Common features of carpet lesions at CT colonography include a broad-based flat morphology with superficially raised edges, rectal or cecal location, surface coating with oral contrast material, and multiple individual computer-aided detection marks.
Abstract
Purpose
To describe carpet lesions (laterally spreading tumors ≥ 3 cm) detected at computed tomographic (CT) colonography, including their clinical, imaging, and pathologic features.
Materials and Methods
The imaging reports for 9152 consecutive adults undergoing initial CT colonography at a tertiary center were reviewed in this HIPAA-compliant, institutional review board–approved retrospective study to identify all potential carpet lesions detected at CT colonography. Carpet lesions were defined as morphologically flat, laterally spreading tumors 3 cm or larger. For those patients with neoplastic carpet lesions, CT colonography studies were analyzed to determine maximal lesion width and height, oral contrast material coating, segmental location, and computer-aided detection (CAD) findings. Demographic data and details of clinical treatment in these patients were reviewed.
Results
Eighteen carpet lesions in 18 patients (0.2%; mean age, 67.1 years; eight men, 10 women) were identified and were subsequently confirmed at colonoscopy and pathologic examination among 20 potential flat masses (≥3 cm) prospectively identified at CT colonography (there were two nonneoplastic rectal false-positive findings). No additional neoplastic carpet lesions were found in the cohort undergoing colonoscopy after CT colonography and/or surgery (there were no false-negatives). Mean lesion width was 46.5 mm (range, 30–80 mm); mean lesion height was 7.9 mm (range, 4–14 mm). Surface retention of oral contrast material was noted in all 18 cases. All but two lesions were located in the distal rectosigmoid or proximal right colon. At CAD, 17 (94.4%) lesions were detected (mean, 6.2 CAD marks per lesion). Sixteen lesions (88.9%) demonstrated advanced histologic features, including a villous component (n = 11), high-grade dysplasia (n = 4), and invasive cancer (n = 5). Sixteen patients (88.9%) required surgical treatment for complete excision.
Conclusion
CT colonography can effectively depict carpet lesions. Common features in this series included older patient age, rectal or cecal location, surface coating with oral contrast material, multiple CAD hits, advanced yet typically benign histologic features, and surgical treatment.
© RSNA, 2013
Introduction
The clinical importance of flat or nonpolypoid colorectal lesions continues to be a major source of controversy, especially as it pertains to the U.S. population (1–10). Although nonpolypoid colorectal lesions are less common than their polypoid counterparts, substantial debate exists in terms of their epidemiology and biology. Confounding factors that further obfuscate the issue have included variable morphologic definitions, geographic and/or ethnic differences, varying detection strategies, and subjective differences in histopathologic assessment. In addition, the term “flat” may at first seem to be somewhat of a misnomer, because the majority of nonpolypoid lesions are superficially elevated and are not completely flat or even with the mucosa (2,11). With rare exceptions, the edges of flat colorectal lesions tend to be slightly raised from the surrounding normal mucosa. Controversy aside, one important subset of nonpolypoid colorectal lesions for which the clinical importance is not in dispute is large, relatively flat laterally spreading tumors, which are generally referred to as carpet lesions when they reach 3 cm or larger (10,12–14).
Although a relatively flat or plaquelike morphology decreases conspicuity compared with that of polypoid lesions, both standard optical colonoscopy and computed tomographic (CT) colonography are capable of depicting most superficially elevated nonpolypoid lesions (1,7,9,15–17). Information regarding the detection of carpet lesions at CT colonography is sparse and is limited to individual case reports or to the few cases that are buried within series that include smaller flat polyps (7,12,15). The purpose of this study was to describe carpet lesions (laterally spreading tumors ≥ 3 cm) depicted at CT colonography with regard to their clinical, imaging, and pathologic features.
Materials and Methods
Patients and Procedures
This Health Insurance Portability and Accountability Act–compliant retrospective analysis was approved by our institutional review board; the need to obtain signed informed consent was waived. The data for the study cohort were derived from reports for 9152 consecutive unique adults (mean age, 57.1 years ± 7.9 [standard deviation]; 4126 men, 5026 women) who underwent initial CT colonography at a single U.S.-based tertiary care institution (University of Wisconsin Hospital and Clinics) in a 105-month period from April 2004 through December 2012. Routine screening was by far the most frequent clinical indication (n = 8357 [91.3%]), followed by incomplete optical colonoscopy (n = 756 [8.3%]). Our protocol for bowel preparation, colonic distention, multidetector CT scanning, and CT colonography study interpretation has been previously described in detail (18–20). To briefly recap the key protocol elements, bowel preparation usually consisted of a saline laxative (magnesium citrate or sodium phosphate) in conjunction with positive oral contrast material tagging (with 2% wt/vol barium sulfate and iodine-based diatrizoate) the evening before the examination. Colonic distention was achieved with automated carbon dioxide delivery (21), followed by the acquisition of supine and prone low-radiation-dose multidetector CT images with 1.25-mm collimation, 120 kVp, variable tube current settings, and image reconstruction with standard filtered back projection at 1-mm intervals. A series of images with the patient in the decubitus position was obtained in patients with persistent inadequate distention of a colonic segment (22). CT data sets were then processed with a dedicated CT colonography software system (V3D Colon; Viatronix, Stony Brook, NY) for interpretation. This system includes combined three-dimensional (3D) and two-dimensional (2D) lesion detection with 2D confirmation (19,20,23).
For all colorectal lesions 6 mm or larger that were detected at prospective CT colonography, a morphologic category was assigned (sessile, flat, or pedunculated). Flat (nonpolypoid) colorectal polyps are characterized at CT colonography as superficially elevated, broad-based, plaquelike lesions. In general, smaller flat (nonpolypoid) lesions measuring 1–2 cm or smaller will typically measure less than 3 mm in height. However, when flat lesions exceed 3 cm in size, they may also exceed 3 mm in maximal height but will maintain a flat, plaquelike morphology without evidence of luminal constriction or compromise (13). For the purpose of this study, all detected flat (nonpolypoid) lesions 3 cm or larger that met these criteria were of potential interest, and the term “carpet lesion” was applied. In Asia, the term “laterally spreading tumor” is often used for flat lesions exceeding 20 mm in width, which may be further subdivided into “granular” and “nongranular” subtypes (10,13,14). Of note, endoscopic classification of nonpolypoid neoplasms according to the Paris system does not explicitly use the terms “carpet lesion” or “laterally spreading tumor” and is more useful for describing smaller lesions (24). Although some discourage the use of these terms (9), we believe the terms are instructive for larger flat lesions.
