of saccular aneurysms. The initial events, occurring within 1 day of device placement, include complete denudation of endothelial cells where the device contacts the parent artery as well as adherence of inflammatory cells to scattered intersections of the device at the neck. Endothelialization of the parent artery is quite rapid but is delayed over the aneurysm neck. Endothelialization of the neck of the aneurysm proceeds from the parent artery and depends on an underlying smooth muscle cell substrate. Circulating progenitor cells are not a factor in aneurysm healing.
Although device size and pore density have received substantial interest and investigation (13–15), the role of optimal wall apposition has been poorly studied. On the basis of the supposition that poor wall apposition diminishes the hemodynamic effects of flow diverters, many practitioners routinely inflate balloons to fully expand devices that are clearly malapposed to the vessel wall (9). Our findings suggest that, in addition to potential hemodynamic considerations, optimal wall apposition is a key modulator of the healing of the device. Because the smooth muscle and endothelial cells that grow over the struts of the device itself and provide final aneurysm closure are derived from the adjacent parent artery, full apposition of the device to the wall may be necessary for ideal healing.
It has been hypothesized that flow diverters disturb the flow and induce thrombosis in the aneurysm cavity to segregate the aneurysm from the circulation (14). However, our findings suggest that endothelialization of the flow diverter is more important than thrombus formation in the aneurysm cavity in the complete occlusion of aneurysms. We acknowledge that the domes of aneurysms may undergo substantial degrees of thrombosis, and this dome-related thrombosis likely is related to diminished flow resulting from placement of the flow diverter. We further propose that the adherence of inflammatory cells in the intersection of the device at the neck could alter the hemodynamic changes in the aneurysm. However, the constellation of findings in our study strongly suggests that complete aneurysm occlusion occurs only as a result of healing at the neck of the aneurysm, which is histologically characterized by a contiguous layer of endothelial cells overlying a smooth muscle cell substrate.
Numerous studies (16–18) have demonstrated the importance of endothelialization in the healing of aneurysms following endovascular coil embolization. Endothelial cells can be derived from bone marrow–derived endothelial progenitor cells or from the local vessel wall. Frosen et al (19) demonstrated that endothelial and neointimal cells were mostly originated from the aneurysm wall, with only a minor contribution from the bone marrow in a mouse model of saccular aneurysm. Li et al (20) transfused fluorescence-labeled cultured endothelial progenitor cells in rabbits treated with flow diverters and observed fluorescence-positive endothelial progenitor cells in the subendothelial space and around flow diverter struts. However, in our immunohistochemical analysis, we did not notice any CD34-positive cells, suggesting that the endothelial cells do not originate from the circulating progenitor cells.
Our study had some limitations. The origin of bone marrow–derived cells is typically confirmed with bone marrow transplantation experiments, which are difficult in rabbits. En face and immunohistochemical studies are qualitatively assessed. Rabbits sacrificed at early time points were not given antiplatelet therapy. We acknowledge that supraphysiologic doses of clopidogrel were used in our study. These high doses may have afffected outcomes, and, thus, our findings should be interpreted carefully. Finally, we were not able to fully analyze the relationship between thrombus formation and endothelialization. Although this study lends clarity to the cellular processes occurring at the aneurysm neck, our data do not allow us to confidently conclude the relative importance of flow disruption versus endothelialization.
The initial event following flow diversion treatment is adherence of clusters of inflammatory cells across the aneurysm neck. Endothelialization is relatively delayed, is derived exclusively from cells in the adjacent parent artery, and is dependent on an underlying smooth muscle cell substrate.
Practical application: Endothelialization of the neck of the aneurysm proceeds from cells derived from the parent artery as opposed to circulating progenitor cells in the blood. These findings can provide guidance for rational design of next-generation flow diverters, especially regarding efforts to speed endothelialization over the aneurysm neck.
Advances in Knowledge
■ The initial event following flow diversion treatment is adherence of clusters of inflammatory cells across the aneurysm neck.
■ Endothelialization of the flow diverter is derived exclusively from cells in the adjacent parent artery.
■ Aneurysm occlusion following flow diverter implantation is achieved primary by endothelialization rather than localized thrombosis.
Implication for Patient Care
■ Endothelialization of flow diverters overcomes platelet aggregation and thrombus formation after flow diverter placement and may improve the healing of intracranial saccular aneurysms.
Acknowledgments
Acknowledgments
We are grateful for stimulating discussions with Juan Cebral, PhD. We thank Covidien (Irvine, Calif) for generously providing the flow diverters for our study.
Received April 3, 2013; revision requested May 13; revision received June 11; accepted July 15; final version accepted July 19.