Data in all patients with potential carpet lesions that were detected prospectively at CT colonography were reviewed by two of the coauthors (P.J.P., with more than 10 years of CT colonography experience, and V.P.L., with 1 year of CT colonography experience) in consensus. The prospective diagnostic confidence for each lesion detected at CT colonography was scored by the interpreting radiologist by using a validated three-point scale (where a score of 3 indicated highest confidence; a score of 2, intermediate confidence, and a score of 1, lowest confidence) (25). For final inclusion, a potential lesion had to have fulfilled the morphologic criteria at CT colonography that are described above, had to have been subsequently confirmed at optical colonoscopy, and had to have shown neoplastic histologic features at pathologic examination. Advanced histologic features were defined as a prominent villous component (tubulovillous or villous), high-grade dysplasia, or invasive cancer. All confirmed carpet lesions were retrospectively reassessed by the two reviewers at CT colonography to determine segmental location, maximal lesion height and maximal lesion width (lesion size), the presence or absence of lesion surface coating with positive oral contrast material (26), and for stand-alone identification by a computer-aided detection (CAD) system. For maximal lesion size (width), both 2D and 3D CT colonography displays were used by the two reviewers, as previously described (27). Maximal lesion height relative to the surrounding normal mucosa was determined on 2D displays. In general, careful lesion measurement at optical colonoscopy can closely match the CT colonography measurement (28). To assess contrast material coating, both polyp window (width, 2000 HU; length, 0 HU) and soft-tissue window (width, 350 HU; length, 40 HU) settings were used. Subjective assessment of carpet lesion conspicuity was performed during the retrospective review to look for features that could present a diagnostic challenge. For CAD analysis, a U.S. Food and Drug Administration–approved system was used (VeraLook; iCAD, Nashua, NH). The number of individual CAD marks on each carpet lesion was recorded for each positional series (supine and prone). Finally, a detailed review of the electronic medical record was performed to extract personal and family history, subsequent clinical treatment, and ultimate patient outcome.
To evaluate for potential false-negative results at CT colonography, our entire clinical database was searched for all carpet lesions detected at colonoscopy after CT colonography. We also compared the rate of malignancy of proven carpet lesions with that of nonflat neoplastic masses (ie, polypoid, bulky, or annular masses ≥ 3 cm) detected at CT colonography within the same patient population.
Statistical Analysis
The Fisher exact test was used to assess statistical significance between groups. P < .05 was considered to indicate a statistically significant difference.
Results
Eighteen colorectal carpet neoplasms in 18 patients (mean age, 67.1 years; range, 50–91 years; eight men, 10 women) were prospectively identified in the original reports among 9152 consecutive individuals undergoing initial CT colonography (Figs 1–4, Fig E1 [online]). All of the lesions were subsequently confirmed at colonoscopy and histopathologic examination (Table). No additional neoplastic carpet lesions were found (ie, there were no false-negative findings at CT colonography) among the positive fraction subsequently evaluated at colonoscopy and/or surgery (n = 891 [9.7%]). One additional 4-cm flat cecal lesion not detected at CT colonography but found at subsequent colonoscopy proved to be hyperplastic—not neoplastic. Two additional 3-cm or larger flat rectal lesions identified at CT colonography correlated with hemorrhoids and with posttreatment changes from prostate cancer. The diagnostic confidence at prospective CT colonography study interpretation for the 18 confirmed carpet lesions was rated highest (score = 3) in 16 patients, intermediate (score = 2) in one patient with a cecal carpet lesion, and lowest (score = 1) in one patient with a rectal carpet lesion, whereas diagnostic confidence in the two patients with false-positive rectal lesions was rated lowest (score = 1) in both patients. Of the 18 patients with true-positive lesions, one had been referred for CT colonography because of incomplete optical colonoscopy; the remaining 17 patients had been referred for initial CT colonography evaluation, primarily for screening. Two patients (11.1%) had a first-degree relative with colorectal cancer.
Figure 1:
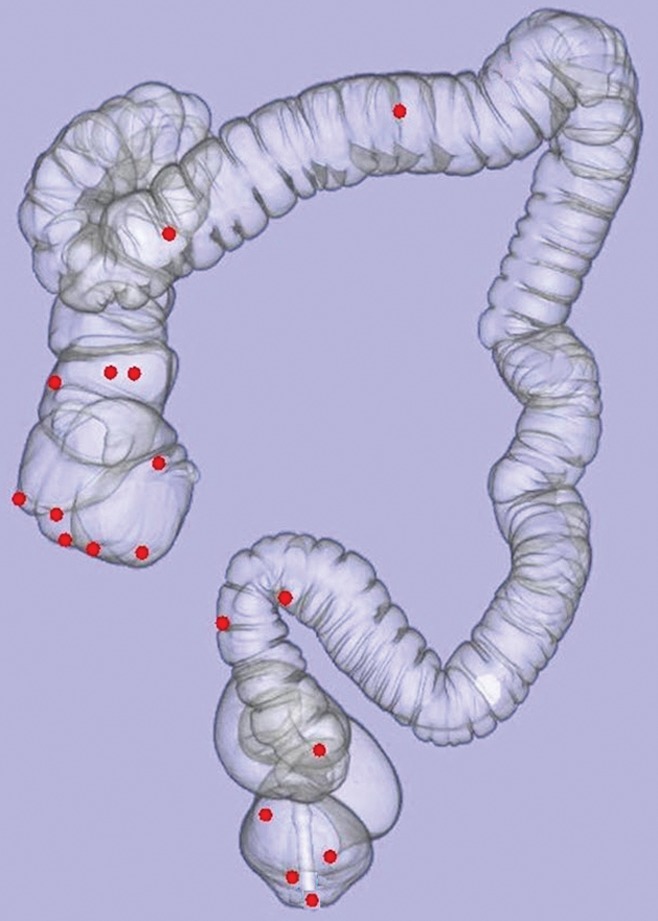
Three-dimensional CT colonography map shows anatomic location (red dot) of each of the 18 colorectal carpet lesions detected at CT colonography. Note that only two tumors (in the transverse colon) are located outside of the rectosigmoid colon (n = 7) or proximal right colon (n = 9).
Figure 4a:

(a–d) Images show sigmoid carpet lesion detected at CT colonography in 56-year-old woman (patient 8). (a) Three-dimensional endoluminal and (b, c) 2D transverse CT colonography images show a 4-cm flat mass (arrowheads). The 3D view in a shows a central depression with a single CAD mark (blue area with arrow). Note oral contrast material coating the lesion in c, whereas there is no contrast material clinging to the healthy nondependent colonic walls. This lesion measured 4 mm in maximal height at CT colonography. (d) Same-day colonoscopy confirmed the carpet lesion, which proved to be a tubulovillous adenoma with high-grade dysplasia after laparoscopic sigmoid resection.
Demographic, CT Colonography, and Histologic Data for 18 Patients with Colorectal Carpet Lesions

Note.—Lesion size and height are the maximal linear width and height of the lesion, respectively.
Please see the Materials and Methods section of the text for a description of the prospective diagnostic confidence scoring system.
Total number of marks on lesion (with patient in supine and prone positions).
HGD = high-grade dysplasia, SA = serrated adenoma, TVA = tubulovillous adenoma, VA = villous adenoma.
Figure 2b:
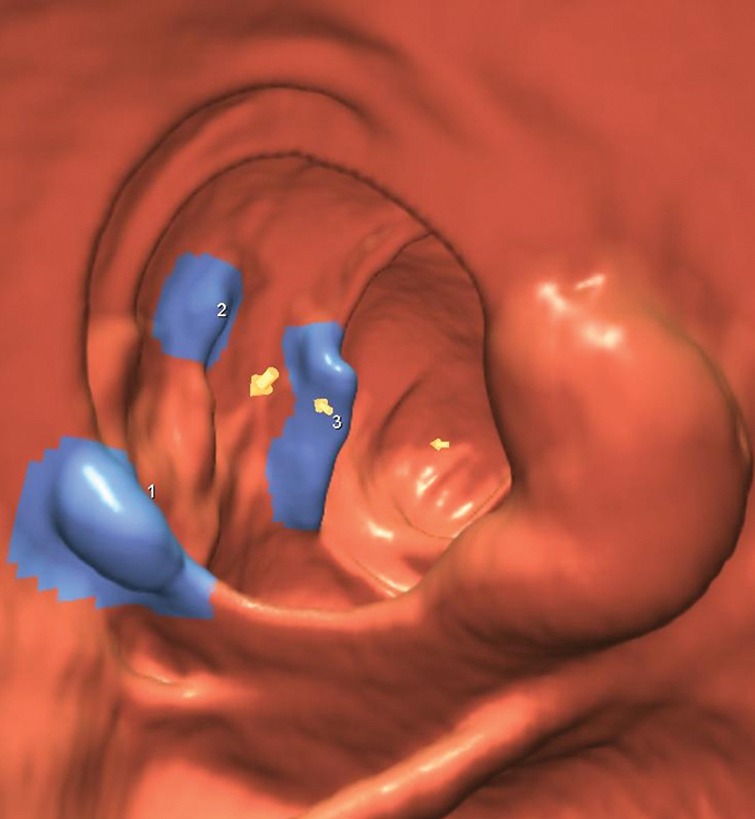
(a–d) Images show cecal carpet lesion detected at screening CT colonography in 50-year-old man (patient 3). (a) Three-dimensional colon map and (b) endoluminal CT colonography view show three CAD marks in the cecum (yellow dots in a; blue regions with arrows in b), which identify focal areas of a 3.5-cm carpet lesion. The lesion is located across from the normal-appearing ileocecal valve. (c, d) Transverse 2D images in (c) polyp and (d) soft-tissue windows confirm a flat soft-tissue lesion (arrows). Note the etching of positive oral contrast material on the surface of the lesion, which is better seen in d. (e) The lesion was confirmed at same-day endoscopy and proved to be a tubulovillous adenoma without high-grade dysplasia after laparoscopic right hemicolectomy.
Figure 2c:
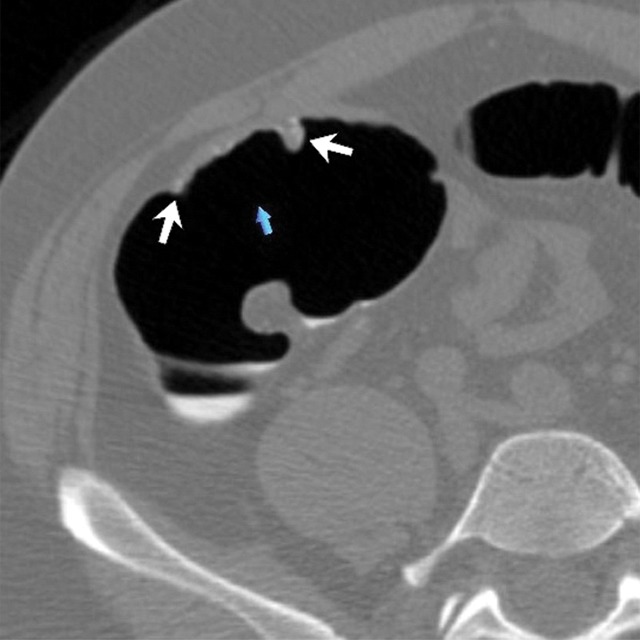
(a–d) Images show cecal carpet lesion detected at screening CT colonography in 50-year-old man (patient 3). (a) Three-dimensional colon map and (b) endoluminal CT colonography view show three CAD marks in the cecum (yellow dots in a; blue regions with arrows in b), which identify focal areas of a 3.5-cm carpet lesion. The lesion is located across from the normal-appearing ileocecal valve. (c, d) Transverse 2D images in (c) polyp and (d) soft-tissue windows confirm a flat soft-tissue lesion (arrows). Note the etching of positive oral contrast material on the surface of the lesion, which is better seen in d. (e) The lesion was confirmed at same-day endoscopy and proved to be a tubulovillous adenoma without high-grade dysplasia after laparoscopic right hemicolectomy.