Funding: This research was supported by the National Institutes of Health (grant NS076491).
Disclosures of Conflicts of Interest: R.K. No relevant conflicts of interest to disclose. Y.H.D. No relevant conflicts of interest to disclose. D.D. No relevant conflicts of interest to disclose. I.R. No relevant conflicts of interest to disclose. D.A.L. No relevant conflicts of interest to disclose. D.F.K. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received consulting fees for clinical trials planning and implementation from eV3/Covidien and Codman; institution received funds for preclinical research from MicroVention, Sequent, and Codman; institution received funds for clinical trials from MicroVention and Codman; receives royalties for patent from UVA Patent Foundation. Other relationships: none to disclose.
Abbreviations:
- DSA
- digital subtraction angiography
- SMA
- smooth muscle actin
References
- 1.Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007;38(8):2346–2352. [DOI] [PubMed] [Google Scholar]
- 2.Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol 2009;30(6):1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadasivan C, Cesar L, Seong J, et al. An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke 2009;40(3):952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cebral JR, Castro MA, Appanaboyina S, Putman CM, Millan D, Frangi AF. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans Med Imaging 2005;24(4):457–467. [DOI] [PubMed] [Google Scholar]
- 5.Altes TA, Cloft HJ, Short JG, et al. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. AJR Am J Roentgenol 2000;174(2):349–354. [DOI] [PubMed] [Google Scholar]
- 6.Hoefer IE, Grundmann S, Schirmer S, et al. Aspirin, but not clopidogrel, reduces collateral conductance in a rabbit model of femoral artery occlusion. J Am Coll Cardiol 2005;46(6):994–1001. [DOI] [PubMed] [Google Scholar]
- 7.Schlitt A, Hauroeder B, Buerke M, et al. Effects of combined therapy of clopidogrel and aspirin in preventing thrombus formation on mechanical heart valves in an ex vivo rabbit model. Thromb Res 2002;107(1-2):39–43. [DOI] [PubMed] [Google Scholar]
- 8.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 2008;52(5):333–342. [DOI] [PubMed] [Google Scholar]
- 9.Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012;54(4):369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubicz B, Collignon L, Raphaeli G, De Witte O. Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: preliminary experience in 20 patients with 27 aneurysms. World Neurosurg 2011;76(1-2):114–119. [DOI] [PubMed] [Google Scholar]
- 11.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 2010;41(10):2247–2253. [DOI] [PubMed] [Google Scholar]
- 12.Wong GK, Kwan MC, Ng RY, Yu SC, Poon WS. Flow diverters for treatment of intracranial aneurysms: current status and ongoing clinical trials. J Clin Neurosci 2011;18(6):737–740. [DOI] [PubMed] [Google Scholar]
- 13.Kizilkilic O, Kocer N, Metaxas GE, Babic D, Homan R, Islak C. Utility of VasoCT in the treatment of intracranial aneurysm with flow-diverter stents. J Neurosurg 2012;117(1):45–49. [DOI] [PubMed] [Google Scholar]
- 14.Sadasivan C, Lieber BB, Cesar L, Miskolczi L, Seong J, Wakhloo AK. Angiographic assessment of the performance of flow divertors to treat cerebral aneurysms. Conf Proc IEEE Eng Med Biol Soc 2006;1:3210–3213. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Huang Q, Hong B, Li Z, Fang X, Liu J. Correlation of aneurysm occlusion with actual metal coverage at neck after implantation of flow-diverting stent in rabbit models. Neuroradiology 2012;54(6):607–613. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara S, Mawad ME, Ogata K, et al. Histopathologic findings in human cerebral aneurysms embolized with platinum coils: report of two cases and review of the literature. AJNR Am J Neuroradiol 2002;23(6):970–974. [PMC free article] [PubMed] [Google Scholar]
- 17.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91(2):284–293. [DOI] [PubMed] [Google Scholar]
- 18.Ozawa T, Tamatani S, Koike T, et al. Histological evaluation of endothelial reactions after endovascular coil embolization for intracranial aneurysm: clinical and experimental studies and review of the literature. Interv Neuroradiol 2003;9(Suppl 1):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frosen J, Marjamaa J, Myllarniemi M, et al. Contribution of mural and bone marrow–derived neointimal cells to thrombus organization and wall remodeling in a microsurgical murine saccular aneurysm model. Neurosurgery 2006;58(5):936–944; discussion 944. [DOI] [PubMed] [Google Scholar]
- 20.Li ZF, Fang XG, Yang PF, et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neurosci Ther 2013;19(5):352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]