Figure 2d:
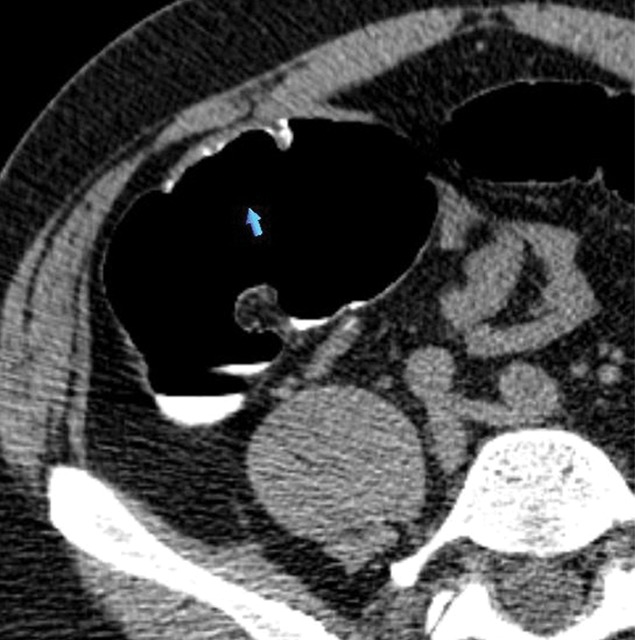
(a–d) Images show cecal carpet lesion detected at screening CT colonography in 50-year-old man (patient 3). (a) Three-dimensional colon map and (b) endoluminal CT colonography view show three CAD marks in the cecum (yellow dots in a; blue regions with arrows in b), which identify focal areas of a 3.5-cm carpet lesion. The lesion is located across from the normal-appearing ileocecal valve. (c, d) Transverse 2D images in (c) polyp and (d) soft-tissue windows confirm a flat soft-tissue lesion (arrows). Note the etching of positive oral contrast material on the surface of the lesion, which is better seen in d. (e) The lesion was confirmed at same-day endoscopy and proved to be a tubulovillous adenoma without high-grade dysplasia after laparoscopic right hemicolectomy.
Figure 2e:

(a–d) Images show cecal carpet lesion detected at screening CT colonography in 50-year-old man (patient 3). (a) Three-dimensional colon map and (b) endoluminal CT colonography view show three CAD marks in the cecum (yellow dots in a; blue regions with arrows in b), which identify focal areas of a 3.5-cm carpet lesion. The lesion is located across from the normal-appearing ileocecal valve. (c, d) Transverse 2D images in (c) polyp and (d) soft-tissue windows confirm a flat soft-tissue lesion (arrows). Note the etching of positive oral contrast material on the surface of the lesion, which is better seen in d. (e) The lesion was confirmed at same-day endoscopy and proved to be a tubulovillous adenoma without high-grade dysplasia after laparoscopic right hemicolectomy.
Figure 3a:
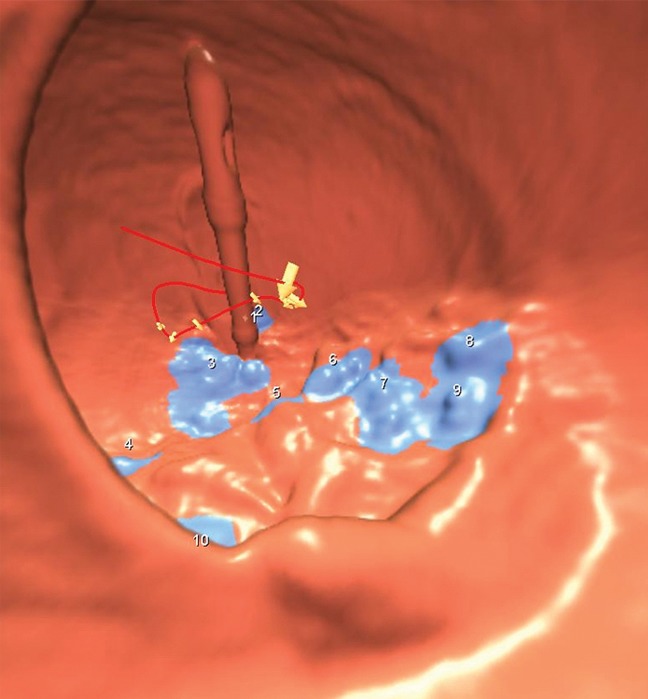
(a–c) Images show rectal carpet lesion detected at CT colonography in 65-year-old woman (patient 4). (a) Three-dimensional endoluminal CT colonography image (obtained with the patient in the prone position) that simulates a retroflexed rectal view at endoscopy shows a large 8-cm multinodular lesion carpeting the lower rectum, with multiple individual CAD marks (blue regions with arrows). Red line = automated centerline for navigation. (b) Sagittal 2D image confirms a flat soft-tissue lesion (arrow), with an area of contrast material coating (arrowheads). (c) This large yet relatively subtle lesion was confirmed at same-day endoscopy and proved to be a villous adenoma without high-grade dysplasia after transanal surgical excision.
Figure 3b:

(a–c) Images show rectal carpet lesion detected at CT colonography in 65-year-old woman (patient 4). (a) Three-dimensional endoluminal CT colonography image (obtained with the patient in the prone position) that simulates a retroflexed rectal view at endoscopy shows a large 8-cm multinodular lesion carpeting the lower rectum, with multiple individual CAD marks (blue regions with arrows). Red line = automated centerline for navigation. (b) Sagittal 2D image confirms a flat soft-tissue lesion (arrow), with an area of contrast material coating (arrowheads). (c) This large yet relatively subtle lesion was confirmed at same-day endoscopy and proved to be a villous adenoma without high-grade dysplasia after transanal surgical excision.
Figure 3c:
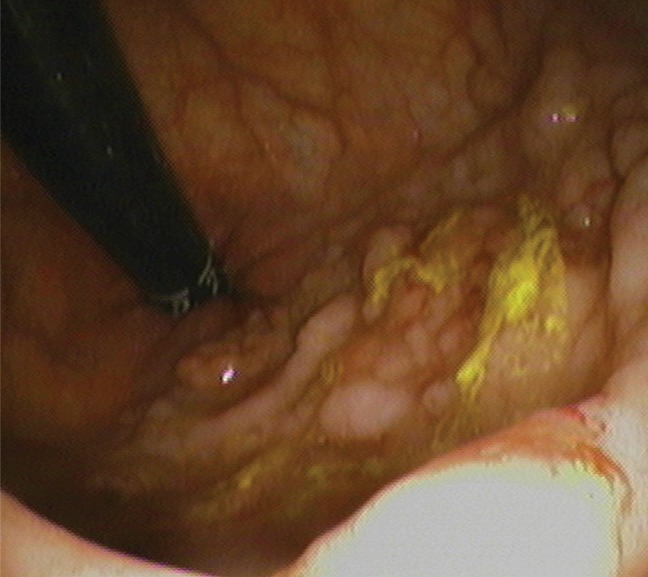
(a–c) Images show rectal carpet lesion detected at CT colonography in 65-year-old woman (patient 4). (a) Three-dimensional endoluminal CT colonography image (obtained with the patient in the prone position) that simulates a retroflexed rectal view at endoscopy shows a large 8-cm multinodular lesion carpeting the lower rectum, with multiple individual CAD marks (blue regions with arrows). Red line = automated centerline for navigation. (b) Sagittal 2D image confirms a flat soft-tissue lesion (arrow), with an area of contrast material coating (arrowheads). (c) This large yet relatively subtle lesion was confirmed at same-day endoscopy and proved to be a villous adenoma without high-grade dysplasia after transanal surgical excision.
Figure 4b:

(a–d) Images show sigmoid carpet lesion detected at CT colonography in 56-year-old woman (patient 8). (a) Three-dimensional endoluminal and (b, c) 2D transverse CT colonography images show a 4-cm flat mass (arrowheads). The 3D view in a shows a central depression with a single CAD mark (blue area with arrow). Note oral contrast material coating the lesion in c, whereas there is no contrast material clinging to the healthy nondependent colonic walls. This lesion measured 4 mm in maximal height at CT colonography. (d) Same-day colonoscopy confirmed the carpet lesion, which proved to be a tubulovillous adenoma with high-grade dysplasia after laparoscopic sigmoid resection.
Figure 4c:
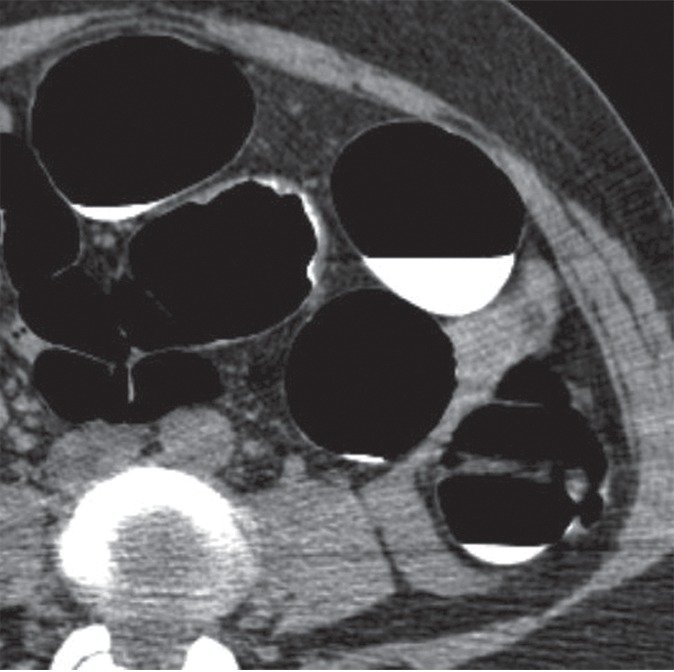
(a–d) Images show sigmoid carpet lesion detected at CT colonography in 56-year-old woman (patient 8). (a) Three-dimensional endoluminal and (b, c) 2D transverse CT colonography images show a 4-cm flat mass (arrowheads). The 3D view in a shows a central depression with a single CAD mark (blue area with arrow). Note oral contrast material coating the lesion in c, whereas there is no contrast material clinging to the healthy nondependent colonic walls. This lesion measured 4 mm in maximal height at CT colonography. (d) Same-day colonoscopy confirmed the carpet lesion, which proved to be a tubulovillous adenoma with high-grade dysplasia after laparoscopic sigmoid resection.
Figure 4d:
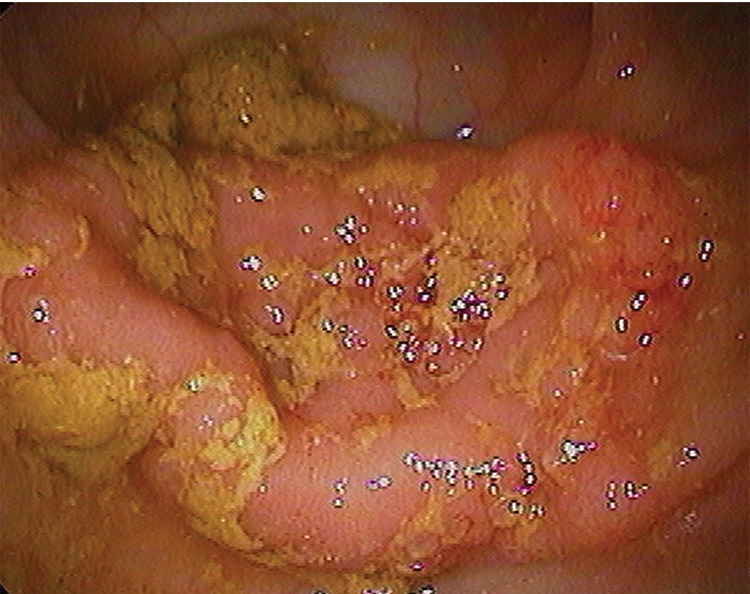
(a–d) Images show sigmoid carpet lesion detected at CT colonography in 56-year-old woman (patient 8). (a) Three-dimensional endoluminal and (b, c) 2D transverse CT colonography images show a 4-cm flat mass (arrowheads). The 3D view in a shows a central depression with a single CAD mark (blue area with arrow). Note oral contrast material coating the lesion in c, whereas there is no contrast material clinging to the healthy nondependent colonic walls. This lesion measured 4 mm in maximal height at CT colonography. (d) Same-day colonoscopy confirmed the carpet lesion, which proved to be a tubulovillous adenoma with high-grade dysplasia after laparoscopic sigmoid resection.
The overall prevalence of proven carpet lesions at CT colonography in this patient population was 0.2% (18 of 9152), or one carpet lesion for every 508 patients evaluated at CT colonography. Compared with the entire positive CT colonography cohort of 1390 patients with any lesion 6 mm or larger, patients with proven carpet lesions were older (67.1 vs 58.9 years, P = .023) and slightly more often female (55.6% vs 46.6%, P = .243). The mean age for men and women with carpet lesions was 59.0 years (range, 50–75 years) and 72.3 years (range, 52–91 years), respectively. The mean size (greatest width) of the 18 carpet lesions was 46.5 mm, with a range of 30–80 mm (Table). Mean lesion height was 7.9 mm, with a range of 4–14 mm; all lesions exceeded 3 mm in height in at least one area. There was a strong predilection for carpet lesions to be located in either the proximal or the distal large intestine, with five in the cecum, five in the rectum, four in the proximal ascending colon, and two in the distal sigmoid near the rectosigmoid junction (Fig 1). The remaining two lesions were found in the transverse colon.
At 3D endoluminal CT colonography, a slightly raised, lobulated, or subtle multifocal polypoid appearance with distortion of the normal underlying fold pattern was observed in all cases (Figs 2–4, E1 [online]). At retrospective review of lesion conspicuity, low rectal lesions were found to be the most challenging, in part because of the rectal balloon catheter (Fig E1 [online]), as well as the possibility of nonneoplastic anorectal disease. All 18 carpet lesions demonstrated a thin coating of positive oral contrast material on at least a portion of their mucosal surface. This coating was more conspicuous in soft-tissue windows (Figs 2–4). A few lesions showed areas of central depression (Fig 4), but all carpet lesions had slightly raised or nodular edges. CAD detected the carpet lesions in 94.4% of patients (17 of 18), with marks on the tumor in both the supine and prone series in 11 patients, in the supine series only in three patients, and in the prone series only in three patients (Figs 2–4, E1 [online]). Multiple CAD marks often tagged the detected lesions (Table), with an overall average of 6.2 marks per CAD-detected lesion (range, 0–16 marks per series). The one CAD false-negative result was in the one patient with a cecal carpet lesion that was designated with intermediate diagnostic confidence at the primary human interpretation.
Figure 2a:

(a–d) Images show cecal carpet lesion detected at screening CT colonography in 50-year-old man (patient 3). (a) Three-dimensional colon map and (b) endoluminal CT colonography view show three CAD marks in the cecum (yellow dots in a; blue regions with arrows in b), which identify focal areas of a 3.5-cm carpet lesion. The lesion is located across from the normal-appearing ileocecal valve. (c, d) Transverse 2D images in (c) polyp and (d) soft-tissue windows confirm a flat soft-tissue lesion (arrows). Note the etching of positive oral contrast material on the surface of the lesion, which is better seen in d. (e) The lesion was confirmed at same-day endoscopy and proved to be a tubulovillous adenoma without high-grade dysplasia after laparoscopic right hemicolectomy.
At histopathologic examination (Table), 16 (88.9%) carpet lesions were advanced, including a villous component in 11 lesions, high-grade dysplasia in four lesions, and invasive cancer in five lesions. The two carpet lesions without advanced histologic features were classified as a tubular adenoma and as a serrated adenoma. One invasive cancer was metastatic at presentation. Compared with nonflat neoplastic masses (polypoid, bulky, or annular masses ≥ 3 cm) detected at CT colonography, the rate of malignancy was cut in half for carpet lesions (27.8% vs 55.1%, P = .058), despite their slightly larger size (mean, 4.7 vs 4.0 cm, P = .115).
Sixteen patients (88.9%) ultimately required surgery for definitive treatment (Table). Thirteen patients underwent partial colectomy (nine laparoscopic, four open), and three underwent transanal surgical excision. The two other carpet lesions were removed endoscopically in piecemeal fashion by using a hot snare. Residual or recurrent tumor was identified at follow-up endoscopy 4 months later in one of these patients; the other patient died of unknown causes before endoscopic follow-up was performed.
Discussion
Carpet lesions, which are flat laterally spreading colorectal masses measuring 3 cm or greater in size, are relatively uncommon tumors in our Midwestern U.S. population—seen about once for every 500 adults undergoing initial CT colonography. On the basis of the subset of cases referred to colonoscopy, CT colonography appears to be a sensitive and specific test for the detection of these clinically important lesions, particularly when cathartic preparation, positive oral contrast material tagging, and a combined 3D-2D detection strategy are used. Among cases evaluated with colonoscopy, we found very few false-positive or false-negative CT colonography interpretations of flat lesions measuring 3 cm or larger at our institution, which had 11 CT colonography staff radiologists over the study period. Because untagged residual fecal material is known to both mimic and obscure carpet lesions (1,29,30), we believe that both a cathartic preparation and oral contrast material tagging are likely to be necessary to avoid misdiagnosis. Interestingly, the prevalence of unsuspected invasive cancer detected at screening CT colonography is similar, at about 0.2% (31), to the prevalence of carpet lesions, and detection of unsuspected invasive cancer is also optimized by the use of a cathartic preparation supplemented with oral contrast agents (32).
We identified a number of interesting features among the carpet lesions found in our CT colonography cohort. Affected individuals were typically older adults, but the sex distribution was more equal compared with that for most colorectal neoplasia, which has a male predominance. The majority of carpet lesions were located in or near the cecum and rectum, which matches earlier reported experience with barium enema examinations (13). Coating of areas along the broad tumor surface with positive oral contrast material was seen in all 18 patients. CAD successfully identified nearly all carpet lesions confirmed at colonoscopy, often peppering the tumor with multiple individual computer-generated marks. Despite the large lesion sizes (nearly 5 cm on average), these tumors were typically benign, although most demonstrated some form of advanced histologic finding. Finally, even though most carpet lesions were not malignant, almost all of them ultimately required surgical resection. Some of these features will be considered in more detail below.
In the Japanese literature (10,14), two forms of laterally spreading tumors have been described: granular or granulonodular (G-type) and nongranular (NG-type) tumors. Compared with NG-type lesions (also referred to by some as flat or F-type lesions), the G-type is more common, has a more lobulated appearance, and is more histologically benign. Not surprisingly, the granulonodular type of laterally spreading tumor is more readily detectable than the nongranular type at CT colonography in Japan (33). Our carpet lesions more closely resemble the G-type description, whereas the NG (flat) type are rarely if ever detected in our patient population at both CT colonography and colonoscopy. In terms of the Paris classification (24), carpet lesions, which demonstrate superficially elevated edges, all fall into the IIa category or, less frequently, the IIa + IIc category. Completely flat (IIb) or completely depressed (IIc) lesions were not seen in our screening population, matching other U.S. experiences (2). Although a maximal height of greater than 3 mm is a useful definition for flat lesions measuring less than 3 cm in the largest dimension (7,15,34), carpet lesions (which by definition are > 3 cm in lateral size) will typically exceed 3 mm in maximal height, as seen in all of our patients. Classification systems such as the CT Colonography Reporting and Data System, or C-RADS (34), need to be revised to accommodate carpet lesions as an exception to this 3-mm height requirement, as these colorectal masses are clearly flat (nonpolypoid) in overall morphology.
It is worth emphasizing that superficially elevated flat lesions are generally less aggressive than polypoid lesions of a similar size (3,5,7). One exception to this rule may be the rare depressed lesions (IIc in the Paris classification) described primarily in Asian series (1,10,14). Depressed lesions make up a very small fraction of all flat (nonpolypoid) colorectal lesions and tend to have detectable raised edges when described in U.S. series (ie, IIa + IIc lesions according to the Paris classification system) (2). The carpet lesions in our series were less aggressive than nonflat neoplastic masses, which are more often malignant. Although these lesions are less conspicuous than polypoid lesions, CT colonography has the ability to detect nondiminutive superficially elevated flat and carpet-type colorectal lesions when both 3D and 2D views are used (8,15,17). One feature that increases lesion conspicuity at CT colonography is the tendency for these lesions to coat with oral contrast material—particularly lesions with a villous component (26). This phenomenon serves as a beacon for detection (29) and likely corresponds to the mucoid cap seen at post–CT colonography colonoscopy. In contrast, adherent stool will demonstrate internal tagging of oral contrast material, allowing for its distinction from carpet and other true lesions (35). Nonetheless, flat lesions are overrepresented among discordant cases sent to colonoscopy, representing either false-positive findings at CT colonography or false-negative findings at colonoscopy (36). Of colonoscopy-confirmed flat lesions that are missed at CT colonography, most prove to be hyperplastic at histologic examination (15,37,38), as seen with our one case. For flat lesions measuring 3 cm or larger (carpet lesions), our findings demonstrate that CT colonography is highly effective.
As noted above, the tendency for carpet lesions to arise within the rectum and cecum was initially recognized at barium enema examination (13). This is fortuitous for CT colonography, as the rectum and cecum are generally the most capacious and easily distended segments, allowing for optimized evaluation. The carpet lesions within the cecum and proximal ascending colon may be relevant in terms of primary colonoscopy detection, as such right-sided tumors may be more frequently missed at conventional examination (39,40). Although only one carpet lesion in our series had serrated histologic features, arguably, the large hyperplastic cecal carpet lesion should also now fall in the category of the serrated polyp pathway. In our experience, the vast majority of serrated polyps identified in our CT colonography population are smaller than 3 cm, but do tend to be right sided. Despite the capacious nature of the rectum, which allows easier 3D evaluation at CT colonography in general, the low rectal area near the anorectal junction still presents a diagnostic challenge at CT colonography (29,30). Reasons for this include its specific anatomy, the wide variety of anorectal disease, and the close proximity of the balloon catheter. To minimize misdiagnosis in the low rectum, the CT technologist should deflate the balloon or advance the rectal catheter prior to obtaining the second positional series.
Results of previous studies (15,41–43) have shown that CAD is capable of detecting nondiminutive superficially elevated flat lesions at CT colonography with sensitivities of up to 90% in some series, but with overall performance that is generally decreased relative to that for polypoid lesions. Park et al (15) demonstrated a 90% CAD sensitivity for flat cancers measuring up to 25 mm, whereas Taylor and colleagues (43) showed a CAD sensitivity of up to 83% for flat (IIa and IIa + IIc) cancers with a mean size of 25 mm. Little data in the published literature exist on CAD detection of larger carpet lesions, but our series demonstrates high sensitivity (94.4%). In general, multiple focal areas of the carpet lesions were detected with individual CAD marks, rather than a single detection of the entire lesion. CAD performance would presumably decrease for the uncommon nongranular or IIb types of laterally spreading tumors described predominately in Asian series (43).
The rate of surgical resection in our series (88.9%) was much higher than what has been reported in Asian clinical series, although most procedures were laparoscopic. For example, in a series from Tanaka et al (14), 40% of G-type and nearly 80% of NG-type laterally spreading tumors were treated with endoscopic mucosal resection (EMR). Two of the lesions in our series were removed endoscopically with a piecemeal technique, but none were resected by means of EMR. Reasons for this difference are likely multifactorial but reflect more recent adoption of EMR techniques in the United States.
We acknowledge limitations to the current study. Because most patients evaluated with CT colonography in our clinical practice are not sent to optical colonoscopy, we cannot formally assess population prevalence and accuracy (ie, sensitivity and specificity) for the entire cohort. Furthermore, it is well recognized that carpet lesions can be missed at colonoscopy (44), which serves as our necessary reference standard, such that there is no true standard of reference. The endoscopists at our center now more frequently use narrow band imaging for suspected flat lesions but rarely use chromoendoscopy (dye spraying), which could conceivably reveal the extremely rare IIb lesion (completely flat), of which we have no proven cases. Nonetheless, we have shown that CT colonography is sensitive for the detection of carpet lesions confirmed at colonoscopy, which were all morphologically flat but superficially raised masses. Similarly, our reported prevalence of carpet lesions refers strictly to prevalence at CT colonography, which could be slightly lower than the true prevalence if any lesions are missed. Finally, this study represents a single-center experience in a fairly homogeneous Midwestern population, and, hence, prevalence statistics may vary for other cohorts.
In conclusion, we found that CT colonography is an effective tool for the detection of colorectal carpet lesions, which are uncommon but typically affect older adults without a clear male predominance. Common features of carpet lesions at CT colonography include a broad-based flat morphology with superficially raised edges, rectal or cecal location, surface coating with oral contrast material, and multiple individual CAD marks. Although advanced histologic features are typical, most carpet lesions are benign yet often require surgical management.
Advances in Knowledge
■ Carpet lesions are a relatively uncommon finding at CT colonography, with an approximate rate of one case per 500 patient examinations.
■ CT colonography can depict carpet lesions with high positive predictive value, and no false-negative findings were identified in the patient cohort that subsequently underwent colonoscopy.
■ Oral contrast material from bowel preparation tends to coat the surface of carpet lesions, increasing their conspicuity at CT colonography.
■ Computer-aided detection is successful in identifying most (>90%) carpet lesions that are detected by the human reader at CT colonography, typically with multiple “hits” on each lesion.
Implication for Patient Care
■ Our experience with carpet lesions at CT colonography could help raise awareness of the imaging manifestations of these uncommon but important lesions.
SUPPLEMENTAL FIGURE
Received April 4, 2013; revision requested May 7; revision received May 29; accepted June 18; final version accepted July 17.
Disclosures of Conflicts of Interest: P.J.P. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for Viatronix, Check-Cap, Mindways, and Braintree; is cofounder of VirtuoCTC. Other relationships: none to disclose. V.P.L. No relevant conflicts of interest to disclose. J.M.W. No relevant conflicts of interest to disclose. G.D.K. No relevant conflicts of interest to disclose. D.H.K. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for Viatronix; holds stock and/or stock options in Digital Artforms; is cofounder of VirtuoCTC. Other relationships: none to disclose.
Funding: This research was supported by the National Institutes of Health (grants 1R01CA144835-01 and 1R01CA169331-01).
Abbreviations:
- CAD
- computer-aided detection
- 3D
- three-dimensional
- 2D
- two-dimensional
References
- 1.Park SH, Lee SS, Choi EK, et al. Flat colorectal neoplasms: definition, importance, and visualization on CT colonography. AJR Am J Roentgenol 2007;188(4):953–959. [DOI] [PubMed] [Google Scholar]
- 2.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 2008;299(9):1027–1035. [DOI] [PubMed] [Google Scholar]
- 3.Pickhardt PJ, Levin B, Bond JH. Screening for nonpolypoid colorectal neoplasms. JAMA 2008;299(23):2743; author reply 2743–2744. [DOI] [PubMed] [Google Scholar]
- 4.Bond JH. Small flat adenomas appear to have little clinical importance in Western countries. Gastrointest Endosc 1995;42(2):184–187. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien MJ, Winawer SJ, Zauber AG, et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol 2004;2(10):905–911. [DOI] [PubMed] [Google Scholar]
- 6.Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS. Detection of flat lesions in the colon with CT colonography. Abdom Imaging 2002;27(3):292–300. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Kim DH, Robbins JB. Flat (nonpolypoid) colorectal lesions identified at CT colonography in a U.S. screening population. Acad Radiol 2010;17(6):784–790. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol 2004;183(5):1343–1347. [DOI] [PubMed] [Google Scholar]
- 9.Soetikno R, Friedland S, Kaltenbach T, Chayama K, Tanaka S. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology 2006;130(2):566–576; quiz 588–589. [DOI] [PubMed] [Google Scholar]
- 10.Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg 2000;24(9):1081–1090. [DOI] [PubMed] [Google Scholar]
- 11.Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. AJR Am J Roentgenol 2009;193(1):40–46. [DOI] [PubMed] [Google Scholar]
- 12.Galdino GM, Yee J. Carpet lesion on CT colonography: a potential pitfall. AJR Am J Roentgenol 2003;180(5):1332–1334. [DOI] [PubMed] [Google Scholar]
- 13.Rubesin SE, Saul SH, Laufer I, Levine MS. Carpet lesions of the colon. RadioGraphics 1985;5(4):537–552. [Google Scholar]
- 14.Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001;54(1):62–66. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Kim SY, Lee SS, et al. Sensitivity of CT colonography for nonpolypoid colorectal lesions interpreted by human readers and with computer-aided detection. AJR Am J Roentgenol 2009;193(1):70–78. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol 2004;183(5):1343–1347. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto T, Mitsuzaki K, Utsunomiya D, et al. Detection of flat colorectal polyps at screening CT colonography in comparison with conventional polypoid lesions. Acta Radiol 2012;53(7):714–719. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357(14):1403–1412. [DOI] [PubMed] [Google Scholar]
- 19.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol 2007;189(2):290–298. [DOI] [PubMed] [Google Scholar]
- 20.Pickhardt PJ, Kim DH. CT colonography: principles and practice of virtual colonoscopy. Philadelphia, Pa: Saunders, 2010. [Google Scholar]
- 21.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR Am J Roentgenol 2006;186(6):1491–1496. [DOI] [PubMed] [Google Scholar]
- 22.Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdom Imaging 2011;36(5):538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT colonography. AJR Am J Roentgenol 2007;189(6):1451–1456. [DOI] [PubMed] [Google Scholar]
- 24.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon—November 30 to December 1, 2002. Gastrointest Endosc 2003;58(6 Suppl):S3–S43. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Choi JR, Nugent PA, Schindler WR. The effect of diagnostic confidence on the probability of optical colonoscopic confirmation of potential polyps detected on CT colonography: prospective assessment in 1,339 asymptomatic adults. AJR Am J Roentgenol 2004;183(6):1661–1665. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. J Comput Assist Tomogr 2006;30(1):51–57. [DOI] [PubMed] [Google Scholar]
- 27.Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology 2005;236(3):872–878. [DOI] [PubMed] [Google Scholar]
- 28.Barancin C, Pickhardt PJ, Kim DH, et al. Prospective blinded comparison of polyp size on computed tomography colonography and endoscopic colonoscopy. Clin Gastroenterol Hepatol 2011;9(5):443–445. [DOI] [PubMed] [Google Scholar]
- 29.Pickhardt PJ, Kim DHCT. CT colonography: pitfalls in interpretation. Radiol Clin North Am 2013;51(1):69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickhardt PJ. Missed lesions at CT colonography: lessons learned. Abdom Imaging 2013;38(1):82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickhardt PJ, Kim DH, Meiners RJ, et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology 2010;255(1):83–88. [DOI] [PubMed] [Google Scholar]
- 32.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and meta-analysis. Radiology 2011;259(2):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iinuma G, Miyake M, Uchida K, Arai Y, Muramatsu Y, Moriyama N. The challenge: detection of early-stage superficial colorectal lesions. In: Lefere P, Gryspeerdt S, eds. Virtual colonoscopy: a practical guide. 2nd ed. Berlin, Germany: Springer-Verlag, 2010; 153–163. [Google Scholar]
- 34.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology 2005;236(1):3–9. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Choi JHR. Electronic cleansing and stool tagging in CT colonography: advantages and pitfalls with primary three-dimensional evaluation. AJR Am J Roentgenol 2003;181(3):799–805. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. Eur Radiol 2010;20(7):1651–1656. [DOI] [PubMed] [Google Scholar]
- 37.Pickhardt PJ, Choi JR, Hwang I, Schindler WR. Nonadenomatous polyps at CT colonography: prevalence, size distribution, and detection rates. Radiology 2004;232(3):784–790. [DOI] [PubMed] [Google Scholar]
- 38.Cornett D, Barancin C, Roeder B, et al. Findings on optical colonoscopy after positive CT colonography exam. Am J Gastroenterol 2008;103(8):2068–2074. [DOI] [PubMed] [Google Scholar]
- 39.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150(1):1–8. [DOI] [PubMed] [Google Scholar]
- 40.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154(1):22–30. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence EM, Pickhardt PJ, Kim DH, Robbins JB. Colorectal polyps: stand-alone performance of computer-aided detection in a large asymptomatic screening population. Radiology 2010;256(3):791–798. [DOI] [PubMed] [Google Scholar]
- 42.Summers RM, Yao JH, Pickhardt PJ, et al. Computed tomographic virtual colonoscopy computer-aided polyp detection in a screening population. Gastroenterology 2005;129(6):1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor SA, Iinuma G, Saito Y, Zhang J, Halligan S. CT colonography: computer-aided detection of morphologically flat T1 colonic carcinoma. Eur Radiol 2008;18(8):1666–1673. [DOI] [PubMed] [Google Scholar]
- 44.Glick SN, Teplick SK, Balfe DM, et al. Large colonic neoplasms missed by endoscopy. AJR Am J Roentgenol 1989;152(3):513–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


